CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6196-6217.DOI: 10.11949/0438-1157.20250331
• Reviews and monographs • Previous Articles Next Articles
Menghan WANG1,2,3,4( ), Miao YU1,2,3,4, Tong WU1,2,3,4(
), Miao YU1,2,3,4, Tong WU1,2,3,4( )
)
Received:2025-04-01
Revised:2025-05-22
Online:2026-01-23
Published:2025-12-31
Contact:
Tong WU
王梦涵1,2,3,4( ), 于淼1,2,3,4, 吴桐1,2,3,4(
), 于淼1,2,3,4, 吴桐1,2,3,4( )
)
通讯作者:
吴桐
作者简介:王梦涵(2001—),女,硕士研究生,1146532814@qq.com
基金资助:CLC Number:
Menghan WANG, Miao YU, Tong WU. Research progress of electrolyte for lithium-sulfur batteries: molecular design and application[J]. CIESC Journal, 2025, 76(12): 6196-6217.
王梦涵, 于淼, 吴桐. 锂硫电池电解液研究进展:分子设计与应用[J]. 化工学报, 2025, 76(12): 6196-6217.
Add to citation manager EndNote|Ris|BibTeX
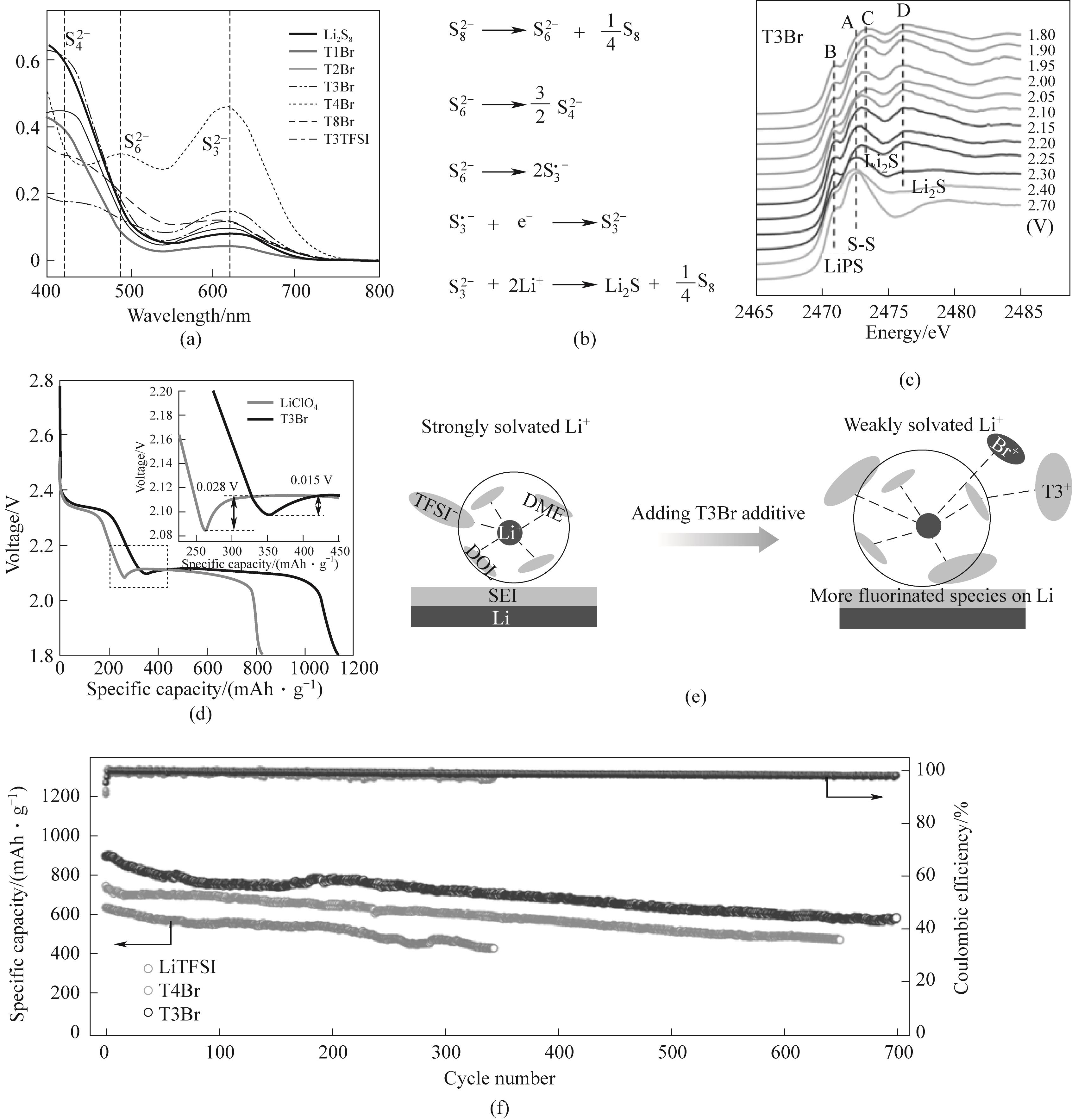
Fig.2 (a) Ultraviolet-visible spectra of Li2S8 solution with QASs, T1Br, T2Br, T3Br, T4Br, T8Br and T3TFSI; (b) Reaction equation; (c) S-K-edge Near Edge Structure (XANES) Spectrum during Positive Electrode Discharge in