CIESC Journal ›› 2023, Vol. 74 ›› Issue (2): 784-795.DOI: 10.11949/0438-1157.20221488
• Separation engineering • Previous Articles Next Articles
Xiaowan PENG( ), Xiaonan GUO, Chun DENG(
), Xiaonan GUO, Chun DENG( ), Bei LIU, Changyu SUN, Guangjin CHEN
), Bei LIU, Changyu SUN, Guangjin CHEN
Received:2022-11-15
Revised:2023-01-14
Online:2023-03-21
Published:2023-02-05
Contact:
Chun DENG
彭晓婉( ), 郭笑楠, 邓春(
), 郭笑楠, 邓春( ), 刘蓓, 孙长宇, 陈光进
), 刘蓓, 孙长宇, 陈光进
通讯作者:
邓春
作者简介:彭晓婉(1995—),女,博士研究生,1836160461@qq.com
基金资助:CLC Number:
Xiaowan PENG, Xiaonan GUO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Modeling and simulation of CH4/N2 separation process with two absorption-adsorption columns using ZIF-8 slurry[J]. CIESC Journal, 2023, 74(2): 784-795.
彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795.
Add to citation manager EndNote|Ris|BibTeX
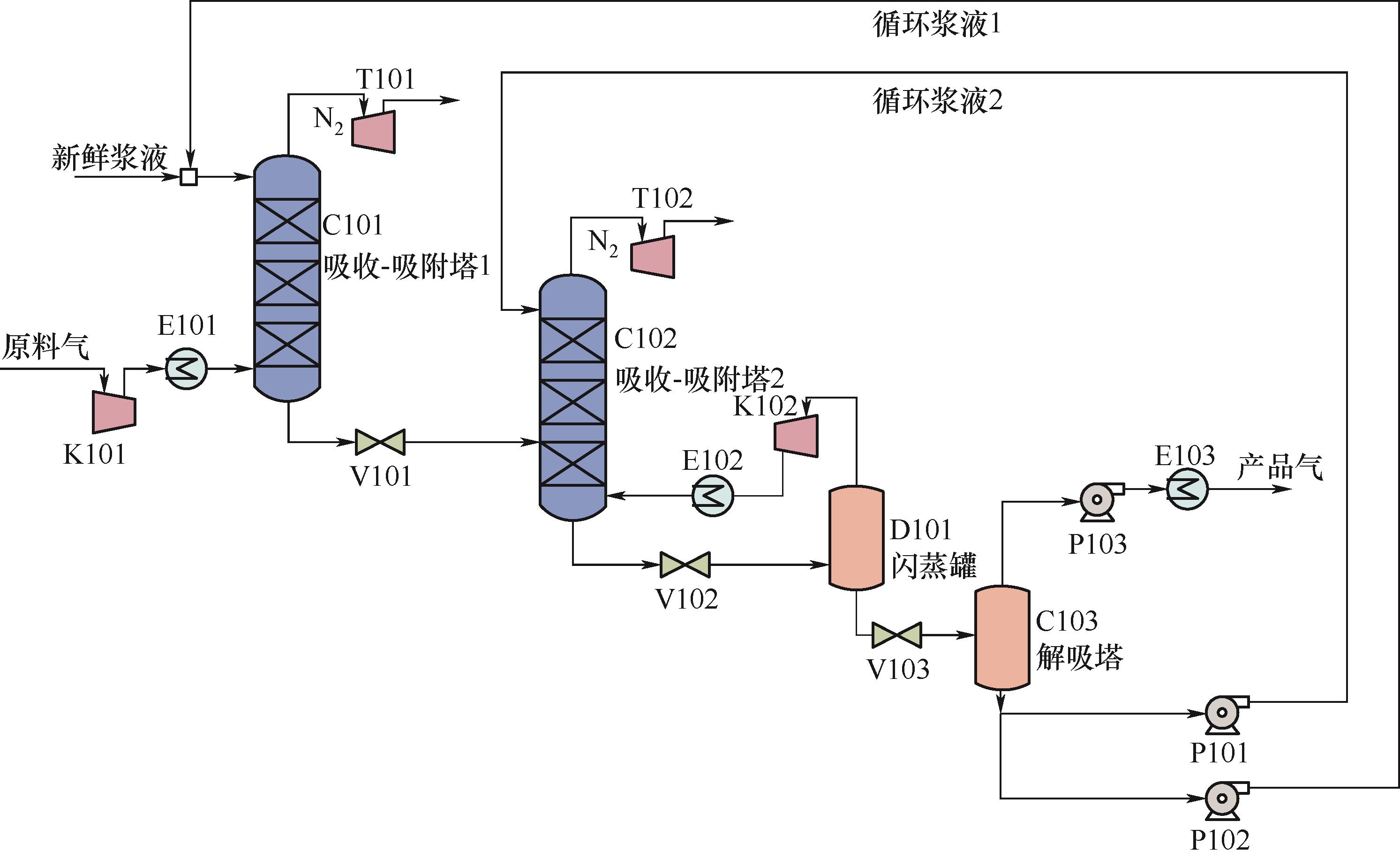
Fig.1 Schematic diagram of twin columns of ZIF-8 slurry absorption-adsorption separation of CH4/N2 C101, C102—absorption-adsorption column; D101—flash tank; C103—desorber; K101, K102—gas compressor; P101, P102—circulation slurry pump; P103—vacuum pump; T101、T102—turbine; V101, V102, V103—valve; E101, E102, E103—heat exchanger
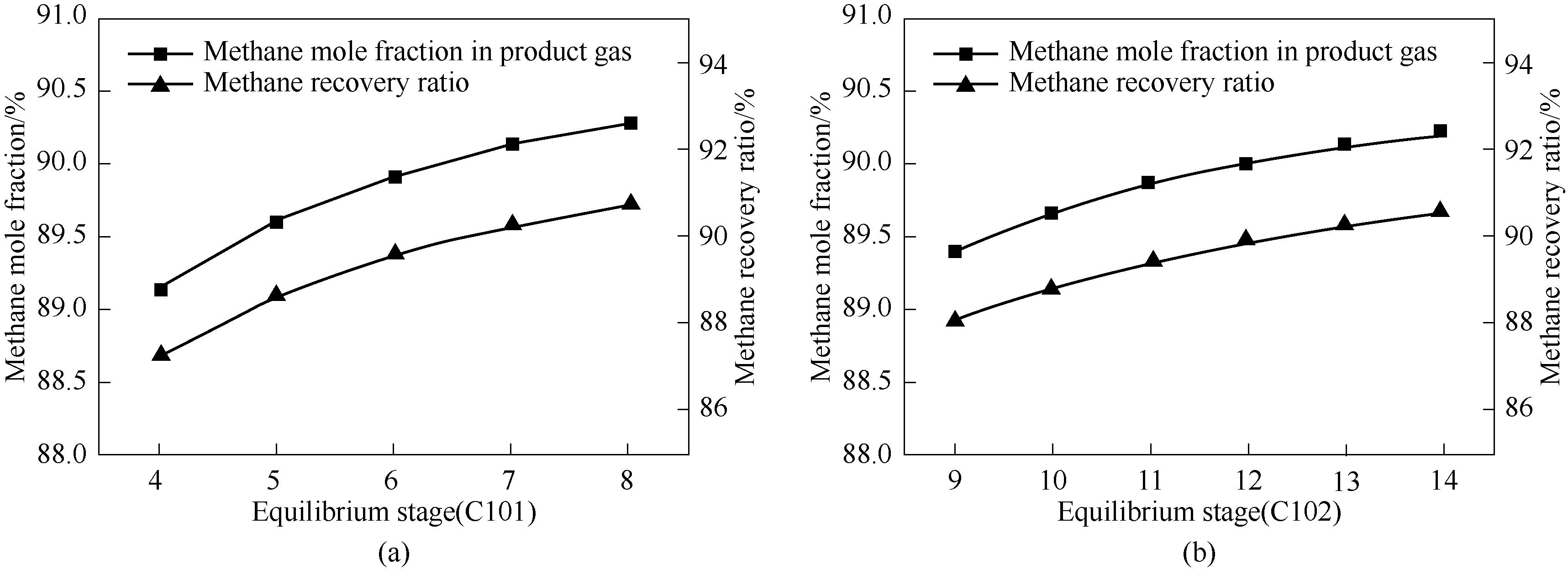
Fig.2 Effect of equilibrium stage numbers of absorption-adsorption column on methane mole fraction in the product gas stream and the methane recovery ratio
| 气液比 | 吸收-吸附塔塔顶气相流量(C102)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C102)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 12 | 0.4080 | 3.520 | 0.3117 | 87.66 | 91.08 |
| 14 | 0.4190 | 3.974 | 0.3004 | 90.13 | 90.25 |
| 16 | 0.4279 | 4.511 | 0.2914 | 91.98 | 89.34 |
Table 1 Simulation results of different gas-slurry ratios of C102
| 气液比 | 吸收-吸附塔塔顶气相流量(C102)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C102)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 12 | 0.4080 | 3.520 | 0.3117 | 87.66 | 91.08 |
| 14 | 0.4190 | 3.974 | 0.3004 | 90.13 | 90.25 |
| 16 | 0.4279 | 4.511 | 0.2914 | 91.98 | 89.34 |
| 气液比 | 吸收-吸附塔塔顶气相流量 (C101)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C101)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 17 | 0.1706 | 3.409 | 0.3075 | 88.10 | 90.30 |
| 20 | 0.2806 | 4.486 | 0.3004 | 90.13 | 90.25 |
| 23 | 0.3624 | 5.867 | 0.2917 | 91.19 | 88.67 |
