CIESC Journal ›› 2022, Vol. 73 ›› Issue (6): 2289-2305.DOI: 10.11949/0438-1157.20220062
• Reviews and monographs • Previous Articles Next Articles
Wenjing ZHANG( ),Jing LI(
),Jing LI( ),Zidong WEI(
),Zidong WEI( )
)
Received:2022-01-12
Revised:2022-03-13
Online:2022-06-30
Published:2022-06-05
Contact:
Jing LI,Zidong WEI
通讯作者:
李静,魏子栋
作者简介:张文静(1996—),女,博士研究生,基金资助:CLC Number:
Wenjing ZHANG, Jing LI, Zidong WEI. Electrocatalysis from a mesoscale perspective: interface, membrane and porous electrode[J]. CIESC Journal, 2022, 73(6): 2289-2305.
张文静, 李静, 魏子栋. 介尺度视角下的电催化:从界面、隔膜到多孔电极[J]. 化工学报, 2022, 73(6): 2289-2305.
Add to citation manager EndNote|Ris|BibTeX
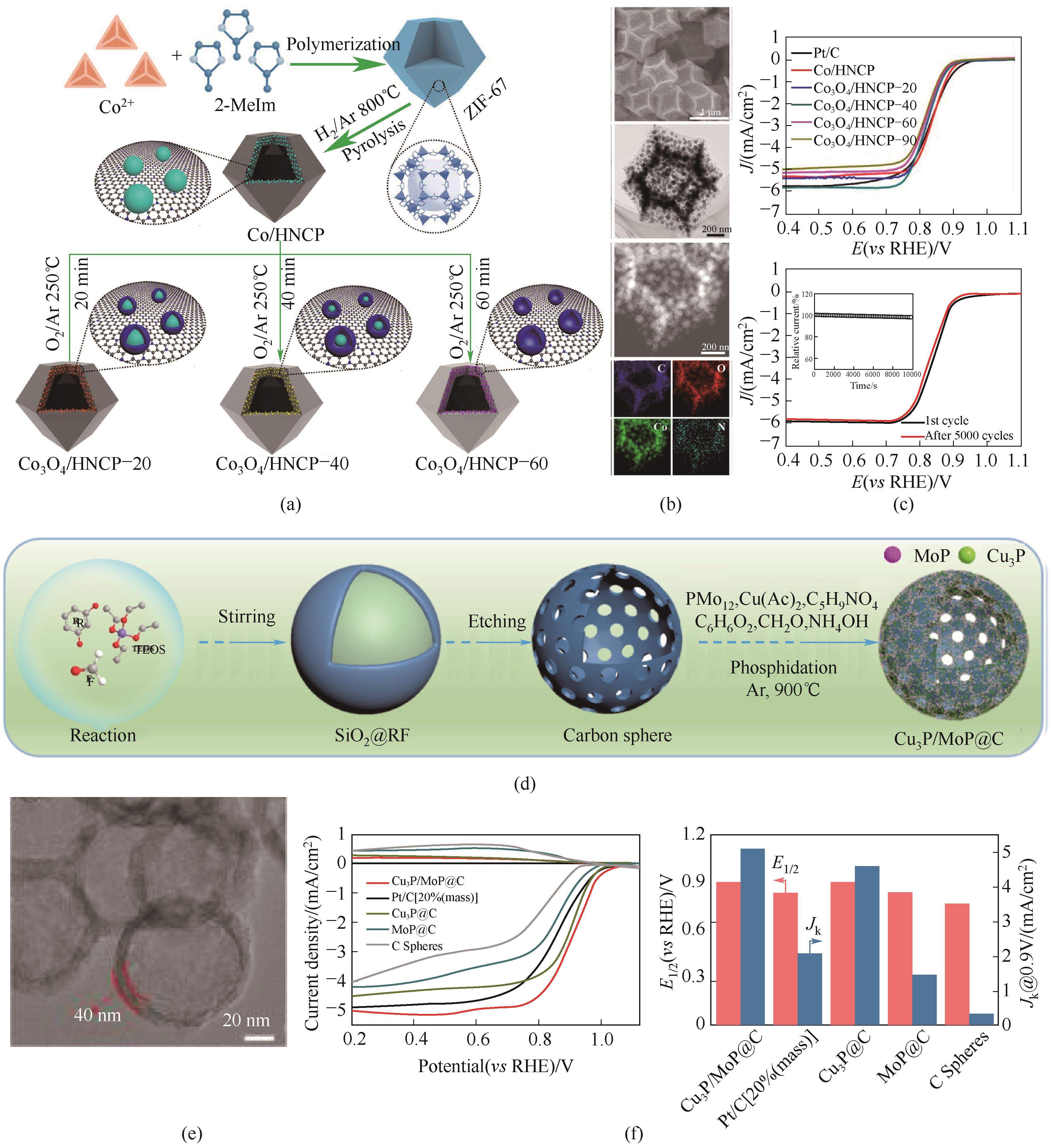
Fig.3 (a) Illustration of Co-Co3O4-based nanoarchitectures embedded in hollow nitrogen-doped carbon polyhedron; (b) SEM, TEM, STEM, and EDS mappings of Co3O4/HNCP-40; (c) ORR polarization curves and before and after 5000 cycles for the ORR[15]; (d) Schematic illustration of preparation Cu3P/MoP@C; (e) HR-TEM image; (f) ORR polarization and the corresponding half-wave potential (E1/2) and kinetic current density (Jk) at 0.9 V[16]
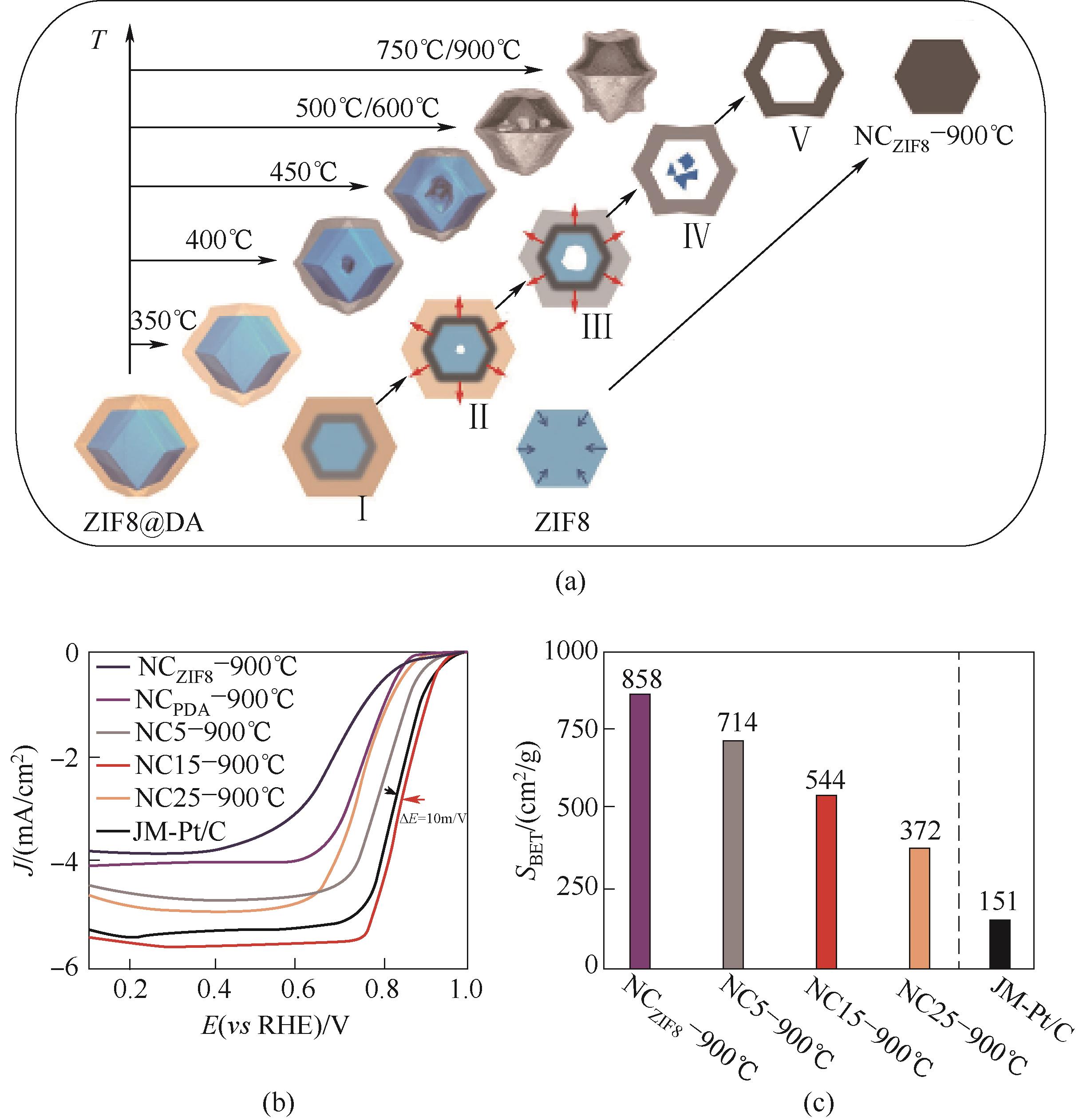
Fig.4 (a) Schematic illustration of the stresses induced orientation contraction mechanism for constructing the hollow structures; (b) ORR polarization curves measured in O2-saturated 0.1 mol/L KOH solution; (c) The comparison of BET specific surfaces areas[17]
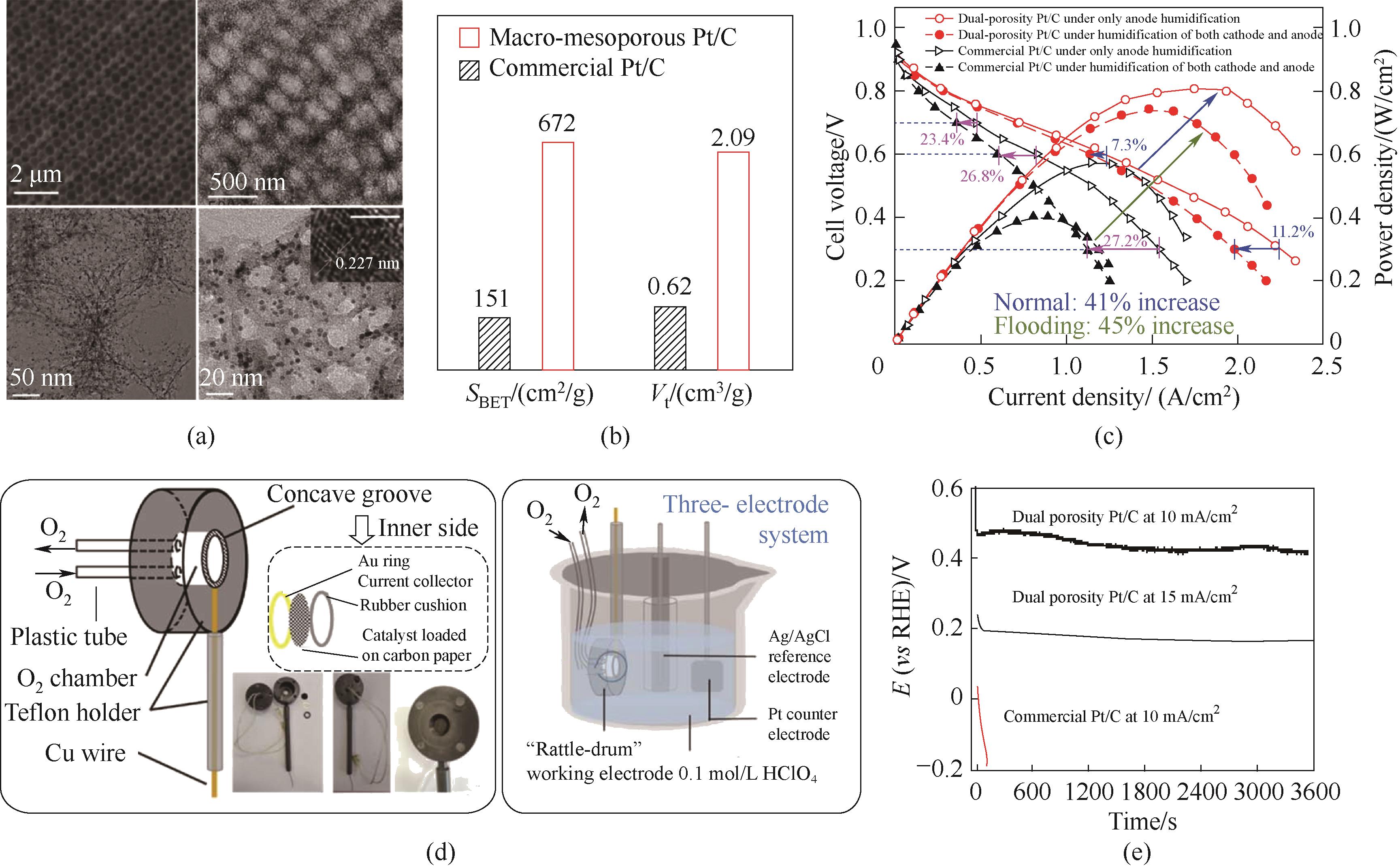
Fig.5 (a) SEM and TEM images of the catalysts; (b) Comparison of the specific surface areas and total pore volumes for the two catalysts; (c) Polarization and power densities curves of MEA; (d) The schematic and optical pictures of the self-made rattle-drum -like working electrode; (e) Chronopotentiometry curves recorded at 10/15 mA/cm2, the catalyst was loaded on the “rattle-drum” working electrode [22]
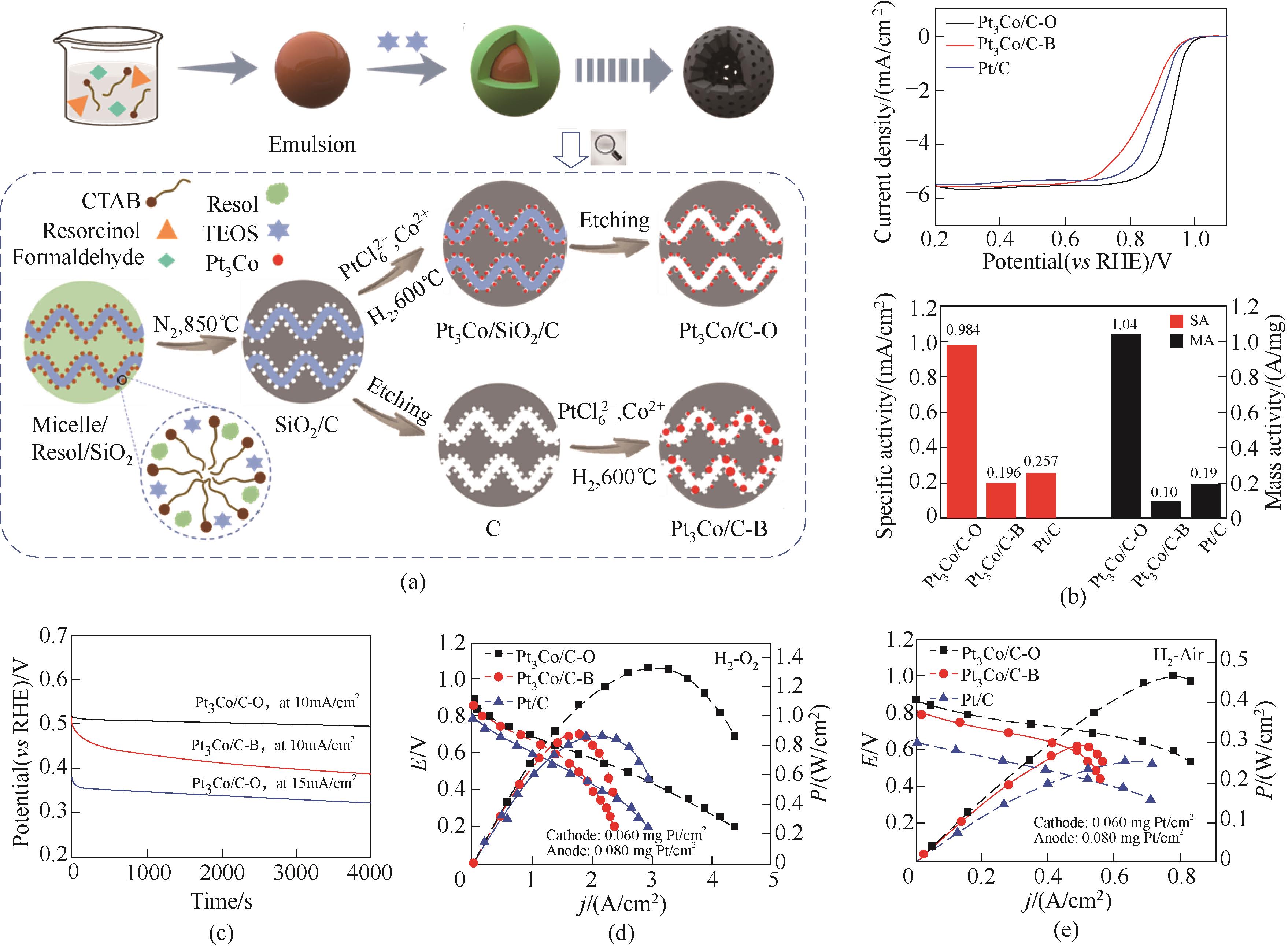
Fig.6 (a) Schematic illustration of the preparation of Pt3Co/C-O and Pt3Co/C-B catalysts; (b) ORR polarization curves and specific activity and mass activity; (c) Chronopotentiometry curves recorded at 10/15 mA/cm2, the catalyst was loaded on the “rattle-drum” working electrode; Polarization curves and power density of H2-O2 (d) and H2-Air (e) fuel cells[26]
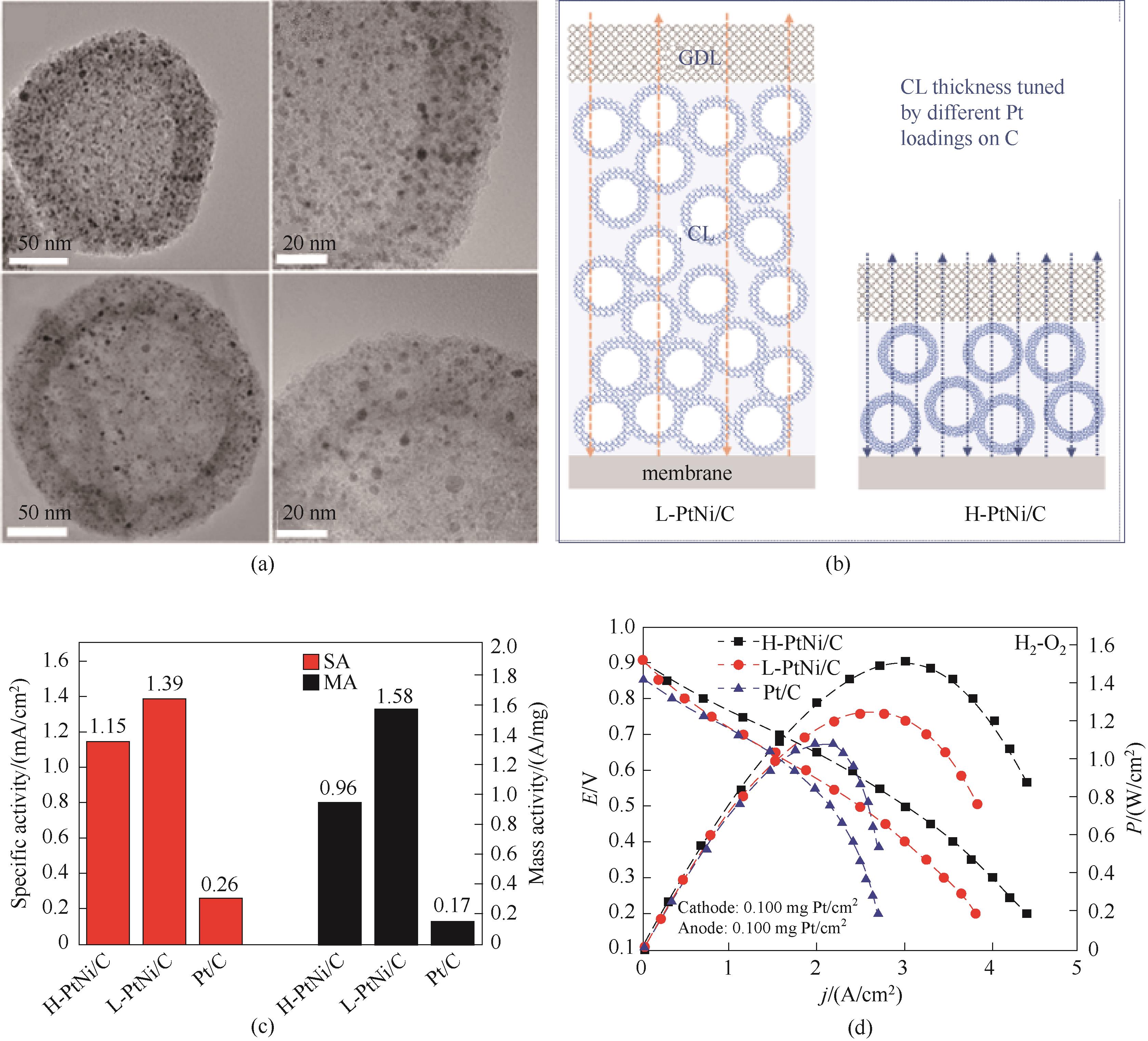
Fig.7 (a) TEM images of H-PtNi/C (top) and L-PtNi/C (down); (b) Schematic illustration of MEA cathodes constructed by catalysts with a high Pt loading (H-PtNi/C) and a low Pt loading (L-PtNi/C) on carbon (GDL is gas diffusion layer; CL is catalyst layer); (c) Specific activity and mass activity estimated at 0.9 V versus reversible hydrogen electrode (RHE); (d) Polarization curves and power densities of H2-O2 fuel cells[27]

