CIESC Journal ›› 2022, Vol. 73 ›› Issue (3): 1343-1350.DOI: 10.11949/0438-1157.20211344
• Energy and environmental engineering • Previous Articles Next Articles
Xiaoxi WANG( ),Xiaoyan LI,Baowei WANG(
),Xiaoyan LI,Baowei WANG( )
)
Received:2021-09-16
Revised:2021-11-23
Online:2022-03-14
Published:2022-03-15
Contact:
Baowei WANG
通讯作者:
王保伟
作者简介:王小西(1992—),男,硕士研究生,助理工程师,基金资助:CLC Number:
Xiaoxi WANG, Xiaoyan LI, Baowei WANG. Decomposition of carbon dioxide via dielectric barrier discharge microplasma[J]. CIESC Journal, 2022, 73(3): 1343-1350.
王小西, 李笑艳, 王保伟. 介质阻挡放电微等离子体分解二氧化碳研究[J]. 化工学报, 2022, 73(3): 1343-1350.
Add to citation manager EndNote|Ris|BibTeX
| 输入功率/W | 放电频率/kHz | 停留时间/s | 放电间距/mm | 放电长度/mm |
|---|---|---|---|---|
| 10.0~60.0 | 7.0~10.0 | 1.0~4.0 | 0.5~1.5 | 60.0~120.0 |
Table 1 The main parameters and range of CO2 decomposition by DBD plasma
| 输入功率/W | 放电频率/kHz | 停留时间/s | 放电间距/mm | 放电长度/mm |
|---|---|---|---|---|
| 10.0~60.0 | 7.0~10.0 | 1.0~4.0 | 0.5~1.5 | 60.0~120.0 |

Fig.2 Influence of the input power on discharge current waveforms (frequency: 7.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
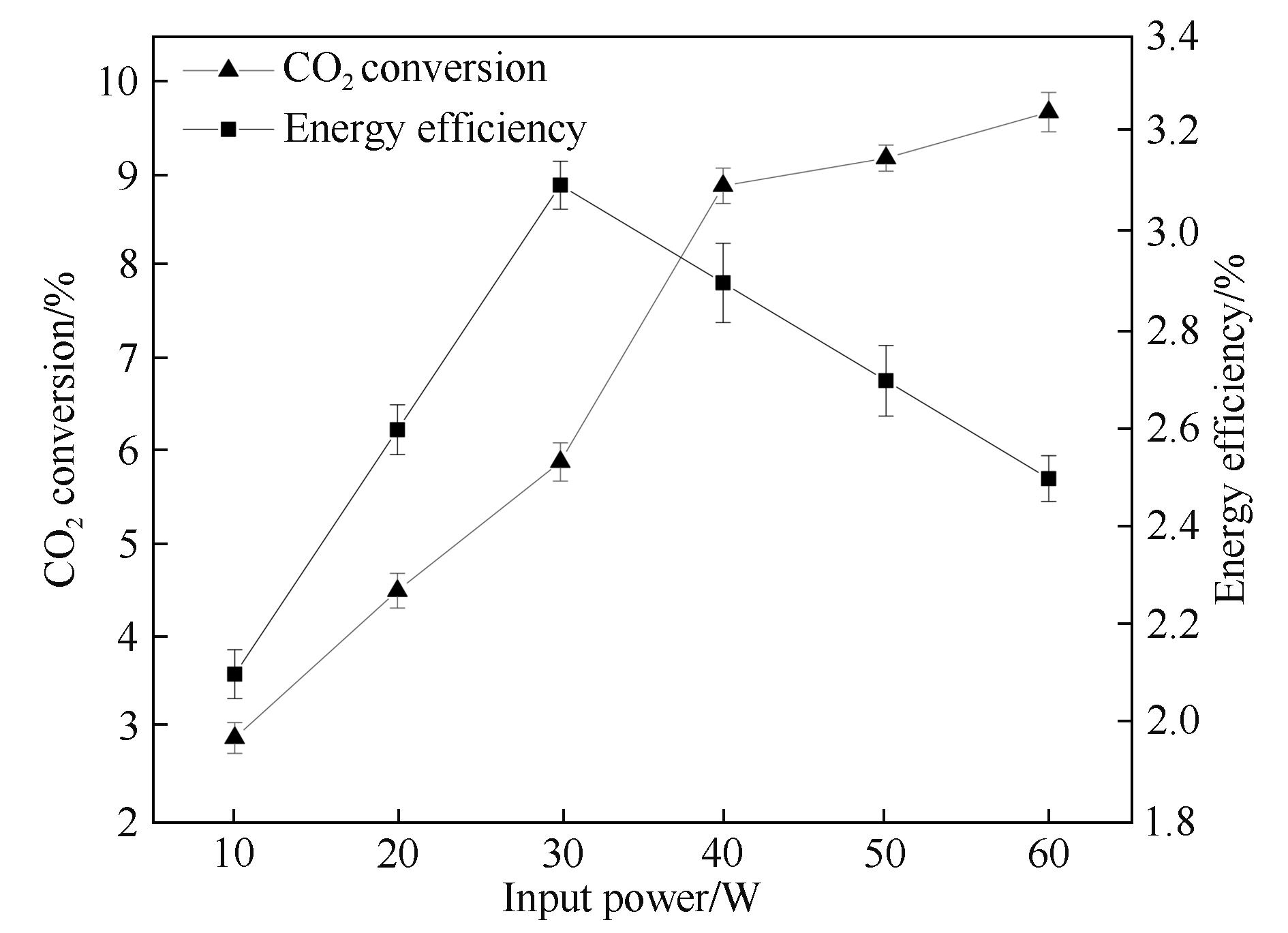
Fig.3 Influence of the input power on CO2 conversion and energy efficiency (frequency: 7.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
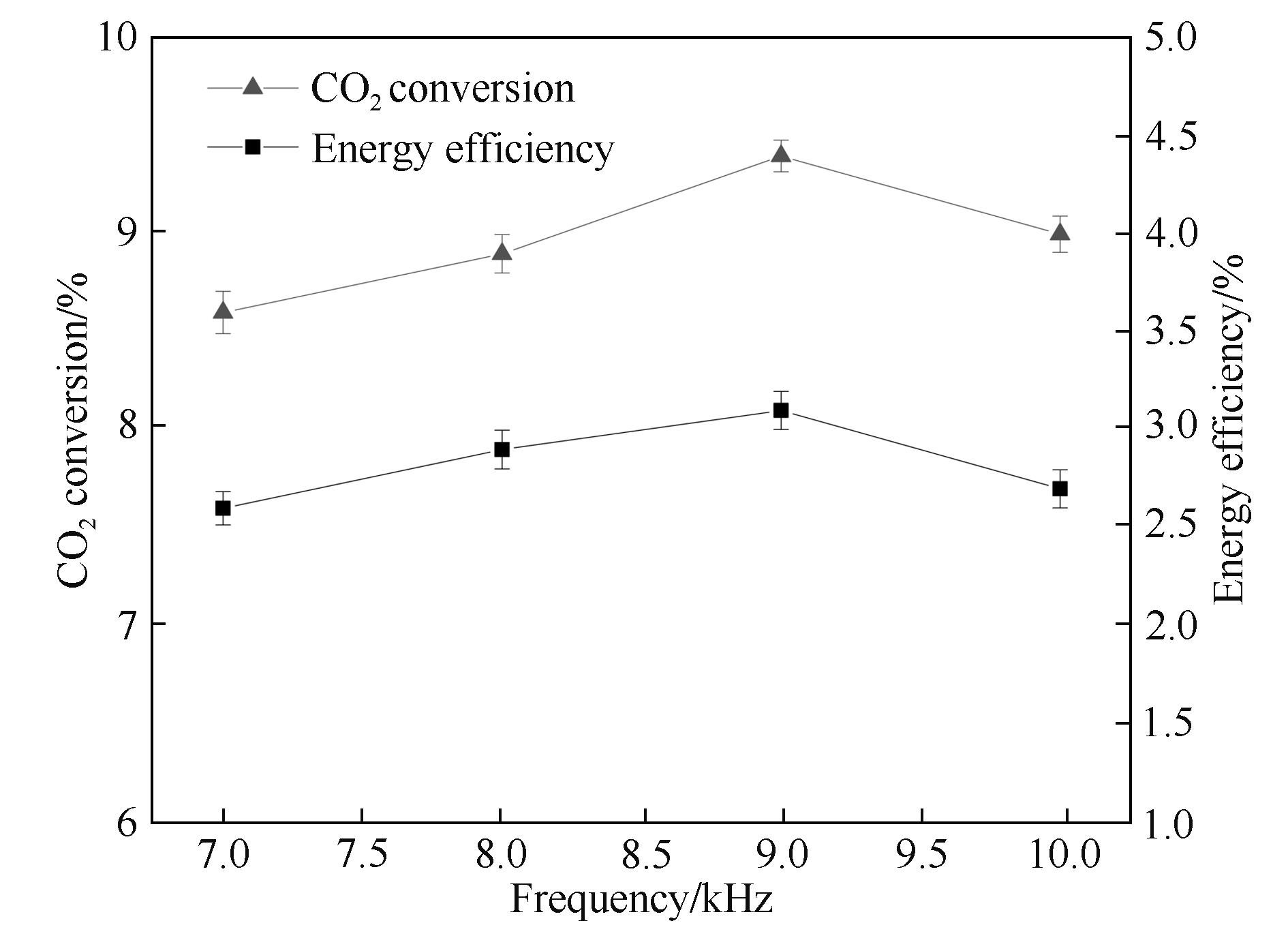
Fig.4 Influence of the frequency on CO2 conversion and energy efficiency (input power: 40 W; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
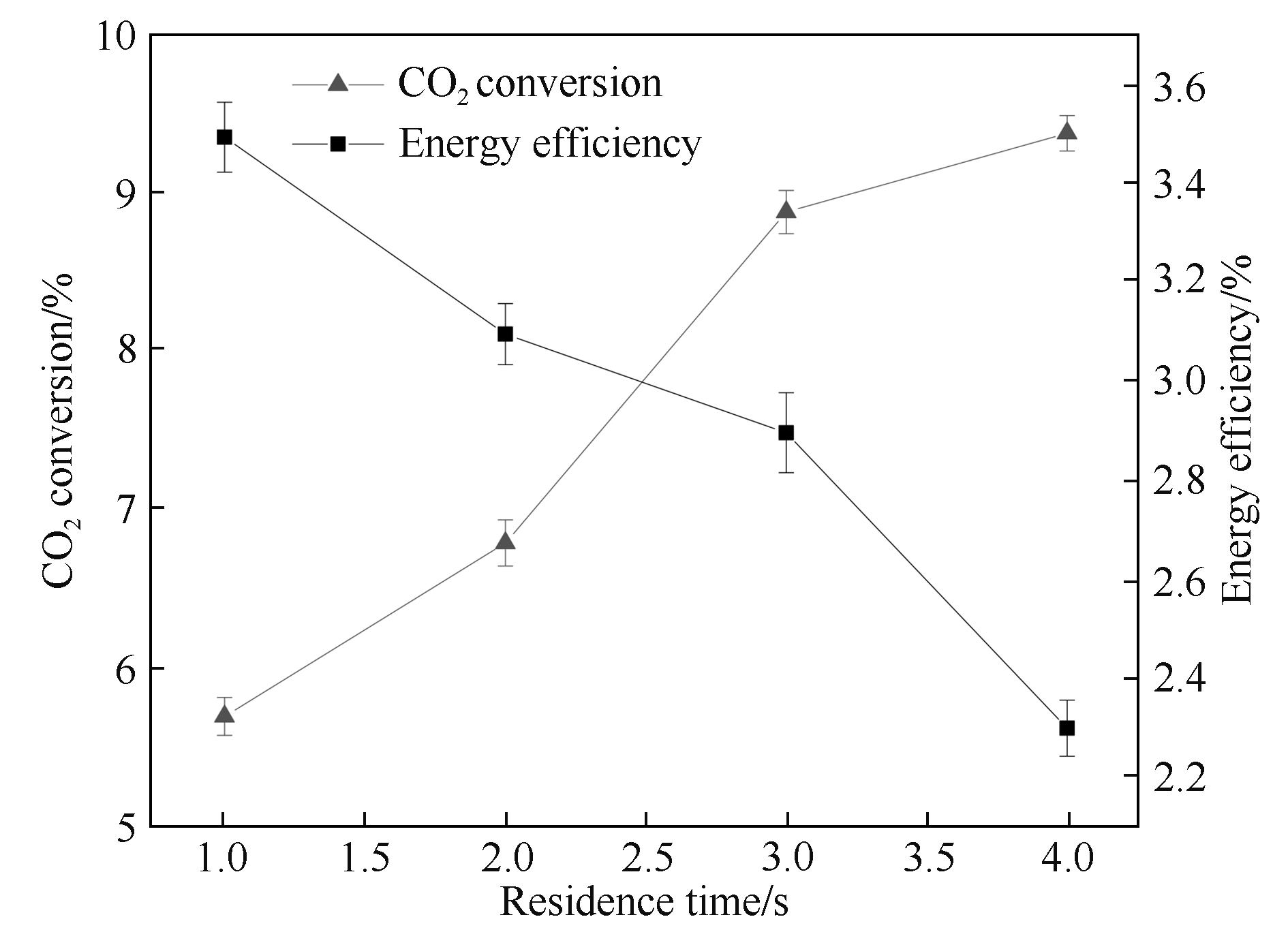
Fig.5 Influence of the residence time on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm)

Fig.6 Influence of the discharge length on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; τ: 2.5 s; discharge gap: 0.5 mm; barrier thickness: 1.6 mm)
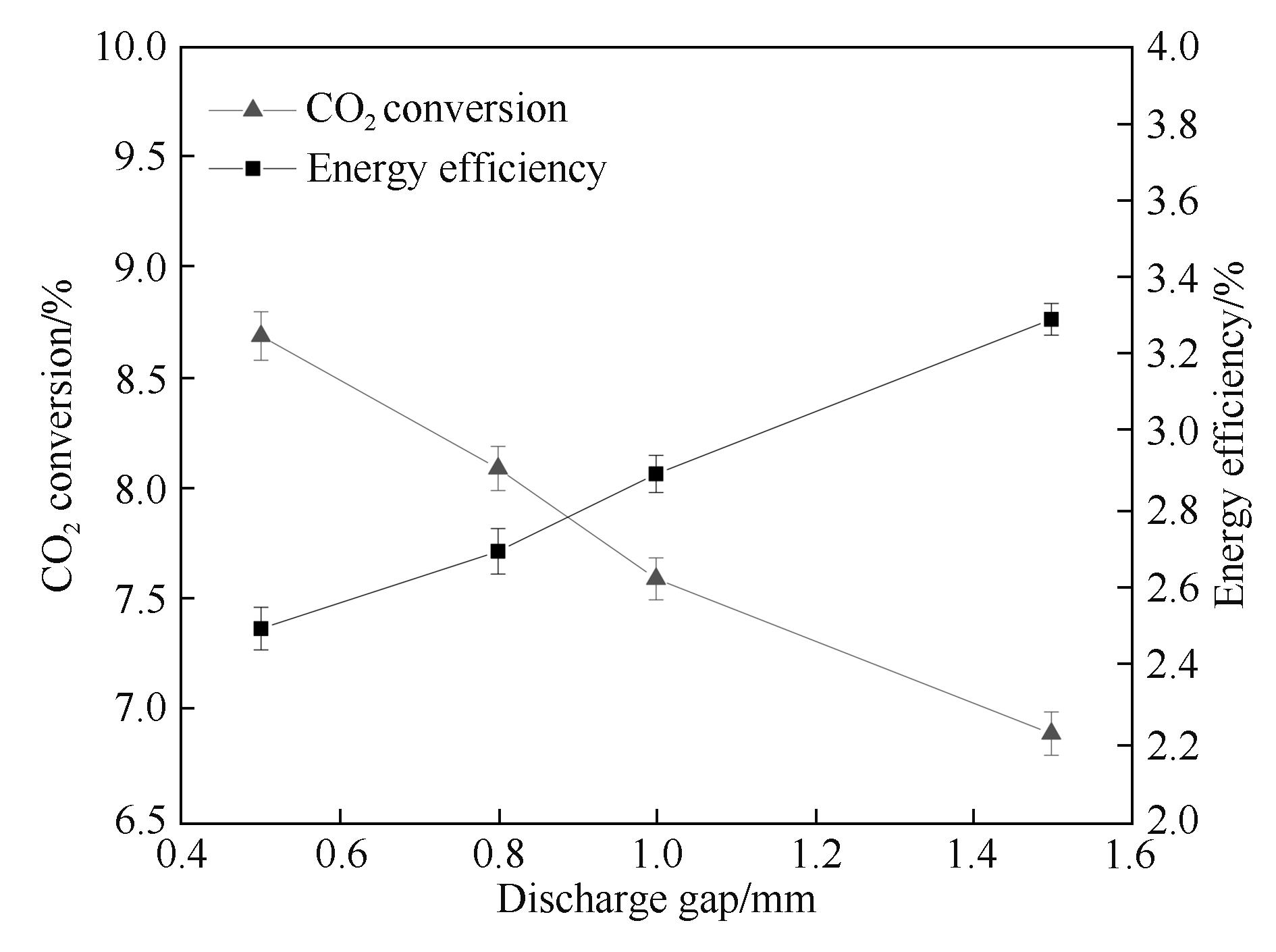
Fig.7 Influence of the discharge gap on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; τ: 2.5 s; discharge length: 80 mm; barrier thickness: 1.6 mm)
| 水平 | 因素 | |||||
|---|---|---|---|---|---|---|
| 输入功率/W | 放电间距/mm | 放电频率/kHz | 停留时间/s | 放电长度/mm | 介质厚度/mm | |
| 1 | 40.0 | 1.0 | 8.0 | 1.5 | 100 | 1.0 |
| 2 | 50.0 | 0.8 | 9.0 | 2.5 | 80 | 1.6 |
| 3 | 60.0 | 0.5 | 10.0 | 3.5 | 60 | 2.1 |
Table 2 Factors and levels