Table 2 Simulation results of different gas-slurry ratios of C101
| 气液比 | 吸收-吸附塔塔顶气相流量 (C101)/(kmol∙h-1) | 吸收-吸附塔塔顶气相中 甲烷摩尔分数(C101)/% | 产品气流量/ (kmol∙h-1) | 产品气中甲烷 摩尔分数/% | 甲烷回收率/% |
|---|---|---|---|---|---|
| 17 | 0.1706 | 3.409 | 0.3075 | 88.10 | 90.30 |
| 20 | 0.2806 | 4.486 | 0.3004 | 90.13 | 90.25 |
| 23 | 0.3624 | 5.867 | 0.2917 | 91.19 | 88.67 |
内蒸 压力/MPa | 吸收-吸附塔(C101)塔顶LV1中甲烷 吸附量/ (mol∙L-1) | 吸收-吸附塔(C102)塔顶 吸附量/ (mol∙L-1) | 贫液 吸附量/(mol∙L-1) | 贫液 流量/ (kmol∙h-1) | 贫液 摩尔 分数/% | 浆液 | 吸收- 吸附塔(C101) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C101) 塔顶气流中甲烷摩尔分数/% | 吸收- 吸附塔(C102) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C102)塔顶气流中甲烷摩尔分数/% | 产品气流量/(kmol∙h-1) | 产品气中甲烷摩尔 分数/% | 甲烷 回收率/ % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.0459 | 0.0326 | 0.0100 | 0.0279 | 97.56 | 90.76 | 0.2806 | 4.486 | 0.4190 | 3.973 | 0.3004 | 90.13 | 90.25 |
| 0.03 | 0.0808 | 0.0686 | 0.0303 | 0.0842 | 97.95 | 93.13 | 0.2986 | 7.618 | 0.4368 | 7.980 | 0.2646 | 91.59 | 80.78 |
| 0.05 | 0.1148 | 0.1032 | 0.0513 | 0.1417 | 98.43 | 95.34 | 0.3168 | 10.48 | 0.4536 | 11.52 | 0.2296 | 93.43 | 71.51 |
Table 3 Simulation results under different desorption pressures
内蒸 压力/MPa | 吸收-吸附塔(C101)塔顶LV1中甲烷 吸附量/ (mol∙L-1) | 吸收-吸附塔(C102)塔顶 吸附量/ (mol∙L-1) | 贫液 吸附量/(mol∙L-1) | 贫液 流量/ (kmol∙h-1) | 贫液 摩尔 分数/% | 浆液 | 吸收- 吸附塔(C101) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C101) 塔顶气流中甲烷摩尔分数/% | 吸收- 吸附塔(C102) 塔顶气流流量/(kmol∙h-1) | 吸收- 吸附塔(C102)塔顶气流中甲烷摩尔分数/% | 产品气流量/(kmol∙h-1) | 产品气中甲烷摩尔 分数/% | 甲烷 回收率/ % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 0.0459 | 0.0326 | 0.0100 | 0.0279 | 97.56 | 90.76 | 0.2806 | 4.486 | 0.4190 | 3.973 | 0.3004 | 90.13 | 90.25 |
| 0.03 | 0.0808 | 0.0686 | 0.0303 | 0.0842 | 97.95 | 93.13 | 0.2986 | 7.618 | 0.4368 | 7.980 | 0.2646 | 91.59 | 80.78 |
| 0.05 | 0.1148 | 0.1032 | 0.0513 | 0.1417 | 98.43 | 95.34 | 0.3168 | 10.48 | 0.4536 | 11.52 | 0.2296 | 93.43 | 71.51 |
| 项目 | 入口压力/MPa | 出口压力/MPa | 流量 | 有效功率/kW | 电功率/kW | |
|---|---|---|---|---|---|---|
| 合计 | 9.9786 | |||||
| 压缩机 | 原料气 | 0.12 | 2 | 1 kmol·h-1 | 2.3827 | 3.5299 |
| 闪蒸循环气 | 0.15 | 1.5 | 1.5191 kmol·h-1 | 2.6058 | 3.8604 | |
| 产品气 | 0.01 | 0.11 | 0.3004 kmol·h-1 | 0.5418 | 0.8027 | |
| 压缩机电功率合计 | 8.1930 | |||||
| 浆液循环泵P101 | 0.01 | 2 | 1.12 m3·h-1 | 0.8258 | 0.9176 | |
| 浆液循环泵P102 | 0.01 | 1.5 | 1.60 m3·h-1 | 0.8834 | 0.9815 | |
