CIESC Journal ›› 2021, Vol. 72 ›› Issue (9): 4786-4795.DOI: 10.11949/0438-1157.20201921
• Separation engineering • Previous Articles Next Articles
Wei WANG( ),Weixing QIAN,Hongfang MA,Weiyong YING,Haitao ZHANG(
),Weixing QIAN,Hongfang MA,Weiyong YING,Haitao ZHANG( )
)
Received:2020-12-28
Revised:2021-06-14
Online:2021-09-05
Published:2021-09-05
Contact:
Haitao ZHANG
通讯作者:
张海涛
作者简介:王伟(1996—),男,硕士研究生,基金资助:CLC Number:
Wei WANG, Weixing QIAN, Hongfang MA, Weiyong YING, Haitao ZHANG. A theoretical study on adsorption-diffusion of dimethyl ether carbonylation on pyridine-modified H-MOR[J]. CIESC Journal, 2021, 72(9): 4786-4795.
王伟, 钱伟鑫, 马宏方, 应卫勇, 张海涛. 吡啶修饰H-MOR上二甲醚羰基化吸附-扩散理论研究[J]. 化工学报, 2021, 72(9): 4786-4795.
Add to citation manager EndNote|Ris|BibTeX
| 分子/模型 | Nads/(mol/mol) | Eads/(kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | |||||
| Py-H-AlMOR (2×1×1) | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | ||||
| Py-H-AlMOR (2×1×2) | 7.039 | 17.434 | 6.154 | -5.179 | -11.421 | -13.177 | ||||
Table 1 The average adsorption capacity (Nads) and adsorption energy (Eads) of the main reactants and product molecules in different periodic models in the 493 K-2.5 MPa z-axis extension direction (Al-T1O7 as an example)
| 分子/模型 | Nads/(mol/mol) | Eads/(kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | |||||
| Py-H-AlMOR (2×1×1) | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | ||||
| Py-H-AlMOR (2×1×2) | 7.039 | 17.434 | 6.154 | -5.179 | -11.421 | -13.177 | ||||
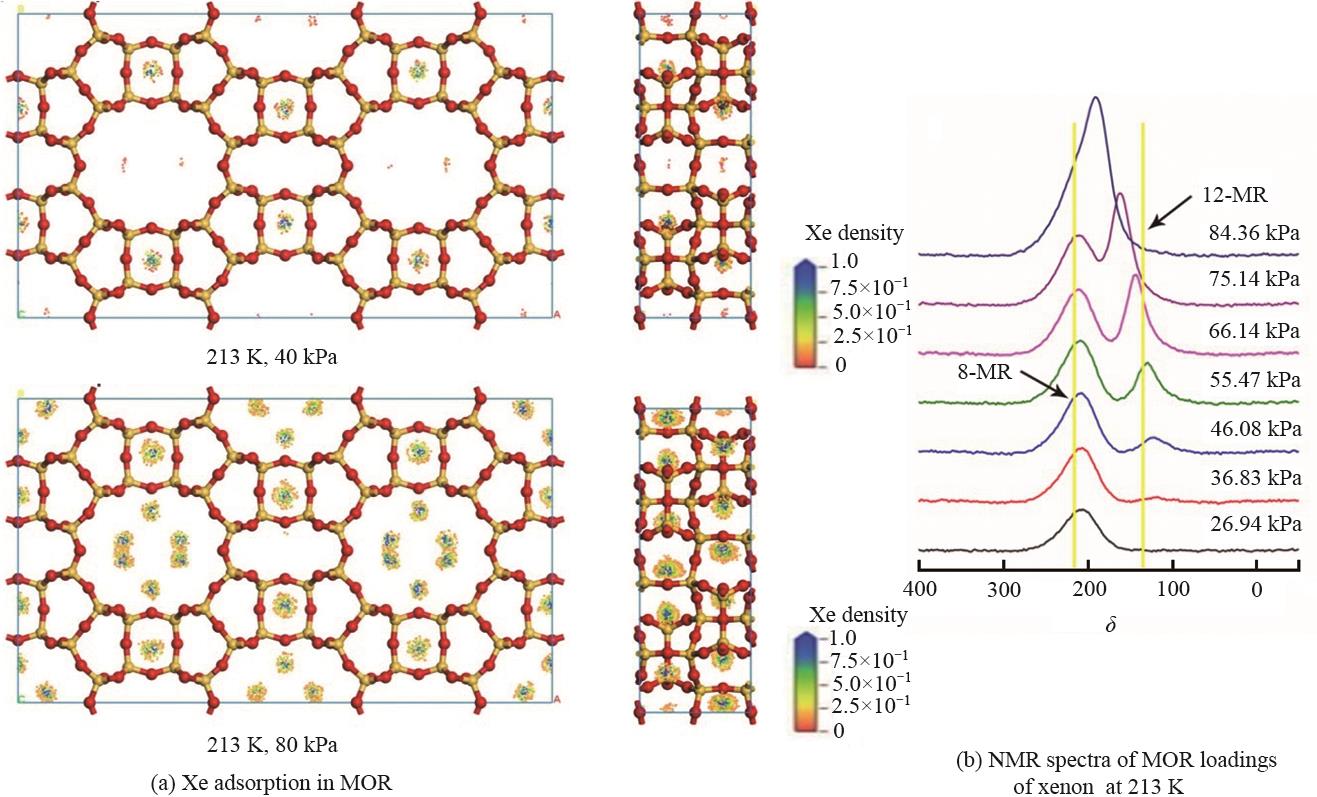
Fig.3 The adsorption density distribution of 129Xe at 213 K on the MOR (2×1×1) periodic model (a) NMR spectra of 129Xe under different pressures in reference experiment (b)
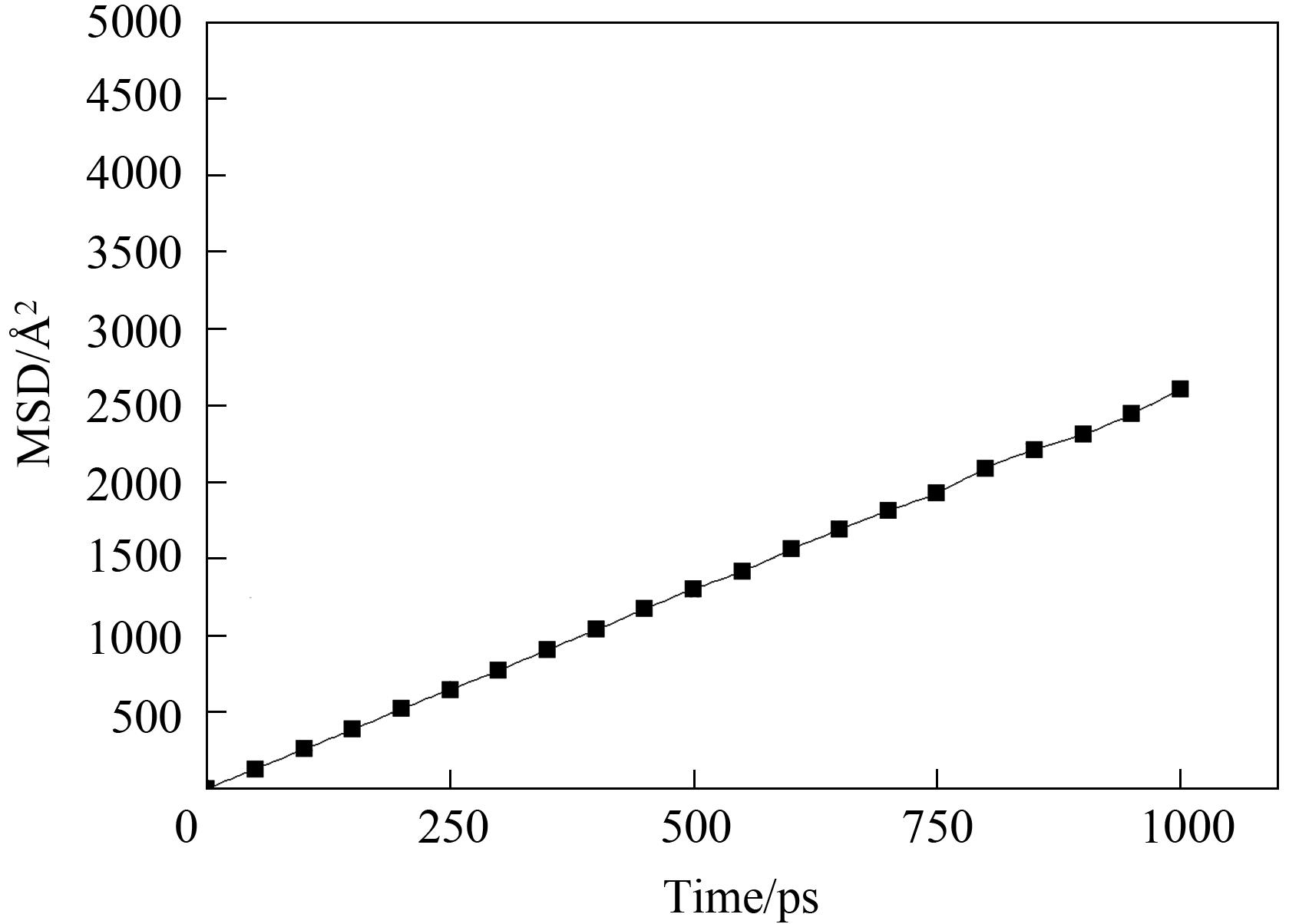
Fig.4 The relationship between the mean square displacement (MSD) of the CH4 molecule at 493 K and the simulation time in the MOR (2×1×1) period model
| 模型/分子 | Nads/(mol/mol) | Eads/(kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | ||||||
| Al-T1O7 | 4.531 | 12.815 | 7.550 | -4.902 | -12.241 | -14.856 | |||||
| Py-Al-T1O7 | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | |||||
| Al-T2O2 | 4.459 | 13.519 | 8.150 | -4.904 | -12.298 | -15.772 | |||||
| Py-Al-T2O2 | 3.462 | 8.988 | 2.785 | -5.198 | -11.313 | -13.085 | |||||
| Al-T3O1 | 4.632 | 12.338 | 7.254 | -4.917 | -12.328 | -15.201 | |||||