| 水平 | 因素 | |||||
|---|---|---|---|---|---|---|
| 输入功率/W | 放电间距/mm | 放电频率/kHz | 停留时间/s | 放电长度/mm | 介质厚度/mm | |
| 1 | 40.0 | 1.0 | 8.0 | 1.5 | 100 | 1.0 |
| 2 | 50.0 | 0.8 | 9.0 | 2.5 | 80 | 1.6 |
| 3 | 60.0 | 0.5 | 10.0 | 3.5 | 60 | 2.1 |
| 序号 | A | B | C | 空白 | D | E | F | χCO2/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5.6 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 7.1 |
| 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 8.5 |
| 4 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 7.7 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 7.3 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 8.7 |
| 7 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 7.9 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 9.3 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 8.3 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 4.9 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 7.4 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 6.9 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 8.2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 6.1 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 9.6 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 7.5 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 6.6 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 9.1 |
| K1 | 30.381 | 26.895 | 35.691 | 35.019 | 31.674 | 31.53 | 33.549 | |
| K2 | 36.576 | 30.444 | 36.06 | 34.665 | 34.326 | 33.126 | 37.77 | |
| K3 | 37.005 | 46.62 | 32.211 | 34.275 | 37.959 | 39.306 | 32.64 | |
| K1 | 10.127 | 8.965 | 11.897 | 11.673 | 10.558 | 10.51 | 11.183 | |
| K2 | 12.192 | 10.148 | 12.02 | 11.555 | 11.442 | 11.042 | 12.59 | |
| K3 | 12.335 | 15.54 | 10.737 | 11.425 | 12.653 | 13.102 | 10.88 | |
| R1 | 2.208 | 6.575 | 1.283 | 0.248 | 2.095 | 2.592 | 1.71 |
Table 3 The result and range analysis of orthogonal experiment
| 序号 | A | B | C | 空白 | D | E | F | χCO2/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5.6 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 7.1 |
| 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 8.5 |
| 4 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 7.7 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 7.3 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 8.7 |