| 泵电功率合计 | 1.8991 | |||||
| 氮气透平T101 | 2 | 0.1 | 0.281 kmol·h-1 | -0.4559 | -0.3077 | |
| 氮气透平T102 | 1.5 | 0.1 | 0.419 kmol·h-1 | -0.6246 | -0.4216 | |
| 透平回收膨胀功合计 | -0.7293 | |||||
| 入口温度/K | 出口温度/K | 流量 | 热负荷/kW | 折合电功率/kW | ||
| 冷却器 | 原料气 | 323.15 | 273.15 | 1 kmol·h-1 | 0.4320 | 0.2160 |
| 闪蒸循环气 | 323.15 | 273.15 | 1.5191 kmol·h-1 | 0.7108 | 0.3554 | |
| 产品气 | 323.15 | 293.15 | 0.3004 kmol·h-1 | 0.0888 | 0.0444 | |
| 冷却器折合电功率合计 | 0.6158 | |||||
Table 4 Summary of energy consumption calculation results
| 项目 | 入口压力/MPa | 出口压力/MPa | 流量 | 有效功率/kW | 电功率/kW | |
|---|---|---|---|---|---|---|
| 合计 | 9.9786 | |||||
| 压缩机 | 原料气 | 0.12 | 2 | 1 kmol·h-1 | 2.3827 | 3.5299 |
| 闪蒸循环气 | 0.15 | 1.5 | 1.5191 kmol·h-1 | 2.6058 | 3.8604 | |
| 产品气 | 0.01 | 0.11 | 0.3004 kmol·h-1 | 0.5418 | 0.8027 | |
| 压缩机电功率合计 | 8.1930 | |||||
| 浆液循环泵P101 | 0.01 | 2 | 1.12 m3·h-1 | 0.8258 | 0.9176 | |
| 浆液循环泵P102 | 0.01 | 1.5 | 1.60 m3·h-1 | 0.8834 | 0.9815 | |
| 泵电功率合计 | 1.8991 | |||||
| 氮气透平T101 | 2 | 0.1 | 0.281 kmol·h-1 | -0.4559 | -0.3077 | |
| 氮气透平T102 | 1.5 | 0.1 | 0.419 kmol·h-1 | -0.6246 | -0.4216 | |
| 透平回收膨胀功合计 | -0.7293 | |||||
| 入口温度/K | 出口温度/K | 流量 | 热负荷/kW | 折合电功率/kW | ||
| 冷却器 | 原料气 | 323.15 | 273.15 | 1 kmol·h-1 | 0.4320 | 0.2160 |
| 闪蒸循环气 | 323.15 | 273.15 | 1.5191 kmol·h-1 | 0.7108 | 0.3554 | |
| 产品气 | 323.15 | 293.15 | 0.3004 kmol·h-1 | 0.0888 | 0.0444 | |
| 冷却器折合电功率合计 | 0.6158 | |||||
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 吸收-吸附塔操作条件 | 平衡级数 | 13 | 7/13 |
| 进料级 | 11 | 7/11 | |
| 全塔温度/K | 273.15 | 273.15/273.15 | |
| 压力/MPa | 2 | 2/1.5 | |
| 闪蒸罐操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.15 | 0.15 | |
| 解吸塔操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.01 | 0.01 | |
| 原料气流量/(kmol∙h-1) | 1 | 1 | |
| 原料气中甲烷的摩尔分数/% | 30 | 30 | |
| 循环浆液量/(m3·h-1) | 2.24 | 2.72 | |
| 产品气摩尔分数/% | 95.46 | 90.13 | |
| 甲烷回收率/% | 90.74 | 90.25 | |
Table 5 Comparison of simulation results under different processes
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 吸收-吸附塔操作条件 | 平衡级数 | 13 | 7/13 |
| 进料级 | 11 | 7/11 | |
| 全塔温度/K | 273.15 | 273.15/273.15 | |
| 压力/MPa | 2 | 2/1.5 | |
| 闪蒸罐操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.15 | 0.15 | |
| 解吸塔操作条件 | 温度/K | 273.15 | 273.15 |
| 压力/MPa | 0.01 | 0.01 | |
| 原料气流量/(kmol∙h-1) | 1 | 1 | |
| 原料气中甲烷的摩尔分数/% | 30 | 30 | |
| 循环浆液量/(m3·h-1) | 2.24 | 2.72 | |