| Py-Al-T3O1 | 3.432 | 8.695 | 3.107 | -5.196 | -11.263 | -13.142 | |||||
| Al-T4O2 | 4.489 | 13.444 | 7.949 | -4.893 | -12.143 | -14.516 | |||||
| Py-Al-T4O2 | 3.359 | 8.224 | 2.777 | -5.195 | -11.251 | -13.062 | |||||
Table 2 Average adsorption capacity (Nads) and adsorption energy (Eads) of main reactants and product molecules in different acid models at 493 K-2.5 MPa
| 模型/分子 | Nads/(mol/mol) | Eads/(kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | ||||||
| Al-T1O7 | 4.531 | 12.815 | 7.550 | -4.902 | -12.241 | -14.856 | |||||
| Py-Al-T1O7 | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | |||||
| Al-T2O2 | 4.459 | 13.519 | 8.150 | -4.904 | -12.298 | -15.772 | |||||
| Py-Al-T2O2 | 3.462 | 8.988 | 2.785 | -5.198 | -11.313 | -13.085 | |||||
| Al-T3O1 | 4.632 | 12.338 | 7.254 | -4.917 | -12.328 | -15.201 | |||||
| Py-Al-T3O1 | 3.432 | 8.695 | 3.107 | -5.196 | -11.263 | -13.142 | |||||
| Al-T4O2 | 4.489 | 13.444 | 7.949 | -4.893 | -12.143 | -14.516 | |||||
| Py-Al-T4O2 | 3.359 | 8.224 | 2.777 | -5.195 | -11.251 | -13.062 | |||||
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Al-T1O7 | Al-T2O2 | Al-T3O1 | Al-T4O2 | |
| 1∶1 | 0.045 | 0.058 | 0.048 | 0.055 |
| 2∶1 | 0.082 | 0.098 | 0.082 | 0.092 |
| 5∶1 | 0.227 | 0.222 | 0.302 | 0.280 |
| 10∶1 | 0.417 | 0.450 | 0.422 | 0.402 |
| 20∶1 | 0.794 | 0.847 | 0.800 | 0.758 |
| 27∶1(opt) | 0.990 | 0.971 | 1.020 | 1.042 |
| 50∶1 | 1.754 | 1.724 | 2.000 | 1.754 |
Table 3 Average adsorption ratio under different feed ratios at 493 K-2.5 MPa in H-AlMOR
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Al-T1O7 | Al-T2O2 | Al-T3O1 | Al-T4O2 | |
| 1∶1 | 0.045 | 0.058 | 0.048 | 0.055 |
| 2∶1 | 0.082 | 0.098 | 0.082 | 0.092 |
| 5∶1 | 0.227 | 0.222 | 0.302 | 0.280 |
| 10∶1 | 0.417 | 0.450 | 0.422 | 0.402 |
| 20∶1 | 0.794 | 0.847 | 0.800 | 0.758 |
| 27∶1(opt) | 0.990 | 0.971 | 1.020 | 1.042 |
| 50∶1 | 1.754 | 1.724 | 2.000 | 1.754 |
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Py-Al- T1O7 | Py-Al- T2O2 | Py-Al- T3O1 | Py-Al- T4O2 | |
| 1∶1 | 0.299 | 0.267 | 0.270 | 0.269 |
| 2∶1 | 0.427 | 0.391 | 0.402 | 0.385 |
| 5∶1 | 0.934 | 0.855 | 0.926 | 0.926 |
| 8∶1(opt) | 0.980 | 1.000 | 1.042 | 0.990 |
| 10∶1 | 1.053 | 1.053 | 1.111 | 1.075 |
| 20∶1 | 1.724 | 1.754 | 1.667 | 1.754 |
| 50∶1 | 3.571 | 3.448 | 3.226 | 3.226 |
Table 4 Average adsorption ratio under different feed ratios at 493 K-2.5 MP in Py-H-AlMOR
