CIESC Journal ›› 2021, Vol. 72 ›› Issue (5): 2426-2435.DOI: 10.11949/0438-1157.20201283
• Reviews and monographs • Previous Articles Next Articles
WANG Xin1( ),ZHAO Peng1,LI Qingyang2,TIAN Pingfang1(
),ZHAO Peng1,LI Qingyang2,TIAN Pingfang1( )
)
Received:2020-09-08
Revised:2020-11-25
Online:2021-05-05
Published:2021-05-05
Contact:
TIAN Pingfang
通讯作者:
田平芳
作者简介:王欣(1996—),女,硕士研究生,基金资助:CLC Number:
WANG Xin, ZHAO Peng, LI Qingyang, TIAN Pingfang. Research advances in semiconductor synthetic biology[J]. CIESC Journal, 2021, 72(5): 2426-2435.
王欣, 赵鹏, 李清扬, 田平芳. 半导体合成生物学的研究进展[J]. 化工学报, 2021, 72(5): 2426-2435.
Add to citation manager EndNote|Ris|BibTeX
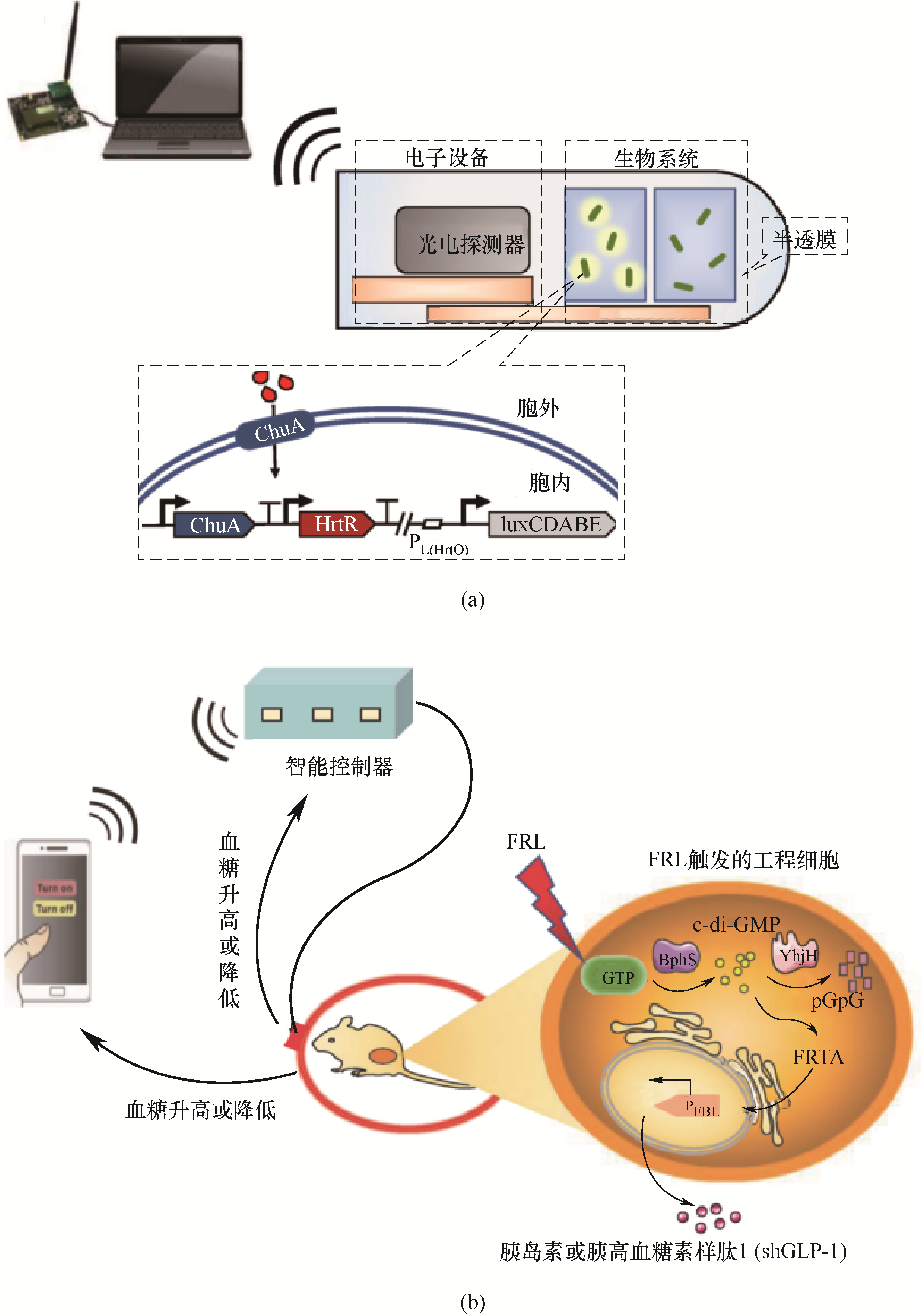
Fig.2 Schematic diagram of absorbable microelectronic equipment (IMBED)(a); Smart phones regulate engineered cells to achieve semi-automatic blood glucose homeostasis (b)ChuA—outer membrane transporter of E. coli; HrtR—heme reactive transcriptional suppressor of Lactococcus lactis; luxCDABE—luciferase gene cluster; FRL—far-red light; BphS—bacterial light-activated cyclic diguanylate monophosphate (c-di-GMP) synthase; YhjH—c-di-GMP-specific phosphodiesterase
| 1 | Bartley B A, Kim K, Medley J K, et al. Synthetic biology: engineering living systems from biophysical principles [J]. Biophysical Journal, 2017, 112(6): 1050-1058. |
| 2 | Cheng A A, Lu T K. Synthetic biology: an emerging engineering discipline [J]. Annual Review of Biomedical Engineering, 2012, 14(1): 155-178. |
| 3 | Tian B, Xu S, Rogers J A, et al. Roadmap on semiconductor-cell biointerfaces [J]. Physical Biology, 2018, 15(3): 031002. |
| 4 | Fu W, Chaiboonchoe A, Khraiwesh B, et al. Intracellular spectral recompositioning of light enhances algal photosynthetic efficiency [J]. Sci. Adv., 2017, 3(9): e1603096. |
| 5 | Royanian S, Ziabari A A, Yousefi R. Efficiency enhancement of ultra-thin CIGS solar cells using bandgap grading and embedding Au plasmonic nanoparticles [J]. Plasmonics, 2020, 15: 1173-1182. |
| 6 | Shockley W, Queisser H J. Detailed balance limit of efficiency of p-n junction solar cells [J]. Journal of Applied Physics, 1961, 32(3): 510-519. |
| 7 | Zhang T, Tremblay P L. Hybrid photosynthesis-powering biocatalysts with solar energy captured by inorganic devices [J]. Biotechnology for Biofuels, 2017, 10(1): 249. |
| 8 | Claassens N J, Sousa D Z, dos Santos V A, et al. Harnessing the power of microbial autotrophy [J]. Nature Reviews Microbiology, 2016, 14(11): 692-706. |
| 9 | Dilek K D, Daniel G N. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis [J]. Acc. Chem. Res., 2019, 52(11): 3143-3148. |
| 10 | Wei W, Sun P, Li Z, et al. A surface-display biohybrid approach to light-driven hydrogen production in air [J]. Sci. Adv., 2018, 4(2): eaap9253. |
| 11 | Guo J, Suastegui M, Sakimoto K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 12 | Liu C, Gallagher J J, Sakimoto K K, et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals [J]. Nano Lett., 2015, 15(5): 3634-3639. |
| 13 | Sakimoto K K, Wong A B, Yang P. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production [J]. Science, 2016, 351(6268): 74-77. |