| 产品气摩尔分数/% | 95.46 | 90.13 | |
| 甲烷回收率/% | 90.74 | 90.25 | |
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 压缩机/kW | 原料气 | 3.5299 | 3.5299 |
| 闪蒸循环气 | 5.3728 | 3.8604 | |
| 产品气 | 0.7621 | 0.8027 | |
| 浆液循环泵合计/kW | 1.8353 | 1.8991 | |
| 冷却器/kW | 原料气 | 0.2160 | 0.2160 |
| 闪蒸循环气 | 0.4412 | 0.3554 | |
| 产品气 | 0.0418 | 0.0444 | |
| 氮气回收合计/kW | -0.7840 | -0.7293 | |
| 合计/kW | 11.4151 | 9.9786 | |
| 单位原料气能耗/(kW·h∙m-3) | 0.510 | 0.445 | |
Table 6 Comparison of energy consumption under different processes
| 项目 | 单吸收-吸附塔工艺 | 双吸收-吸附塔工艺(C101/C102) | |
|---|---|---|---|
| 压缩机/kW | 原料气 | 3.5299 | 3.5299 |
| 闪蒸循环气 | 5.3728 | 3.8604 | |
| 产品气 | 0.7621 | 0.8027 | |
| 浆液循环泵合计/kW | 1.8353 | 1.8991 | |
| 冷却器/kW | 原料气 | 0.2160 | 0.2160 |
| 闪蒸循环气 | 0.4412 | 0.3554 | |
| 产品气 | 0.0418 | 0.0444 | |
| 氮气回收合计/kW | -0.7840 | -0.7293 | |
| 合计/kW | 11.4151 | 9.9786 | |
| 单位原料气能耗/(kW·h∙m-3) | 0.510 | 0.445 | |
| 1 | Xue C L, Cheng W P, Hao W M, et al. CH4/N2 adsorptive separation on zeolite X/AC composites[J]. Journal of Chemistry, 2019, 2019: 1-9. |
| 2 | Li Q Z, Yuan C C, Zhang G Y, et al. Effects of doping Mg2+ on the pore structure of MIL-101 and its adsorption selectivity for CH4/N2 gas mixtures[J]. Fuel, 2019, 240: 206-218. |
| 3 | Yang B, Xu E L, Li M. Purification of coal mine methane on carbon molecular sieve by vacuum pressure swing adsorption[J]. Separation Science and Technology, 2016, 51(6): 909-916. |
| 4 | Li Q Y, Wang L, Ju Y L. Analysis of flammability limits for the liquefaction process of oxygen-bearing coal-bed methane[J]. Applied Energy, 2011, 88(9): 2934-2939. |
| 5 | Anna H R S, Barreto Jr A G, Tavares F W, et al. Methane/nitrogen separation through pressure swing adsorption process from nitrogen-rich streams[J]. Chemical Engineering and Processing: Process Intensification, 2016, 103: 70-79. |
| 6 | Niu Z, Cui X L, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 7 | Lavoie T N, Shepson P B, Gore C A, et al. Assessing the methane emissions from natural gas-fired power plants and oil refineries[J]. Environmental Science & Technology, 2017, 51(6): 3373-3381. |
| 8 | 郑德志. 我国煤层气产业政策评价研究[J]. 煤炭经济研究, 2019, 39(1): 62-65. |
| Zheng D Z. Research on China's coalbed methane industry policy evaluation[J]. Coal Economic Research, 2019, 39(1): 62-65. | |