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Py-Al- T1O7 | Py-Al- T2O2 | Py-Al- T3O1 | Py-Al- T4O2 | |
| 1∶1 | 0.299 | 0.267 | 0.270 | 0.269 |
| 2∶1 | 0.427 | 0.391 | 0.402 | 0.385 |
| 5∶1 | 0.934 | 0.855 | 0.926 | 0.926 |
| 8∶1(opt) | 0.980 | 1.000 | 1.042 | 0.990 |
| 10∶1 | 1.053 | 1.053 | 1.111 | 1.075 |
| 20∶1 | 1.724 | 1.754 | 1.667 | 1.754 |
| 50∶1 | 3.571 | 3.448 | 3.226 | 3.226 |
| 1 | Boronat M, Martínez-Sánchez C, Law D, et al. Enzyme-like specificity in zeolites: a unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO[J]. Journal of the American Chemical Society, 2008, 130(48): 16316-16323. |
| 2 | Rasmussen D B, Christensen J M, Temel B, et al. Ketene as a reaction intermediate in the carbonylation of dimethyl ether to methyl acetate over mordenite[J]. Angewandte Chemie International Edition, 2015, 54(25): 7261-7264. |
| 3 | Li X J, Liu X H, Liu S L, et al. Activity enhancement of ZSM-35 in dimethyl ether carbonylation reaction through alkaline modifications[J]. RSC Advances, 2013, 3(37): 16549. |
| 4 | Zhou H, Zhu W L, Shi L, et al. Promotion effect of Fe in mordenite zeolite on carbonylation of dimethyl ether to methyl acetate[J]. Catalysis Science & Technology, 2015, 5(3): 1961-1968. |
| 5 | 宋庆锋, 张勇, 曾清湖. 合成气直接转化制乙醇工艺路线的技术经济分析[J]. 工业催化, 2013, 21(6): 17-21. |
| Song Q F, Zhang Y, Zeng Q H. Techno-economic analysis of production process of syngas to ethanol[J]. Industrial Catalysis, 2013, 21(6): 17-21. | |
| 6 | 王辉, 吴志连, 邰志军, 等. 合成气经二甲醚羰基化及乙酸甲酯加氢制无水乙醇的研究进展[J]. 化工进展, 2019, 38(10): 4497-4503. |
| Wang H, Wu Z L, Tai Z J, et al. Advances in synthesis of anhydrous ethanol from syngas via carbonylation of dimethyl ether and hydrogenation of methyl acetate[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4497-4503. | |
| 7 | Bhan A, Iglesia E. A link between reactivity and local structure in acid catalysis on zeolites[J]. Accounts of Chemical Research, 2008, 41(4): 559-567. |
| 8 | Cheung P, Bhan A, Sunley G J, et al. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites[J]. Journal of Catalysis, 2007, 245(1): 110-123. |
| 9 | Cheung P, Bhan A, Sunley G J, et al. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites[J]. Angewandte Chemie International Edition, 2006, 45(10): 1617-1620. |