Fig.8 (a) Current densities at η=0.3 V of different metals[37]; (b) Schematic representation of the neutral pH HER on MoP700; (c) HER polarization curves and Tafel plots[38]

Fig.9 (a) Schematic diagram of the synthesis of IO-Ru–TiO2/C, Ru–TiO2/C and Ru/C catalysts[39]; (b) HAADF-STEM images of Ru@ TiO2[40]; (c) H adsorption energy (ΔE*H) and free energy (ΔGads) of H, OH and H2O on Pt/O–TiO2 and Pt/Ti–TiO2[41]

Fig.10 (a) Charge density difference; (b) Projected crystal orbital Hamilton overlap population (pCOHP) curves and partial density of states (PDOS) of interfacial bonds;(c) Ir 4f XPS spectrum; (d) Polarization curves of investigated catalysts for HOR in H2-saturated 0.1 mol/LKOH solutions of Ir/Mo-MoO2, Ir/O-MoO2, and Ir/MoO2[42]

Fig.11 (a) The schematic diagram of the monometallic NiO-Ni3S2 heteronanosheets and the water electrolysis process occurred on its surface[48]; (b) Illustration of the preparation of Fe3O4/FeS2-x samples; (c) OER polarization curves; (d) HRTEM images for the samples of Fe3O4/FeS2-1 and Fe3O4/FeS2-2.5[49]
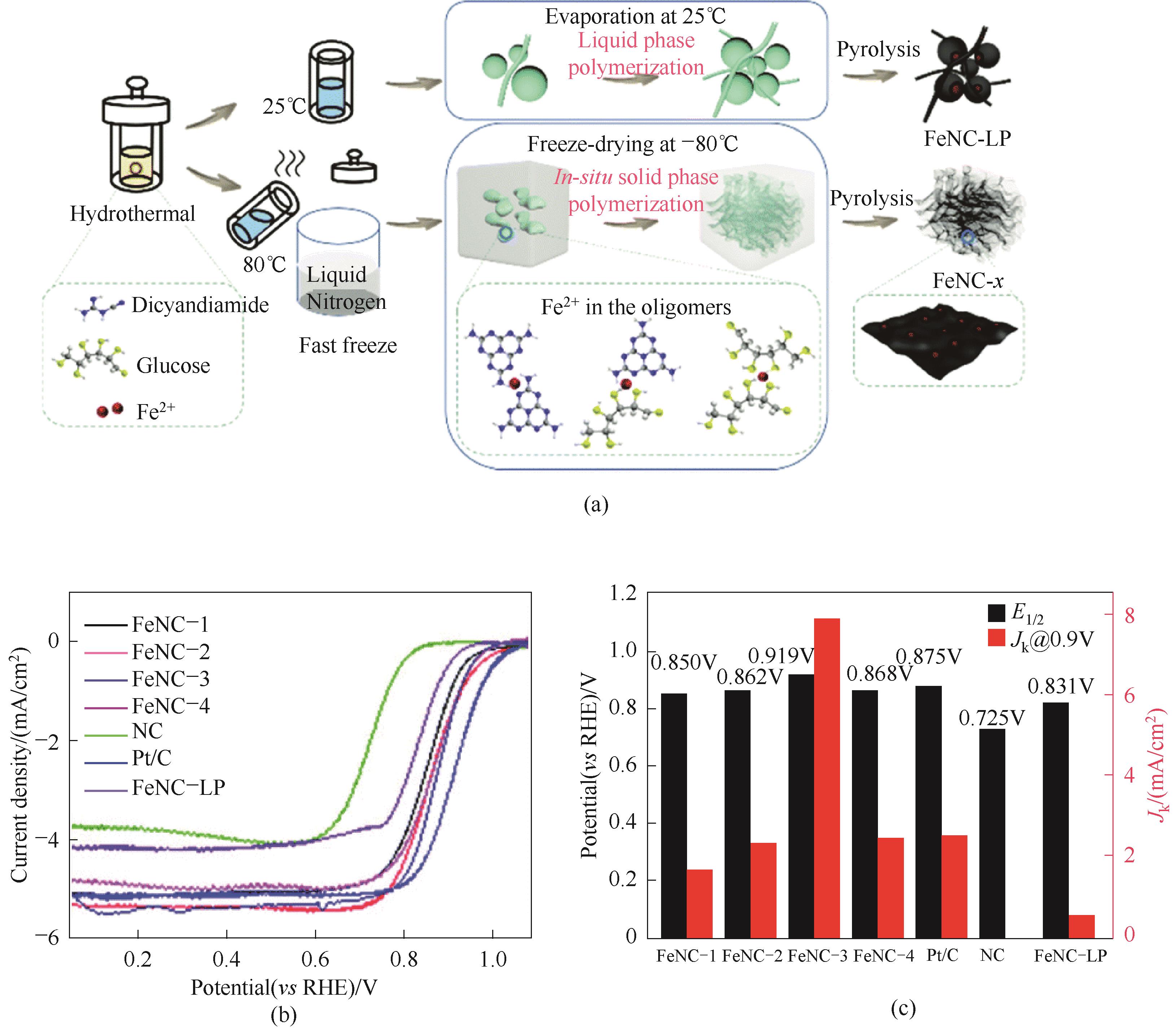
Fig.14 (a) Schematic illustration of the preparation of the aerogel structured FeNC materials; (b) ORR polarization curves;(c) Comparison of half wave potential and dynamic current density [59]
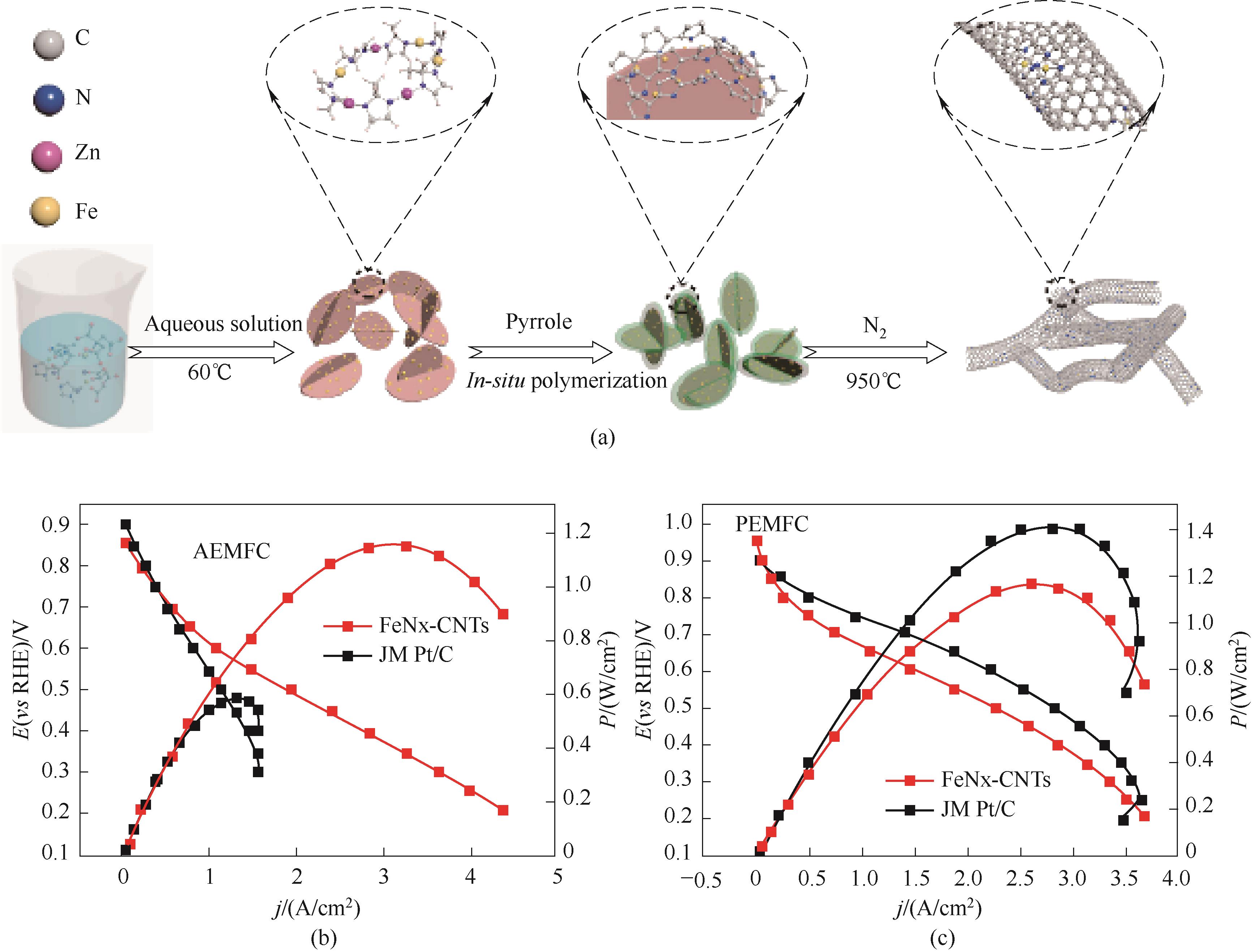
Fig.15 (a) Schematic illustration of the preparation process for the FeNx-CNTs catalysts; (b),(c) Polarization curves and power densities of H2-O2 AEMFC and PEMFC [64]
| 1 | 李宇明, 刘梓烨, 张启扬, 等. 氮掺杂碳材料的制备及其在催化领域中的应用[J]. 化工学报, 2021, 72(8): 3919-3932. |
| Li Y M, Liu Z Y, Zhang Q Y, et al. Preparation of nitrogen-doped carbon materials and their applications in catalysis[J]. CIESC Journal, 2021, 72(8): 3919-3932. | |
| 2 | Rabiee H, Ge L, Zhang X Q, et al. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: a review[J]. Energy & Environmental Science, 2021, 14(4): 1959-2008. |
| 3 | Wang M, Zhang L, He Y J, et al. Recent advances in transition-metal-sulfide-based bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Chemistry A, 2021, 9(9): 5320-5363. |
| 4 | Wu Z Z, Gao F Y, Gao M R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction[J]. Energy & Environmental Science, 2021, 14(3): 1121-1139. |
| 5 | Wang Y, Huang X, Wei Z D. Recent developments in the use of single-atom catalysts for water splitting[J]. Chinese Journal of Catalysis, 2021, 42(8): 1269-1286. |
| 6 | 葛蔚, 刘新华, 任瑛, 等. 从多尺度到介尺度: 复杂化工过程模拟的新挑战[J]. 化工学报, 2010, 61(7): 1613-1620. |
| Ge W, Liu X H, Ren Y, et al. From multi-scale to meso-scale: new challenges for simulation of complex processes in chemical engineering[J]. CIESC Journal, 2010, 61(7): 1613-1620. | |
| 7 | 朱育丹, 陆小华, 郭晓静, 等. 材料化学工程科学内涵及方法初探: 从介观尺度界面流体行为出发认知材料[J]. 化工学报, 2013, 64(1): 148-154. |