| 14 | Xu M, Tremblay P L, Jiang L, et al. Stimulating bioplastic production with light energy by coupling Ralstonia eutropha with the photocatalyst graphitic carbon nitride [J]. Green Chemistry, 2019, 21(9): 2392-2400. |
| 15 | Tremblay P L, Xu M, Chen Y, et al. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction[J]. iScience, 2020, 23(1): 100784. |
| 16 | Kim W, Ng J K, Kunitake M E, et al. Interfacing silicon nanowires with mammalian cells [J]. Journal of the American Chemical Society, 2007, 129(23): 7228-7229. |
| 17 | 张宁, 徐开凯, 陈彦旭, 等.金属-氧化物-半导体硅发光器件在集成电路中的应用前景[J]. 物理学报, 2019, 68(16): 91-96. |
| Zhang N, Xu K K, Chen Y X, et al. Application prospect of metal-oxide-semiconductor silicon light emitting devices in integrated circuits [J]. Acta Physica Sinica, 2019, 68(16): 91-96. | |
| 18 | Brown K A, Harris D F, Wilker M B, et al. Light-driven dinitrogen reduction catalyzed by a CdS: nitrogenase MoFe protein biohybrid [J]. Science, 2016, 352(6284): 448-450. |
| 19 | Liu C, Colon B C, Ziesack M, et al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis [J]. Science, 2016, 352(6290): 1210-1213. |
| 20 | Zhang H, Liu H, Tian Z, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nat. Nanotechnol., 2018, 13(10): 900-905. |
| 21 | Sytnyk M, Jakesova M, Litvinukova M, et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals [J]. Nat. Commun., 2017, 8(1): 91. |
| 22 | Kracke F, Vassilev I, Kromer J O. Microbial electron transport and energy conservation-the foundation for optimizing bioelectrochemical systems [J]. Front. Microbiol., 2015, 6: 575. |
| 23 | Ye J, Yu J, Zhang Y, et al. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid [J]. Applied Catalysis B: Environmental, 2019, 257: 117916. |
| 24 | Kornienko N, Sakimoto K K, Herlihy D M, et al. Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production [J]. Proc. Natl. Acad. Sci. USA, 2016, 113(42): 11750-11755. |
| 25 | Cestellos-Blanco S, Zhang H, Kim J M, et al. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis [J]. Nat. Catal., 2020, 3: 245-255. |
| 26 | Shi L, Dong H, Reguera G, et al. Extracellular electron transfer mechanisms between microorganisms and minerals [J]. Nat. Rev. Microbiol., 2016, 14(10): 651-662. |
| 27 | Jensen H M, Albers A E, Malley K R, et al. Engineering of a synthetic electron conduit in living cells [J]. Proc. Natl. Acad. Sci. USA, 2010, 107(45): 19213-19218. |
| 28 | Sakimoto K K, Kornienko N, Cestellos-Blanco S, et al. Physical biology of the materials-microorganism interface [J]. Journal of the American Chemical Society, 2018, 140(6): 1978-1985. |
| 29 | Gai P, Yu W, Zhao H, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid [J]. Angew. Chem. Int. Ed., 2020, 59(18): 7224-7229. |
| 30 | Suástegui M, Yu Ng C, Chowdhury A, et al. Multilevel engineering of the upstream module of aromatic amino acid biosynthesis in, Saccharomyces cerevisiae, for high production of polymer and drug precursors [J]. Metab. Eng., 2017, 42: 134-144. |