| 10 | Bhan A, Allian A D, Sunley G J, et al. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls[J]. Journal of the American Chemical Society, 2007, 129(16): 4919-4924. |
| 11 | Feng P, Zhang G Q, Zang K L, et al. A theoretical study on the selective adsorption behavior of dimethyl ether and carbon monoxide on H-FER zeolites[J]. Chemical Physics Letters, 2017, 684: 279-284. |
| 12 | Li B J, Xu J, Han B, et al. Insight into dimethyl ether carbonylation reaction over mordenite zeolite from in situ solid-state NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2013, 117(11): 5840-5847. |
| 13 | Boronat M, Martínez C, Corma A. Mechanistic differences between methanol and dimethyl ether carbonylation in side pockets and large channels of mordenite[J]. Physical Chemistry Chemical Physics, 2011, 13(7): 2603-2612. |
| 14 | Rasmussen D B, Christensen J M, Temel B, et al. Reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite-a combined DFT/experimental study[J]. Catalysis Science & Technology, 2017, 7(5): 1141-1152. |
| 15 | 赵娜, 牛君阳, 刘亚华, 等. 预处理条件及金属离子改性对H-MOR分子筛的DME羰基化性能影响[J]. 化工学报, 2015, 66(9): 3504-3510. |
| Zhao N, Niu J Y, Liu Y H, et al. Influence of pretreatment and metal cation modification of H-MOR zeolite on performance of DME carbonylation[J]. CIESC Journal, 2015, 66(9): 3504-3510. | |
| 16 | Wang M X, Huang S Y, Lyu J, et al. Modifying the acidity of H-MOR and its catalytic carbonylation of dimethyl ether[J]. Chinese Journal of Catalysis, 2016, 37(9): 1530-1537. |
| 17 | Liu J L, Xue H F, Huang X M, et al. Stability enhancement of H-mordenite in dimethyl ether carbonylation to methyl acetate by pre-adsorption of pyridine[J]. Chinese Journal of Catalysis, 2010, 31(7): 729-738. |
| 18 | 袁淑萍, 段云波, 王建国, 等. 吡啶在H-MOR分子筛孔道中吸附的量子化学研究[J]. 催化学报, 2006, 27(8): 664-670. |
| Yuan S P, Duan Y B, Wang J G, et al. Study on pyridine adsorption in H-MOR by quantum chemistry[J]. Chinese Journal of Catalysis, 2006, 27(8): 664-670. | |
| 19 | Sano T, Wakabayashi S, Oumi Y, et al. Synthesis of large mordenite crystals in the presence of aliphatic alcohol[J]. Microporous and Mesoporous Materials, 2001, 46(1): 67-74. |
| 20 | Millini R, Frigerio F, Bellussi G, et al. A priori selection of shape-selective zeolite catalysts for the synthesis of 2, 6-dimethylnaphthalene[J]. Journal of Catalysis, 2003, 217(2): 298-309. |