| 9 | Qadir S, Li D F, Gu Y M, et al. Experimental and numerical investigations on the separation performance of [Cu(INA)2] adsorbent for CH4 recovery by VPSA from oxygen-bearing coal mine methane[J]. Chemical Engineering Journal, 2021, 408: 127238. |
| 10 | Yang R T. Adsorbents: Fundamentals and Applications[M]. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2003. |
| 11 | Gao T, Lin W S, Gu A Z, et al. Coalbed methane liquefaction adopting a nitrogen expansion process with propane pre-cooling[J]. Applied Energy, 2010, 87(7): 2142-2147. |
| 12 | Baker R W, Lokhandwala K. Natural gas processing with membranes: an overview[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2109-2121. |
| 13 | Yang J F, Bai H H, Shang H, et al. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets[J]. Chemical Engineering Journal, 2020, 388: 124222. |
| 14 | Feng W R, Wu H, Jin J S, et al. Transformation of Al-CDC from 3D crystals to 2D nanosheets in macroporous polyacrylates with enhanced CH4/N2 separation efficiency and stability[J]. Chemical Engineering Journal, 2022, 429: 132285 |
| 15 | Yousef S, Tuckute S, Tonkonogovas A, et al. Ultra-permeable CNTs/PES membranes with a very low CNTs content and high H2/N2 and CH4/N2 selectivity for clean energy extraction applications[J]. Journal of Materials Research and Technology, 2021, 15: 5114-5127. |
| 16 | Yu H J, Shin J H, Lee A S, et al. Tailoring selective pores of carbon molecular sieve membranes towards enhanced N2/CH4 separation efficiency[J]. Journal of Membrane Science, 2021, 620: 118814. |
| 17 | Gu Z J, Yang Z B, Guo X Y, et al. Vacuum resistance treated ZIF-8 mixed matrix membrane for effective CH4/N2 separation[J]. Separation and Purification Technology, 2021, 272: 118845. |
| 18 | Gu Z J, Yang Z B, Sun Y X, et al. Large-area vacuum-treated ZIF-8 mixed-matrix membrane for highly efficient methane/nitrogen separation[J]. AIChE Journal, 2022, 68(9): e17749. |
| 19 | Sun Q, Zhao Y Y, Liu A X, et al. Continuous separation of CH4/N2 mixture via hydrates formation in the presence of TBAB[J]. Chemical Engineering and Processing: Process Intensification, 2015, 95: 284-288. |
| 20 | Ning X, Koros W J. Carbon molecular sieve membranes derived from Matrimid® polyimide for nitrogen/methane separation[J]. Carbon, 2014, 66: 511-522. |