| Zhu Y D, Lu X H, Guo X J, et al. Preliminary discussion on scientific connotation and research method of aterial-oriented chemical engineering: understanding materials based on confined interfacial fluid behavior on mesoscale[J]. CIESC Journal, 2013, 64(1): 148-154. | |
| 8 | 李静海, 胡英, 袁权. 探索介尺度科学: 从新角度审视老问题[J]. 中国科学: 化学, 2014, 44(3): 277-281. |
| Li J H, Hu Y, Yuan Q. Mesoscience: exploring old problems from a new angle[J]. Scientia Sinica Chimica, 2014, 44(3): 277-281. | |
| 9 | 叶艳玲, 李静海. 介科学: 探索介尺度共性原理[J]. 中国科技术语, 2017, 19(5): 69. |
| Ye Y L, Li J H. Mesoscience: exploring the common principle at mesoscales[J]. China Terminology, 2017, 19(5): 69. | |
| 10 | Peng L S, Wei Z D. Recent progress of mesoscience in design of electrocatalytic materials for hydrogen energy conversion[J]. Particuology, 2020, 48: 19-33. |
| 11 | 张文静, 李静, 魏子栋. 燃料电池空气电极的孔道结构调控[J]. 化工学报, 2020, 71(10): 4553-4574. |
| Zhang W J, Li J, Wei Z D. Strategies for tuning porous structures of air electrode in fuel cells[J]. CIESC Journal, 2020, 71(10): 4553-4574. | |
| 12 | Yang Z C, Zhang Y, Kong J H, et al. Hollow carbon nanoparticles of tunable size and wall thickness by hydrothermal treatment of α-cyclodextrin templated by F127 block copolymers[J]. Chemistry of Materials, 2013, 25(5): 704-710. |
| 13 | Hwang J, Kim S, Wiesner U, et al. Generalized access to mesoporous inorganic particles and hollow spheres from multicomponent polymer blends[J]. Advanced Materials, 2018, 30(27): 1801127. |
| 14 | Li B Q, Zhang S Y, Kong L, et al. Porphyrin organic framework hollow spheres and their applications in lithium-sulfur batteries[J]. Advanced Materials, 2018, 30(23): e1707483. |
| 15 | Ding D N, Shen K, Chen X D, et al. Multi-level architecture optimization of MOF-templated co-based nanoparticles embedded in hollow N-doped carbon polyhedra for efficient OER and ORR[J]. ACS Catalysis, 2018, 8(9): 7879-7888. |
| 16 | Guo M, Xu M J, Qu Y, et al. Electronic/mass transport increased hollow porous Cu3P/MoP nanospheres with strong electronic interaction for promoting oxygen reduction in Zn-air batteries[J]. Applied Catalysis B: Environmental, 2021, 297: 120415. |
| 17 | Wang M J, Mao Z X, Liu L, et al. Preparation of hollow nitrogen doped carbon via stresses induced orientation contraction[J]. Small, 2018, 14(52): e1804183. |
| 18 | Borup R, Meyers J, Pivovar B, et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation[J]. Chemical Reviews, 2007, 107(10): 3904-3951. |
| 19 | Choi J Y, Yang L J, Kishimoto T, et al. Is the rapid initial performance loss of Fe/N/C non precious metal catalysts due to micropore flooding? [J]. Energy & Environmental Science, 2017, 10(1): 296-305. |