| 21 | Cox S D, Gier T E, Stucky G D, et al. Inclusion tuning of nonlinear optical materials: switching the SHG of p-nitroaniline and 2-methyl-p-nitroaniline with molecular sieve hosts[J]. Journal of the American Chemical Society, 1988, 110(9): 2986-2987. |
| 22 | Chibani S, Chebbi M, Lebègue S, et al. A DFT investigation of the adsorption of iodine compounds and water in H-, Na-, Ag-, and Cu-mordenite[J]. The Journal of Chemical Physics, 2016, 144(24): 244705. |
| 23 | Liu Z Q, Yi X F, Wang G R, et al. Roles of 8-ring and 12-ring channels in mordenite for carbonylation reaction: from the perspective of molecular adsorption and diffusion[J]. Journal of Catalysis, 2019, 369: 335-344. |
| 24 | Baerlocher C, McCusker L B, Liu Z. Database of Zeolite Structures[EB/OL]. [2019-12-10]. . |
| 25 | Meunier M. Introduction to Materials Studio[J]. EPJ Web of Conferences, 2012, 30: 04001. |
| 26 | Akten E D, Siriwardane R, Sholl D S. Monte Carlo simulation of single-and binary-component adsorption of CO2, N2, and H2 in zeolite Na-4A[J]. Energy & Fuels, 2003, 17(4): 977-983. |
| 27 | Dunne L J, Manos G, Du Z M. Exact statistical mechanical one-dimensional lattice model of alkane binary mixture adsorption in zeolites and comparision with Monte-Carlo simulations[J]. Chemical Physics Letters, 2003, 377(5/6): 551-556. |
| 28 | 殷开梁, 邹定辉, 杨波, 等. Materials Studio软件涉及力场中氢键的研究[J]. 计算机与应用化学, 2006, 23(12): 1335-1340. |
| Yin K L, Zou D H, Yang B, et al. Investigation of H-bonding for the related force fields in materials studio software[J]. Computers and Applied Chemistry, 2006, 23(12): 1335-1340. | |
| 29 | Wu C F, Xu W J. Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin[J]. Polymer, 2007, 48(18): 5440-5448. |
| 30 | Sun H. COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds[J]. The Journal of Physical Chemistry B, 1998, 102(38): 7338-7364. |
| 31 | Wang C M, Li B W, Wang Y D, et al. Insight into the topology effect on the diffusion of ethene and propene in zeolites: a molecular dynamics simulation study[J]. Journal of Energy Chemistry, 2013, 22(6): 914-918. |
| 32 | Wu J Y, Liu Q L, Xiong Y, et al. Molecular simulation of water/alcohol mixtures' adsorption and diffusion in zeolite 4A membranes[J]. The Journal of Physical Chemistry B, 2009, 113(13): 4267-4274. |
| 33 | Zhou J, Zhou T R. Material structure simulation techniques and applications[J]. Materials Science Forum, 2007, 561/562/563/564/565: 1793-1796. |