| 31 | Snyder P J, LaJeunesse D R, Reddy P, et al. Bioelectronics communication: encoding yeast regulatory responses using nanostructured gallium nitride thin films [J]. Nanoscale, 2018, 10: 11506–11516. |
| 32 | Kladko D V, Zakharzhevskii M A, Vinogradov V V. Magnetic field-mediated control of whole-cell biocatalysis [J]. J. Phys. Chem. Lett., 2020, 11: 8989-8996. |
| 33 | Updike S J, Hicks G P. The enzyme electrode [J]. Nature, 1967, 214(5092): 986-988. |
| 34 | Mimee M, Nadeau P, Hayward A, et al. An ingestible bacterial-electronic system to monitor gastrointestinal health [J]. Science, 2018, 360(6391): 915-918. |
| 35 | Shao J, Xue S, Yu G, et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice [J]. Sci. Transl. Med., 2017, 9(387): eaal2298. |
| 36 | Li F, Tang J, Geng J, et al. Polymeric DNA hydrogel: design, synthesis and applications [J]. Progress in Polymer Science, 2019, 98: 101163. |
| 37 | Han J, Cui Y, Han X, et al. Super-soft DNA/dopamine-grafted-dextran hydrogel as dynamic wire for electric circuits switched by a microbial metabolism process [J]. Adv. Sci. (Weinh), 2020, 7(13): 2000684. |
| 38 | Tang J, Yao C, Gu Z, et al. Super-soft and super-elastic DNA robot with magnetically driven navigational locomotion for cell delivery in confined space [J]. Angew. Chem. Int. Ed., 2020, 59(6): 2490-2495. |
| 39 | Hamada S, Yancey K G, Pardo Y, et al. Dynamic DNA material with emergent locomotion behavior powered by artificial metabolism [J]. Science Robotics, 2019, 4(29): eaaw3512. |
| 40 | Nawroth J C, Lee H, Feinberg A W, et al. A tissue-engineered jellyfish with biomimetic propulsion [J]. Nat. Biotechnol., 2012, 30(8): 792-797. |
| 41 | Cvetkovic C, Raman R, Chan V, et al. Three-dimensionally printed biological machines powered by skeletal muscle [J]. Proc. Natl. Acad. Sci. USA, 2014, 111(28): 10125-10130. |
| 42 | Feinberg A W, Feigel A, Shevkoplyas S S, et al. Muscular thin films for building actuators and powering devices[J]. Science, 2007, 317(5843): 1366-1370. |
| 43 | Justus K B, Hellebrekers T, Lewis D D, et al. A biosensing soft robot: autonomous parsing of chemical signals through integrated organic and inorganic interfaces [J]. Science Robotics, 2019, 4(31): eaax0765. |
| 44 | Strukov D B, Likharev K K. Defect-tolerant architectures for nanoelectronic crossbar memories [J]. Journal of Nanoscience and Nanotechnology, 2007, 7(1): 151-167. |
| 45 | Jiang Y, Tian B. Inorganic semiconductor biointerfaces[J]. Nat. Rev. Mater., 2018, 3(12): 473-490. |
| 46 | Allentoft M E, Collins M, Harker D, et al. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils [J]. Proc. Biol. Sci., 2012, 279(1748): 4724-4733. |
| 47 | Grass R N, Heckel R, Puddu M, et al. Robust chemical preservation of digital information on DNA in silica with error-correcting codes [J]. Angew. Chem. Int. Ed., 2015, 54(8): 2552-2555. |
| 48 | Bonnet J, Colotte M, Coudy D, et al. Chain and conformation stability of solid-state DNA: implications for room temperature storage [J]. Nucleic Acids Research, 2010, 38(5): 1531-1546. |