| 7 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 7.9 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 9.3 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 8.3 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 4.9 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 7.4 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 6.9 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 8.2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 6.1 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 9.6 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 7.5 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 6.6 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 9.1 |
| K1 | 30.381 | 26.895 | 35.691 | 35.019 | 31.674 | 31.53 | 33.549 | |
| K2 | 36.576 | 30.444 | 36.06 | 34.665 | 34.326 | 33.126 | 37.77 | |
| K3 | 37.005 | 46.62 | 32.211 | 34.275 | 37.959 | 39.306 | 32.64 | |
| K1 | 10.127 | 8.965 | 11.897 | 11.673 | 10.558 | 10.51 | 11.183 | |
| K2 | 12.192 | 10.148 | 12.02 | 11.555 | 11.442 | 11.042 | 12.59 | |
| K3 | 12.335 | 15.54 | 10.737 | 11.425 | 12.653 | 13.102 | 10.88 | |
| R1 | 2.208 | 6.575 | 1.283 | 0.248 | 2.095 | 2.592 | 1.71 |
| 参数 | 局部方差总和 | 自由度 | 方差比 | F临界值 | 重要性 |
|---|---|---|---|---|---|
| 输入功率 | 18.323 | 2 | 99.043 | 99 | * |
| 放电间距 | 147.402 | 2 | 796.768 | 99 | * |
| 放电频率 | 6.016 | 2 | 32.519 | 99 | |
| 停留时间 | 13.275 | 2 | 71.757 | 99 | |
| 放电长度 | 22.486 | 2 | 121.546 | 99 | * |
| 介质厚度 | 9.99 | 2 | 54 | 99 | |
| 误差 | 0.18 | 2 | — | — |
Table 4 The variance analysis
| 参数 | 局部方差总和 | 自由度 | 方差比 | F临界值 | 重要性 |
|---|---|---|---|---|---|
| 输入功率 | 18.323 | 2 | 99.043 | 99 | * |
| 放电间距 | 147.402 | 2 | 796.768 | 99 | * |
| 放电频率 | 6.016 | 2 | 32.519 | 99 | |
| 停留时间 | 13.275 | 2 | 71.757 | 99 | |
| 放电长度 | 22.486 | 2 | 121.546 | 99 | * |
| 介质厚度 | 9.99 | 2 | 54 | 99 | |
| 误差 | 0.18 | 2 | — | — |
| 输入 功率/W | 放电 间距/mm | 放电 频率/kHz | 停留 时间/s | 放电 长度/mm | 介质 厚度/mm |
|---|---|---|---|---|---|
| 60.0 | 0.5 | 9.0 | 1.5 | 60 | 1.6 |
Table 5 The best factor level combination
| 输入 功率/W | 放电 间距/mm | 放电 频率/kHz | 停留 时间/s | 放电 长度/mm | 介质 厚度/mm |
|---|---|---|---|---|---|
| 60.0 | 0.5 | 9.0 | 1.5 | 60 | 1.6 |
| CO2转化技术 | 转化率/% | 文献 |
|---|---|---|
| 热催化法 | 0.5 | [ |