| 34 | Frenkel D, Smit B. Monte Carlo simulations in various ensembles[M]//Understanding Molecular Simulation. Amsterdam: Elsevier, 2002: 111-137. |
| 35 | He T, Liu X C, Xu S T, et al. Role of 12-ring channels of mordenite in DME carbonylation investigated by solid-state NMR[J]. The Journal of Physical Chemistry C, 2016, 120(39): 22526-22531. |
| [1] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [2] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [3] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [4] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [5] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [6] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [7] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [8] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [9] | Zijian WANG, Ming KE, Jiahan LI, Shuting LI, Jinru SUN, Yanbing TONG, Zhiping ZHAO, Jiaying LIU, Lu REN. Progress in preparation and application of short b-axis ZSM-5 molecular sieve [J]. CIESC Journal, 2023, 74(4): 1457-1473. |
| [10] | Rong WANG, Yonghong WANG, Xinru ZHANG, Jinping LI. Construction of 6FDA-based polyimide carbon molecular sieve membranes for gas separation and its application [J]. CIESC Journal, 2023, 74(4): 1433-1445. |
| [11] | Xiangning HU, Yuanbo YIN, Chen YUAN, Yun SHI, Cuiwei LIU, Qihui HU, Wen YANG, Yuxing LI. Experimental study on visualization of refined oil migration in soil [J]. CIESC Journal, 2023, 74(4): 1827-1835. |
| [12] | Yu PAN, Zihang WANG, Jiayun WANG, Ruzhu WANG, Hua ZHANG. Heat and moisture performance study of Cur-LiCl coated heat exchanger [J]. CIESC Journal, 2023, 74(3): 1352-1359. |
| [13] | Xuanjun WU, Chao WANG, Zijian CAO, Weiquan CAI. Deep learning model of fixed bed adsorption breakthrough curve hybrid-driven by data and physical information [J]. CIESC Journal, 2023, 74(3): 1145-1160. |
| [14] | Xiaowan PENG, Xiaonan GUO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Modeling and simulation of CH4/N2 separation process with two absorption-adsorption columns using ZIF-8 slurry [J]. CIESC Journal, 2023, 74(2): 784-795. |
| [15] | Jinlin MENG, Yu WANG, Qunfeng ZHANG, Guanghua YE, Xinggui ZHOU. Pore network model of low-temperature nitrogen adsorption-desorption in mesoporous materials [J]. CIESC Journal, 2023, 74(2): 893-903. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||