| 20 | Guo L, Jiang W J, Zhang Y, et al. Embedding Pt nanocrystals in N-doped porous carbon/carbon nanotubes toward highly stable electrocatalysts for the oxygen reduction reaction[J]. ACS Catalysis, 2015, 5(5): 2903-2909. |
| 21 | Takenaka S, Miyamoto H, Utsunomiya Y, et al. Catalytic activity of highly durable Pt/CNT catalysts covered with hydrophobic silica layers for the oxygen reduction reaction in PEFCs[J]. The Journal of Physical Chemistry C, 2014, 118(2): 774-783. |
| 22 | Wang M J, Zhao T, Luo W, et al. Quantified mass transfer and superior antiflooding performance of ordered macro-mesoporous electrocatalysts[J]. AIChE Journal, 2018, 64(7): 2881-2889. |
| 23 | Ao X, Zhang W, Zhao B T, et al. Atomically dispersed Fe–N–C decorated with Pt-alloy core–shell nanoparticles for improved activity and durability towards oxygen reduction[J]. Energy & Environmental Science, 2020, 13(9): 3032-3040. |
| 24 | Zhao W Y, Ye Y K, Jiang W J, et al. Mesoporous carbon confined intermetallic nanoparticles as highly durable electrocatalysts for the oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2020, 8(31): 15822-15828. |
| 25 | Galeano C, Meier J C, Peinecke V, et al. Toward highly stable electrocatalysts via nanoparticle pore confinement[J]. Journal of the American Chemical Society, 2012, 134(50): 20457-20465. |
| 26 | Hong W, Shen X R, Wang F Z, et al. A bimodal-pore strategy for synthesis of Pt3Co/C electrocatalyst toward oxygen reduction reaction[J]. Chemical Communications, 2021, 57(35): 4327-4330. |
| 27 | Hong W, Shen X R, Wang J, et al. High-loading Pt-alloy catalysts for boosted oxygen reduction reaction performance[J]. Chinese Journal of Chemical Engineering, 2021 |
| 28 | Zhang X, Wang Y T, Liu C B, et al. Recent advances in non-noble metal electrocatalysts for nitrate reduction[J]. Chemical Engineering Journal, 2021, 403: 126269. |
| 29 | Lim K R G, Handoko A D, Nemani S K, et al. Rational design of two-dimensional transition metal carbide/nitride (MXene) hybrids and nanocomposites for catalytic energy storage and conversion[J]. ACS Nano, 2020, 14(9): 10834-10864. |
| 30 | Guo Y H, Bae J, Fang Z W, et al. Hydrogels and hydrogel-derived materials for energy and water sustainability[J]. Chemical Reviews, 2020, 120(15): 7642-7707. |
| 31 | Wang Q C, Lei Y P, Wang Y C, et al. Atomic-scale engineering of chemical-vapor-deposition-grown 2D transition metal dichalcogenides for electrocatalysis[J]. Energy & Environmental Science, 2020, 13(6): 1593-1616. |
| 32 | Xu H, Shang H Y, Wang C, et al. Ultrafine Pt-based nanowires for advanced catalysis[J]. Advanced Functional Materials, 2020, 30(28): 2000793. |
| 33 | 郑星群, 李莉, 魏子栋. 介尺度视角下的电催化剂调控策略[J]. 化工学报, 2020, 71(10): 4445-4461. |