| 电化学法 | 16.1 | [ |
| 滑动弧光放电 | <15 | [ |
| 电晕放电 | 15.2 | [ |
| DBD等离子体技术 | 10.6 | 本实验 |
Table 6 Comparison of CO2 conversion with different methods
| CO2转化技术 | 转化率/% | 文献 |
|---|---|---|
| 热催化法 | 0.5 | [ |
| 电化学法 | 16.1 | [ |
| 滑动弧光放电 | <15 | [ |
| 电晕放电 | 15.2 | [ |
| DBD等离子体技术 | 10.6 | 本实验 |
| 1 | Zhou W, Zhou C, Yin H R, et al. Direct conversion of syngas into aromatics over a bifunctional catalyst: inhibiting net CO2 release[J]. Chemical Communications, 2020, 56(39): 5239-5242. |
| 2 | 刘昌俊, 郭秋婷, 叶静云, 等. 二氧化碳转化催化剂研究进展及相关问题思考[J]. 化工学报, 2016, 67(1): 6-13. |
| Liu C J, Guo Q T, Ye J Y, et al. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC Journal, 2016, 67(1): 6-13. | |
| 3 | Thomas H, Bozec Y, Elkalay K, et al. Enhanced open ocean storage of CO2 from shelf sea pumping[J]. Science, 2004, 304(5673): 1005-1008. |
| 4 | Mac Dowell N, Fennell P S, Shah N, et al. The role of CO2 capture and utilization in mitigating climate change[J]. Nature Climate Change, 2017, 7(4): 243-249. |
| 5 | Sun Y, Lin Z, Peng S H, et al. A critical perspective on CO₂ conversions into chemicals and fuels[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(6): 3097-3109. |
| 6 | Ahmed R, Liu G J, Yousaf B, et al. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—a review[J]. Journal of Cleaner Production, 2020, 242: 118409. |
| 7 | Kozák T, Bogaerts A. Splitting of CO2 by vibrational excitation in non-equilibrium plasmas: a reaction kinetics model[J]. Plasma Sources Science & Technology, 2014, 23(4): 045004. |
| 8 | Kondratenko E V, Mul G, Baltrusaitis J, et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes[J]. Energy & Environmental Science, 2013, 6(11): 3112. |
| 9 | Appel A M, Bercaw J E, Bocarsly A B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chemical Reviews, 2013, 113(8): 6621-6658. |
| 10 | Whipple D T, Kenis P J A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction[J]. The Journal of Physical Chemistry Letters, 2010, 1(24): 3451-3458. |
| 11 | Saravanan A, Senthilkumar P, Vo D V N, et al. A comprehensive review on different approaches for CO2 utilization and conversion pathways[J]. Chemical Engineering Science, 2021, 236: 116515. |