| 21 | Zhong D L, Lu Y Y, Sun D J, et al. Performance evaluation of methane separation from coal mine gas by gas hydrate formation in a stirred reactor and in a fixed bed of silica sand[J]. Fuel, 2015, 143: 586-594. |
| 22 | 刘佳奇, 尚华, 唐轩, 等. 分子筛基CH4-N2分离材料的研究进展[J]. 化工进展, 2019, 38(1): 449-456. |
| Liu J Q, Shang H, Tang X, et al. Zeolite based materials for CH4-N2 separation[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 449-456. | |
| 23 | Lu B, Shen Y H, Tang Z L, et al. Vacuum pressure swing adsorption process for coalbed methane enrichment[J]. Chinese Journal of Chemical Engineering, 2021, 32: 264-280. |
| 24 | 陈高飞, 汪亚燕, 张东辉, 等. 基于平衡效应的多种吸附剂对CH4/N2分离性能研究[J]. 天然气化工(C1化学与化工), 2020, 45(2): 17-23, 37. |
| Chen G F, Wang Y Y, Zhang D H, et al. Separation performance of CH4/N2 on various adsorbents based on equilibrium effect[J]. Natural Gas Chemical Industry, 2020, 45(2): 17-23, 37. | |
| 25 | 尚华, 白洪灏, 刘佳奇, 等. CH4-N2在自支撑颗粒型Silicalite-1上的吸附分离及PSA模拟[J]. 化工学报, 2020, 71(5): 2088-2098. |
| Shang H, Bai H H, Liu J Q, et al. PSA simulation and adsorption separation of CH4-N2 by self-supporting pellets Silicalite-1[J]. CIESC Journal, 2020, 71(5): 2088-2098. | |
| 26 | Li T, Jia X X, Chen H, et al. Tuning the pore environment of MOFs toward efficient CH4/N2 separation under humid conditions[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15830-15839. |
| 27 | Chang M, Zhao Y J, Liu D H, et al. Methane-trapping metal-organic frameworks with an aliphatic ligand for efficient CH4/N2 separation[J]. Sustainable Energy & Fuels, 2020, 4(1): 138-142. |
| 28 | Wang S M, Shivanna M, Yang Q Y. Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(15): e202201017. |
| 29 | 杨江峰, 赵强, 于秋红, 等. 煤层气回收及CH4/N2分离PSA材料的研究进展[J]. 化工进展, 2011, 30(4): 793-801. |
| Yang J F, Zhao Q, Yu Q H, et al. Progress of recovery of coal bed methane and adsorption materials for separation of CH4/N2 by pressure swing adsorption[J]. Chemical Industry and Engineering Progress, 2011, 30(4): 793-801. | |
| 30 | Yang Z X, Hussain M Z, MarÃn P, et al. Enrichment of low concentration methane: an overview of ventilation air methane[J]. Journal of Materials Chemistry A, 2022, 10(12): 6397-6413. |
| 31 | 郭武杰, 李媛, 李世帅, 等. 高硅沸石分子筛ZSM-11用于CH4与N2吸附分离性能的研究[J]. 天然气化工—C1化学与化工, 2022, 47(1): 67-72. |
| Guo W J, Li Y, Li S S, et al. Study on adsorption and separation performance of CH4/N2 by high-silica ZSM-11 zeolite[J]. Natural Gas Chemical Industry, 2022, 47(1): 67-72. | |