| 49 | Akram F, Haq I U, Ali H, et al. Trends to store digital data in DNA: an overview [J]. Mol. Biol. Rep., 2018, 45(5): 1479-1490. |
| 50 | Extance A. How DNA could store all the world's data [J]. Nature, 2016, 537(7618): 22-24. |
| 51 | Panda D, Molla K A, Baig M J, et al. DNA as a digital information storage device: hope or hype? [J]. 3 Biotech, 2018, 8(5): 239. |
| 52 | Church G M, Gao Y, Kosuri S. Next-generation digital information storage in DNA [J]. Science, 2012, 337(6102): 1628. |
| 53 | Greenberg A, Hamilton J, Maltz D A, et al. The cost of a cloud: research problems in data center networks [J]. Acm Sigcomm Computer Communication Review, 2008, 39(1): 68-73. |
| 54 | de Silva P Y, Ganegoda G U. New trends of digital data storage in DNA [J]. Biomed. Res. Int., 2016, 2016: 8072463. |
| 55 | Ceze L, Nivala J, Strauss K. Molecular digital data storage using DNA [J]. Nature Reviews Genetics, 2019, 20(8): 456-466. |
| 56 | Ping Z, Ma D, Huang X, et al. Carbon-based archiving: current progress and future prospects of DNA-based data storage [J]. Gigascience, 2019, 8(6): giz075. |
| 57 | Schwartz J J, Lee C, Shendure J. Accurate gene synthesis with tag-directed retrieval of sequence-verified DNA molecules [J]. Nature Methods, 2012, 9(9): 913-915. |
| 58 | Erlich Y, Zielinski D. DNA fountain enables a robust and efficient storage architecture [J]. Science, 2017, 355(6328): 950-954. |
| 59 | Huffman D A. A method for the construction of minimum-redundancy codes [J]. Resonance, 2006, 11(2): 91-99. |
| 60 | Blawat M, Gaedke K, Huetter I, et al. Forward error correction for DNA data storage [J]. Procedia Computer Science, 2016, 80: 1011-1022. |
| 61 | Goldman N, Bertone P, Chen S, et al. Towards practical, high-capacity, low-maintenance information storage in synthesized DNA [J]. Nature, 2013, 494(7435): 77-80. |
| 62 | Bornholt J, Lopez R, Carmean D, et al. A DNA-based archival storage system [J]. ACM SIGPLAN Notices, 2016, 51(4): 637-649. |
| 63 | Baum E B. Building an associative memory vastly larger than the brain [J]. Science, 1995, 268(5210): 583-585. |
| 64 | Yazdi S M, Yuan Y, Ma J, et al. A rewritable, random-access DNA-based storage system [J]. Sci. Rep., 2015, 5: 14138. |
| 65 | Organick L, Ang S D, Chen Y J, et al. Random access in large-scale DNA data storage [J]. Nature Biotechnology, 2018, 36: 242-248. |
| 66 | Goodwin S, Mcpherson J D, Mccombie W R. Coming of age: ten years of next-generation sequencing technologies [J]. Nat. Rev. Genet., 2016, 17(6): 333-351. |
| 67 | Shendure J, Balasubramanian S, Church G M, et al. DNA sequencing at 40: past, present and future [J]. Nature, 2017, 550(7676): 345-353. |
| 68 | Deamer D, Akeson M, Branton D. Three decades of nanopore sequencing [J]. Nature Biotechnology, 2016, 34(5): 518-524. |
| 69 | Shipman S L, Nivala J, Macklis J D, et al. CRISPR-Cas encoding of a digital movie into the genomes of a population of living bacteria [J]. Nature, 2017, 547(7663): 345-349. |
| 70 | Jain S, Farnoud F, Schwartz M, et al. Duplication-correcting codes for data storage in the DNA of living organisms [J]. IEEE Transactions on Information Theory, 2017, 63(8): 4996-5010. |