| 12 | Kamkeng A D N, Wang M H, Hu J, et al. Transformation technologies for CO2 utilisation: current status, challenges and future prospects[J]. Chemical Engineering Journal, 2021, 409: 128138. |
| 13 | Nigara Y, Cales B. Production of CO by direct thermal splitting of CO2 at high temperature[J]. Bulletin of the Chemical Society of Japan, 1986, 59(6): 1997-2002. |
| 14 | Ganesh I. Conversion of carbon dioxide into methanol—a potential liquid fuel: fundamental challenges and opportunities (a review)[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 221-257. |
| 15 | Liu J L, Wang X, Li X S, et al. CO2 conversion, utilisation and valorisation in gliding arc plasma reactors[J]. Journal of Physics D:Applied Physics, 2020, 53(25): 253001. |
| 16 | Zhang S, Fan Q, Xia R, et al. CO2 reduction: from homogeneous to heterogeneous electrocatalysis[J]. Accounts of Chemical Research, 2020, 53(1): 255-264. |
| 17 | Das S, Wan Daud W M A. A review on advances in photocatalysts towards CO2 conversion[J]. RSC Advances, 2014, 4(40): 20856-20893. |
| 18 | Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products[J]. Renewable and Sustainable Energy Reviews, 2010, 14(2): 557-577. |
| 19 | George A, Shen B X, Craven M, et al. A review of non-thermal plasma technology: a novel solution for CO2 conversion and utilization[J]. Renewable and Sustainable Energy Reviews, 2021, 135: 109702. |
| 20 | Sun S R, Wang H X, Mei D H, et al. CO2 conversion in a gliding arc plasma: performance improvement based on chemical reaction modeling[J]. Journal of CO2 Utilization, 2017, 17: 220-234. |
| 21 | Li L, Zhang H, Li X D, et al. Magnetically enhanced gliding arc discharge for CO2 activation[J]. Journal of CO2 Utilization, 2020, 35: 28-37. |
| 22 | Nagassou D, Mohsenian S, Nallar M, et al. Decomposition of CO2 in a solar-gliding arc plasma reactor: effects of water, nitrogen, methane, and process optimization[J]. Journal of CO2 Utilization, 2020, 38: 39-48. |
| 23 | Ramakers M, Medrano J A, Trenchev G, et al. Revealing the arc dynamics in a gliding arc plasmatron: a better insight to improve CO2 conversion[J]. Plasma Sources Science & Technology, 2017, 26(12): 125002. |
| 24 | Li L, Zhang H, Li X D, et al. Plasma-assisted CO2 conversion in a gliding arc discharge: improving performance by optimizing the reactor design[J]. Journal of CO2 Utilization, 2019, 29: 296-303. |