| 32 | 田军鹏, 沈圆辉, 张东辉, 等. 规整复合吸附剂真空变压吸附分离CH4/N2工艺模拟与分析[J]. 化工学报, 2021, 72(11): 5675-5685. |
| Tian J P, Shen Y H, Zhang D H, et al. Simulation and analysis of CH4/N2 separation by vacuum pressure swing adsorption with structured composite adsorption media[J]. CIESC Journal, 2021, 72(11): 5675-5685. | |
| 33 | Liu H, Liu B, Lin L C, et al. A hybrid absorption–adsorption method to efficiently capture carbon[J]. Nature Communications, 2014, 5: 5147. |
| 34 | Liu H, Pan Y, Liu B, et al. Tunable integration of absorption-membrane-adsorption for efficiently separating low boiling gas mixtures near normal temperature[J]. Scientific Reports, 2016, 6: 21114. |
| 35 | Yan S R, Xiao P, Zhu D, et al. A large-scale experimental study on CO2 capture utilizing slurry-based ab-adsorption approach[J]. Chinese Journal of Chemical Engineering, 2021, 31: 56-66. |
| 36 | Li H, Liu B, Yang M K, et al. CO2 separation performance of zeolitic imidazolate framework-8 porous slurry in a pilot-scale packed tower[J]. Industrial & Engineering Chemistry Research, 2020, 59(13): 6154-6163. |
| 37 | Yan S R, Zhu D, Zhang Z Y, et al. A pilot-scale experimental study on CO2 capture using zeolitic imidazolate framework-8 slurry under normal pressure[J]. Applied Energy, 2019, 248: 104-114. |
| 38 | Li H, Chen W, Liu B, et al. CO2 capture using ZIF-8/water-glycol-2-methylimidazole slurry with high capacity and low desorption heat[J]. Chemical Engineering Science, 2018, 182: 189-199. |
| 39 | Yang M K, Han Y, Zou E B, et al. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat[J]. Energy, 2020, 201: 117605. |
| 40 | Chen W, Zou E B, Zuo J Y, et al. Separation of ethane from natural gas using porous ZIF-8/water-glycol slurry[J]. Industrial & Engineering Chemistry Research, 2019, 58(23): 9997-10006. |
| 41 | Li H, Gao X T, Jia C Z, et al. Enrichment of hydrogen from a hydrogen/propylene gas mixture using ZIF-8/water-glycol slurry[J]. Energies, 2018, 11(7): 1890. |
| 42 | Peng X W, Jia C Z, Qiao Z C, et al. A new energy efficient process for hydrogen purification using ZIF-8/glycol-water slurry: experimental study and process modeling[J]. International Journal of Hydrogen Energy, 2021, 46(63): 32081-32098. |