| Zheng X Q, Li L, Wei Z D. Constructing and regulating electrocatalysts: from perspective of mesoscale[J]. CIESC Journal, 2020, 71(10): 4445-4461. | |
| 34 | Subbaraman R, Tripkovic D, Strmcnik D, et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li⁺-Ni(OH)₂-Pt interfaces[J]. Science, 2011, 334(6060): 1256-1260. |
| 35 | Xiang R, Peng L S, Wei Z D. Tuning interfacial structures for better catalysis of water electrolysis[J]. Chemistry - A European Journal, 2019, 25(42): 9799-9815. |
| 36 | Yang L, Liu R M, Jiao L F. Electronic redistribution: construction and modulation of interface engineering on CoP for enhancing overall water splitting[J]. Advanced Functional Materials, 2020, 30(14): 1909618. |
| 37 | Danilovic N, Subbaraman R, Strmcnik D, et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts[J]. Angewandte Chemie International Edition, 2012, 124(50): 12663-12666. |
| 38 | Xie X H, Song M, Wang L G, et al. Electrocatalytic hydrogen evolution in neutral pH solutions: dual-phase synergy[J]. ACS Catalysis, 2019, 9(9): 8712-8718. |
| 39 | Jiang J X, Tao S C, He Q, et al. Interphase-oxidized ruthenium metal with half-filled d-orbitals for hydrogen oxidation in an alkaline solution[J]. Journal of Materials Chemistry A, 2020, 8(20): 10168-10174. |
| 40 | Zhou Y, Xie Z, Jiang J, et al. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction[J]. Nature Catalysis, 2020, 3(5) : 454-462. |
| 41 | Zheng X Q, Li L, Deng M M, et al. Understanding the effect of interfacial interaction on metal/metal oxide electrocatalysts for hydrogen evolution and hydrogen oxidation reactions on the basis of first-principles calculations[J]. Catalysis Science & Technology, 2020, 10(14): 4743-4751. |
| 42 | Li M T, Xie Z Y, Zheng X Q, et al. Revealing the regulation mechanism of Ir–MoO2 interfacial chemical bonding for improving hydrogen oxidation reaction[J]. ACS Catalysis, 2021, 11(24): 14932-14940. |
| 43 | Peng L S, Liao M S, Zheng X Q, et al. Accelerated alkaline hydrogen evolution on M(OH) x /M-MoPO x (M = Ni, Co, Fe, Mn) electrocatalysts by coupling water dissociation and hydrogen ad-desorption steps[J]. Chemical Science, 2020, 11(9): 2487-2493. |
| 44 | Whittaker T, Kumar K B S, Peterson C, et al. H2 oxidation over supported Au nanoparticle catalysts: evidence for heterolytic H2 activation at the metal-support interface[J]. Journal of the American Chemical Society, 2018, 140(48): 16469-16487. |
| 45 | Miller H A, Vizza F, Marelli M, et al. Highly active nanostructured palladium-ceria electrocatalysts for the hydrogen oxidation reaction in alkaline medium[J]. Nano Energy, 2017, 33: 293-305. |
| 46 | Feng L L, Yu G T, Wu Y Y, et al. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting[J]. Journal of the American Chemical Society, 2015, 137(44): 14023-14026. |