| 25 | Liu J L, Park H W, Chung W J, et al. High-efficient conversion of CO2 in AC-pulsed tornado gliding arc plasma[J]. Plasma Chemistry and Plasma Processing, 2016, 36(2): 437-449. |
| 26 | Ramakers M, Heijkers S, Tytgat T, et al. Combining CO2 conversion and N2 fixation in a gliding arc plasmatron[J]. Journal of CO2 Utilization, 2019, 33: 121-130. |
| 27 | Liu J B, Li X S, Liu J L, et al. Insight into gliding arc (GA) plasma reduction of CO2 with H2: GA characteristics and reaction mechanism[J]. Journal of Physics D-Applied Physics, 2019, 52(28): 284001. |
| 28 | Ramakers M, Trenchev G, Heijkers S, et al. Gliding arc plasmatron: providing an alternative method for carbon dioxide conversion[J]. ChemSusChem, 2017, 10(12): 2642-2652. |
| 29 | Nunnally T, Gutsol K, Rabinovich A, et al. Dissociation of CO2 in a low current gliding arc plasmatron[J]. Journal of Physics D-Applied Physics, 2011, 44(27): 274009. |
| 30 | Kim S C, Lim M S, Chun Y N. Reduction characteristics of carbon dioxide using a plasmatron[J]. Plasma Chemistry and Plasma Processing, 2014, 34(1): 125-143. |
| 31 | Xu W, Li M W, Xu G H, et al. Decomposition of CO2 using DC corona discharge at atmospheric pressure[J]. Japanese Journal of Applied Physics, 2004, 43(12): 8310-8311. |
| 32 | Wen Y Z, Jiang X Z. Decomposition of CO2 using pulsed corona discharges combined with catalyst[J]. Plasma Chemistry and Plasma Processing, 2001, 21(4): 665-678. |
| 33 | 代斌, 宫为民, 张秀玲, 等. 脉冲电晕等离子体活化纯CO2的反应[J]. 中国环境科学, 1999, 19(5): 410-412. |
| Dai B, Gong W M, Zhang X L, et al. Investigation on the conversion of pure CO2 by pulse corona plasma[J]. China Environmental Science, 1999, 19(5): 410-412 | |
| 34 | 李明伟, 许根慧, 刘昌俊, 等. 电晕放电二氧化碳冷等离子体转化特性研究[J]. 燃料化学学报, 2001, 29(3): 243-246. |
| Li M W, Xu G H, Liu C J, et al. Study on corona discharge for carbon dioxide conversion using cold plasma reaction[J]. Journal of Fuel Chemistry, 2001, 29(3): 243-246. | |
| 35 | Aerts R, Somers W, Bogaerts A. Carbon dioxide splitting in a dielectric barrier discharge plasma: a combined experimental and computational study[J]. ChemSusChem, 2015, 8(4): 702-716. |