| 43 | Chen W, Wang M L, Yang S W, et al. Experimental study on breakthrough separation for hydrogen recovery from coke oven gas using ZIF-8 slurry[J]. Energies, 2022, 15(4): 1487. |
| 44 | Li H, Chen W, Liu B, et al. A purely green approach to low-cost mass production of zeolitic imidazolate frameworks[J]. Green Energy & Environment, 2021, DOI:10.1016/j.gee.2021.09.003 . |
| 45 | Chen W, Guo X N, Zou E B, et al. A continuous and high-efficiency process to separate coal bed methane with porous ZIF-8 slurry: experimental study and mathematical modelling[J]. Green Energy & Environment, 2020, 5(3): 347-363. |
| [1] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [2] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [3] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [4] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [5] | Xiaoyang LIU, Jianliang YU, Yujie HOU, Xingqing YAN, Zhenhua ZHANG, Xianshu LYU. Effect of spiral microchannel on detonation propagation of hydrogen-doped methane [J]. CIESC Journal, 2023, 74(7): 3139-3148. |
| [6] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [7] | Chao NIU, Shengqiang SHEN, Yan YANG, Bonian PAN, Yiqiao LI. Flow process calculation and performance analysis of methane BOG ejector [J]. CIESC Journal, 2023, 74(7): 2858-2868. |
| [8] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [9] | Xiaowen ZHOU, Jie DU, Zhanguo ZHANG, Guangwen XU. Study on the methane-pulsing reduction characteristics of Fe2O3-Al2O3 oxygen carrier [J]. CIESC Journal, 2023, 74(6): 2611-2623. |
| [10] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [11] | Jianhua ZHANG, Mengmeng CHEN, Yawen SUN, Yongzhen PENG. Efficient nitrogen and phosphorus removal from domestic wastewater via simultaneous partial nitritation and phosphorus removal combined Anammox [J]. CIESC Journal, 2023, 74(5): 2147-2156. |
| [12] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [13] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [14] | Xuanjun WU, Chao WANG, Zijian CAO, Weiquan CAI. Deep learning model of fixed bed adsorption breakthrough curve hybrid-driven by data and physical information [J]. CIESC Journal, 2023, 74(3): 1145-1160. |
| [15] | Dingping LIU, Aihua CHEN, Xiangyang ZHANG, Wenhao HE, Hai WANG. Study on semi dry hydrolytic denitrification of aluminum ash [J]. CIESC Journal, 2023, 74(3): 1294-1302. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||