| 47 | Choi J, Kim D, Zheng W R, et al. Interface engineered NiFe2O4-x/NiMoO4 nanowire arrays for electrochemical oxygen evolution[J]. Applied Catalysis B: Environmental, 2021, 286: 119857. |
| 48 | Peng L S, Shen J J, Zheng X Q, et al. Rationally design of monometallic NiO-Ni3S2/NF heteronanosheets as bifunctional electrocatalysts for overall water splitting[J]. Journal of Catalysis, 2019, 369: 345-351. |
| 49 | Wang M J, Zheng X Q, Song L L, et al. Fe3O4/FeS2 heterostructures enable efficient oxygen evolution reaction[J]. Journal of Materials Chemistry A, 2020, 8(28): 14145-14151. |
| 50 | Liu L C, Corma A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles[J]. Chemical Reviews, 2018, 118(10): 4981-5079. |
| 51 | Yang F, Deng D H, Pan X L, et al. Understanding nano effects in catalysis[J]. National Science Review, 2015, 2(2): 183-201. |
| 52 | Cheng N C, Sun X L. Single atom catalyst by atomic layer deposition technique[J]. Chinese Journal of Catalysis, 2017, 38(9): 1508-1514. |
| 53 | Qiao B T, Liang J X, Wang A Q, et al. Single atom gold catalysts for low-temperature CO oxidation[J]. Chinese Journal of Catalysis, 2016, 37(10): 1580-1586. |
| 54 | Wang Q M, Chen S G, Shi F, et al. Structural evolution of solid Pt nanoparticles to a hollow PtFe alloy with a Pt-skin surface via space-confined pyrolysis and the nanoscale kirkendall effect[J]. Advanced Materials, 2016, 28(48): 10673-10678. |
| 55 | Zou X, Chen S G, Wang Q M, et al. Leaching- and sintering-resistant hollow or structurally ordered intermetallic PtFe alloy catalysts for oxygen reduction reactions[J]. Nanoscale, 2019, 11(42): 20115-20122. |
| 56 | Wang J, Huang Z Q, Liu W, et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction[J]. Journal of the American Chemical Society, 2017, 139(48): 17281-17284. |
| 57 | Zhang H G, Hwang S, Wang M Y, et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation[J]. Journal of the American Chemical Society, 2017, 139(40): 14143-14149. |
| 58 | Chen Y J, Ji S F, Wang Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2017, 56(24): 6937-6941. |
| 59 | Hong W, Feng X, Tan L Q, et al. Preparation of monodisperse ferrous nanoparticles embedded in carbon aerogels via in situ solid phase polymerization for electrocatalytic oxygen reduction[J]. Nanoscale, 2020, 12(28): 15318-15324. |
| 60 | Jahnke H, Schönborn M, Zimmermann G. Organic dyestuffs as catalysts for fuel cells[J]. Topics in Current Chemistry, 1976, 61:131-181. |
| 61 | Gupta S, Tryk D, Bae I, et al. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction[J]. Journal of Applied Electrochemistry, 1989, 19(1): 19-27. |
| 62 | Masa J, Xia W, Muhler M, et al. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction[J]. Angewandte Chemie International Edition, 2015, 54(35): 10102-10120. |