| 36 | Ozkan A, Bogaerts A, Reniers F. Routes to increase the conversion and the energy efficiency in the splitting of CO2 by a dielectric barrier discharge[J]. Journal of Physics D: Applied Physics, 2017, 50(8): 084004. |
| 37 | Duan X F, Li Y P, Ge W J, et al. Degradation of CO2 through dielectric barrier discharge microplasma[J]. Greenhouse Gases: Science and Technology, 2015, 5(2): 131-140. |
| 38 | Duan X F, Hu Z Y, Li Y P, et al. Effect of dielectric packing materials on the decomposition of carbon dioxide using DBD microplasma reactor[J]. AIChE Journal, 2015, 61(3): 898-903. |
| 39 | Belov I, Paulussen S, Bogaerts A. Appearance of a conductive carbonaceous coating in a CO2 dielectric barrier discharge and its influence on the electrical properties and the conversion efficiency[J]. Plasma Sources Science & Technology, 2016, 25(1): 015023. |
| 40 | Wang J Y, Xia G G, Huang A M, et al. CO2 decomposition using glow discharge plasmas[J]. Journal of Catalysis, 1999, 185(1): 152-159. |
| 41 | Snoeckx R, Heijkers S, van Wesenbeeck K, et al. CO2 conversion in a dielectric barrier discharge plasma: N2 in the mix as a helping hand or problematic impurity?[J]. Energy and Environmental Science, 2016, 9(3): 999-1011. |
| 42 | Li S R, Ongis M, Manzolini G, et al. Non-thermal plasma-assisted capture and conversion of CO2 [J]. Chemical Engineering Journal, 2021, 410: 128335. |
| 43 | Itoh N, Sanchez M A, Xu W C, et al. Application of a membrane reactor system to thermal decomposition of CO2 [J]. Journal of Membrane Science, 1993, 77(2/3): 245-253. |
| 44 | 宋爽, 裘建平, 何志桥, 等. CO2一步电化学转化技术的可行性研究[J]. 航天医学与医学工程, 2006, 19(3): 199-203. |
| Song S, Qiu J P, He Z Q, et al. Study on feasibility of one-step electrochemical conversion of CO2 [J]. Aerospace Medicine and Medical Engineering, 2006, 19(3): 199-203. |
| [1] | Yifei ZHANG, Fangchen LIU, Shuangxing ZHANG, Wenjing DU. Performance analysis of printed circuit heat exchanger for supercritical carbon dioxide [J]. CIESC Journal, 2023, 74(S1): 183-190. |
| [2] | He JIANG, Junfei YUAN, Lin WANG, Guyu XING. Experimental study on the effect of flow sharing cavity structure on phase change flow characteristics in microchannels [J]. CIESC Journal, 2023, 74(S1): 235-244. |