| 71 | Nguyen H H, Park J, Park S J, et al. Long-term stability and integrity of plasmid-based DNA data storage [J]. Polymers (Basel), 2018, 10(1): 28. |
| 72 | Tian B, Lieber C M. Nanowired bioelectric interfaces [J]. Chem. Rev., 2019, 119(15): 9136-9152. |
| [1] | Lingding MENG, Ruqing CHONG, Feixue SUN, Zihui MENG, Wenfang LIU. Immobilization of carbonic anhydrase on modified polyethylene membrane and silica [J]. CIESC Journal, 2023, 74(8): 3472-3484. |
| [2] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [3] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [4] | Chunlei ZHAO, Liang GUO, Cong GAO, Wei SONG, Jing WU, Jia LIU, Liming LIU, Xiulai CHEN. Metabolic engineering of Escherichia coli for chondroitin production [J]. CIESC Journal, 2023, 74(5): 2111-2122. |
| [5] | Xin LIU, Jun GE, Chun LI. Light-driven microbial hybrid systems improve level of biomanufacturing [J]. CIESC Journal, 2023, 74(1): 330-341. |
| [6] | Xue LIU, Lijuan ZHANG, Guangrong ZHAO. Commensalistic Escherichia coli coculture for biosynthesis of daidzein [J]. CIESC Journal, 2022, 73(9): 4015-4024. |
| [7] | Xinzhe ZHANG, Wentao SUN, Bo LYU, Chun LI. Oxidative modification of plant natural products and microbial manufacturing [J]. CIESC Journal, 2022, 73(7): 2790-2805. |
| [8] | Mai ZHANG, Yao TIAN, Zhiqi GUO, Ye WANG, Guangjin DOU, Hao SONG. Design and optimization of photocatalysis-biological hybrid system for green synthesis of fuels and chemicals [J]. CIESC Journal, 2022, 73(7): 2774-2789. |
| [9] | Jiachen SUN, Wentao SUN, Hui SUN, Bo LYU, Chun LI. Licorice flavone synthase Ⅱ catalyzes liquiritigenin to specifically synthesize 7,4′-dihydroxyflavone [J]. CIESC Journal, 2022, 73(7): 3202-3211. |
| [10] | Yi SUN, Teng ZHANG, Bo LYU, Chun LI. Improvement for fine regulation of microbial cell factory by intracellular biosensors [J]. CIESC Journal, 2022, 73(2): 521-534. |
| [11] | Wei SONG, Jinhui WANG, Guipeng HU, Xiulai CHEN, Liming LIU, Jing WU. Cascade catalysis for the synthesis of (R)-β-tyrosine [J]. CIESC Journal, 2022, 73(1): 352-361. |
| [12] | Yukun ZHENG, Qing SUN, Zhen CHEN, Huimin YU. Progress for chemicals production via microbial cell factory: selecting several small molecules and macromolecular products as examples [J]. CIESC Journal, 2021, 72(12): 6109-6121. |
| [13] | Nan SU, Yinan WU, Yinyee TAN, Lihua JIN, Chong ZHANG, Aikawa SHIMPEI, Hasunuma TOMOHISA, Kondo AKIHIKO, Xinhui XING. Comparative omics study of Spirulinaplatensis mutants based on ARTP mutagenesis breeding system [J]. CIESC Journal, 2021, 72(12): 6298-6310. |
| [14] | Chunhui FAN,Zongye GAO,Shanjing YAO,Dongqiang LIN. Quantitative characterization of non-specific adsorption on affinity chromatography resins [J]. CIESC Journal, 2021, 72(10): 5218-5225. |
| [15] | Hui ZHOU,Zhifeng TIAN,Xiaowei TANG,Zhilong XIU. Urease-driven preparation of calcium carbonate micro-nanoparticles with different polymorphs [J]. CIESC Journal, 2021, 72(10): 5319-5329. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||