| 63 | Ren Q, Wang H, Lu X F, et al. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction[J]. Advanced Science, 2018, 5(3): 1700515. |
| 64 | He Q, Zeng L P, Wang J, et al. Polymer-coating-induced synthesis of FeN x enriched carbon nanotubes as cathode that exceeds 1.0 W·cm-2 peak power in both proton and anion exchange membrane fuel cells[J]. Journal of Power Sources, 2021, 489: 229499. |
| 65 | 李存璞, 王建川, 魏子栋. 电化学反应器隔膜材料的分子设计与介尺度策略[J]. 化工学报, 2020, 71(10): 4490-4501. |
| Li C P, Wang J C, Wei Z D. Mesoscopic strategies and molecular design of diaphragm for electrochemical reactors[J]. CIESC Journal, 2020, 71(10): 4490-4501. | |
| 66 | Varcoe J R, Atanassov P, Dekel D R, et al. Anion-exchange membranes in electrochemical energy systems[J]. Energy & Environmental Science, 2014, 7(10): 3135-3191. |
| 67 | Wang Y J, Qiao J L, Baker R, et al. Alkaline polymer electrolyte membranes for fuel cell applications[J]. Chemical Society Reviews, 2013, 42(13): 5768-5787. |
| 68 | Wang L Q, Peng X, Mustain W E, et al. Radiation-grafted anion-exchange membranes: the switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance[J]. Energy & Environmental Science, 2019, 12(5): 1575-1579. |
| 69 | Wang J, Zhao Y, Setzler B P, et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells [J]. Nature Energy, 2019, 4(5): 392-398. |
| 70 | Hamada T, Hasegawa S, Fukasawa H, et al. Poly(ether ether ketone) (PEEK)-based graft-type polymer electrolyte membranes having high crystallinity for high conducting and mechanical properties under various humidified conditions[J]. Journal of Materials Chemistry A, 2015, 3(42): 20983-20991. |
| 71 | Lee W H, Kim Y S, Bae C. Robust hydroxide ion conducting poly(biphenyl alkylene)s for alkaline fuel cell membranes[J]. ACS Macro Letters, 2015, 4(8): 814-818. |
| 72 | Zhao W F, He C, Nie C X, et al. Synthesis and characterization of ultrahigh ion-exchange capacity polymeric membranes[J]. Industrial & Engineering Chemistry Research, 2016, 55(36): 9667-9675. |
| 73 | Park A M, Turley F E, Wycisk R J, et al. Electrospun and cross-linked nanofiber composite anion exchange membranes[J]. Macromolecules, 2014, 47(1): 227-235. |
| 74 | Zeng L P, Liao Y C, Wang J C, et al. Construction of highly efficient ion channel within anion exchange membrane based on interpenetrating polymer network for H2/Air (CO2-free) alkaline fuel cell[J]. Journal of Power Sources, 2021, 486: 229377. |
| 75 | Zeng L P, He Q, Liao Y C, et al. Anion exchange membrane based on interpenetrating polymer network with ultrahigh ion conductivity and excellent stability for alkaline fuel cell[J]. Research, 2020, 2020: 4794706. |