| [3] | Ruitao SONG, Pai WANG, Yunpeng WANG, Minxia LI, Chaobin DANG, Zhenguo CHEN, Huan TONG, Jiaqi ZHOU. Numerical simulation of flow boiling heat transfer in pipe arrays of carbon dioxide direct evaporation ice field [J]. CIESC Journal, 2023, 74(S1): 96-103. |
| [4] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [5] | Wenzhu LIU, Heming YUN, Baoxue WANG, Mingzhe HU, Chonglong ZHONG. Research on topology optimization of microchannel based on field synergy and entransy dissipation [J]. CIESC Journal, 2023, 74(8): 3329-3341. |
| [6] | Rui HONG, Baoqiang YUAN, Wenjing DU. Analysis on mechanism of heat transfer deterioration of supercritical carbon dioxide in vertical upward tube [J]. CIESC Journal, 2023, 74(8): 3309-3319. |
| [7] | Qiyu ZHANG, Lijun GAO, Yuhang SU, Xiaobo MA, Yicheng WANG, Yating ZHANG, Chao HU. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide [J]. CIESC Journal, 2023, 74(7): 2753-2772. |
| [8] | Kexin HUANG, Tong LI, Anqi LI, Mei LIN. Mode decomposition of flow field in T-junction with rotating impeller [J]. CIESC Journal, 2023, 74(7): 2848-2857. |
| [9] | Chenxi LI, Yongfeng LIU, Lu ZHANG, Haifeng LIU, Jin’ou SONG, Xu HE. Quantum chemical analysis of n-heptane combustion mechanism under O2/CO2 atmosphere [J]. CIESC Journal, 2023, 74(5): 2157-2169. |
| [10] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [11] | Lufan JIA, Yiying WANG, Yuman DONG, Qinyuan LI, Xin XIE, Hao YUAN, Tao MENG. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction [J]. CIESC Journal, 2023, 74(3): 1239-1246. |
| [12] | Bingguo ZHU, Jixiang HE, Jinliang XU, Bin PENG. Heat transfer characteristics of supercritical pressure CO2 in diverging/converging tube under cooling conditions [J]. CIESC Journal, 2023, 74(3): 1062-1072. |
| [13] | Xueting ZHANG, Jijiang HU, Jing ZHAO, Bogeng LI. Preparation of high molecular weight polypropylene glycol in microchannel reactor [J]. CIESC Journal, 2023, 74(3): 1343-1351. |
| [14] | Xingyu YANG, You MA, Chunying ZHU, Taotao FU, Youguang MA. Study on liquid-liquid distribution in comb parallel microchannels [J]. CIESC Journal, 2023, 74(2): 698-706. |
| [15] | Renchu HE, Zhaohui ZHANG, Minglei YANG, Cong WANG, Zhenhao XI. Online optimization of gasoline blending considering carbon emissions [J]. CIESC Journal, 2023, 74(2): 818-829. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||