| [1] | Yanpeng WU, Xiaoyu LI, Qiaoyang ZHONG. Experimental analysis on filtration performance of electrospun nanofibers with amphiphobic membrane of oily fine particles [J]. CIESC Journal, 2023, 74(S1): 259-264. |
| [2] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [3] | Xuejin YANG, Jintao YANG, Ping NING, Fang WANG, Xiaoshuang SONG, Lijuan JIA, Jiayu FENG. Research progress in dry purification technology of highly toxic gas PH3 [J]. CIESC Journal, 2023, 74(9): 3742-3755. |
| [4] | Yali HU, Junyong HU, Suxia MA, Yukun SUN, Xueyi TAN, Jiaxin HUANG, Fengyuan YANG. Development of novel working fluid and study on electrochemical characteristics of reverse electrodialysis heat engine [J]. CIESC Journal, 2023, 74(8): 3513-3521. |
| [5] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [6] | Jipeng ZHOU, Wenjun HE, Tao LI. Reaction engineering calculation of deactivation kinetics for ethylene catalytic oxidation over irregular-shaped catalysts [J]. CIESC Journal, 2023, 74(6): 2416-2426. |
| [7] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| [8] | Zhaoguang CHEN, Yuxiang JIA, Meng WANG. Modeling neutralization dialysis desalination driven by low concentration waste acid and its validation [J]. CIESC Journal, 2023, 74(6): 2486-2494. |
| [9] | Lei WANG, Lei WANG, Yunlong BAI, Liuliu HE. Preparation of SA lithium ion sieve membrane and its adsorptive properties [J]. CIESC Journal, 2023, 74(5): 2046-2056. |
| [10] | Hao GU, Fujian ZHANG, Zhen LIU, Wenxuan ZHOU, Peng ZHANG, Zhongqiang ZHANG. Desalination performance and mechanism of porous graphene membrane in temporal dimension under mechanical-electrical coupling [J]. CIESC Journal, 2023, 74(5): 2067-2074. |
| [11] | Yongyao SUN, Qiuying GAO, Wenguang ZENG, Jiaming WANG, Yifei CHEN, Yongzhe ZHOU, Gaohong HE, Xuehua RUAN. Design and optimization of membrane-based integration process for advanced utilization of associated gases in N2-EOR oilfields [J]. CIESC Journal, 2023, 74(5): 2034-2045. |
| [12] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [13] | Laiming LUO, Jin ZHANG, Zhibin GUO, Haining WANG, Shanfu LU, Yan XIANG. Simulation and experiment of high temperature polymer electrolyte membrane fuel cells stack in the 1—5 kW range [J]. CIESC Journal, 2023, 74(4): 1724-1734. |
| [14] | Rong WANG, Yonghong WANG, Xinru ZHANG, Jinping LI. Construction of 6FDA-based polyimide carbon molecular sieve membranes for gas separation and its application [J]. CIESC Journal, 2023, 74(4): 1433-1445. |
| [15] | Yangguang LYU, Peipei ZUO, Zhengjin YANG, Tongwen XU. Triazine framework polymer membranes for methanol/n-hexane separation via organic solvent nanofiltration [J]. CIESC Journal, 2023, 74(4): 1598-1606. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||