T3Br Electrolyte; (d) Voltage curves of the electrode during the discharge process in T3Br and blank electrolyte; (e) Schematic diagram of the influence of blank electrolytes and T3Br electrolytes on the evolution of the electrochemical interface on the lithium surface; (f) Long cycle performance of 1C (Under the corresponding current, one discharge lasts for 1 hour)[45]
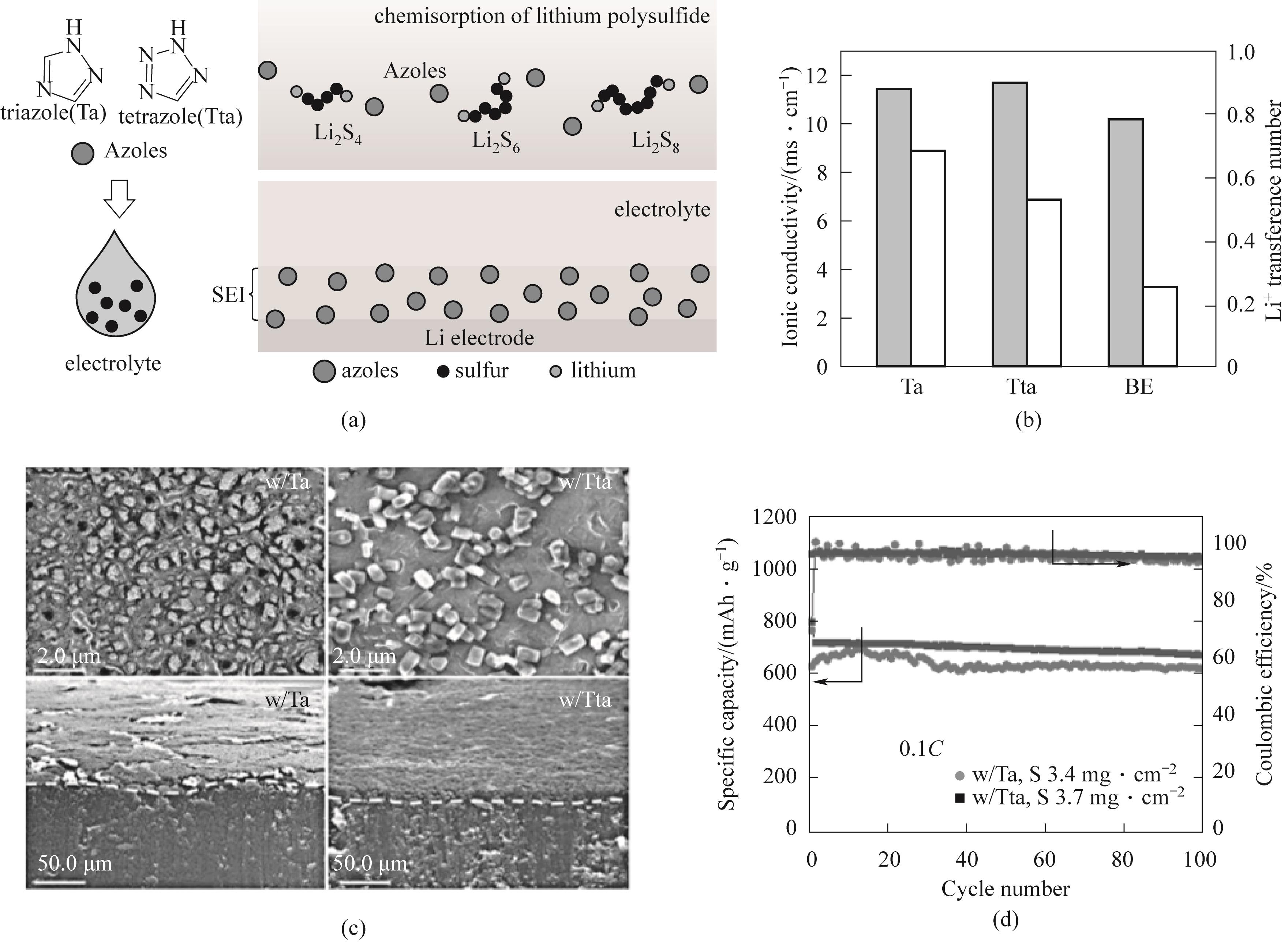
Fig.3 (a) The structure of the oxazole Li2S n (n=4,6,8) complex and the schematic diagram of the oxazole compounds participating in the formation of solid electrolyte interface facial mask and inhibiting the formation of lithium dendrites; (b) Ionic conductivity and Li+transfer number; (c) S After 50 cycles, the scanning electron microscope (SEM) image of the Li | Li symmetric battery’s lithium negative electrode corresponds to the cross-sectional SEM image; (d) 0.1C, Cycle performance of high load Li-S batteries with sulfur loadings of 3.4 mg·cm-2 and 3.7 mg·cm-2 using Ta and Tta based electrolytes[46]
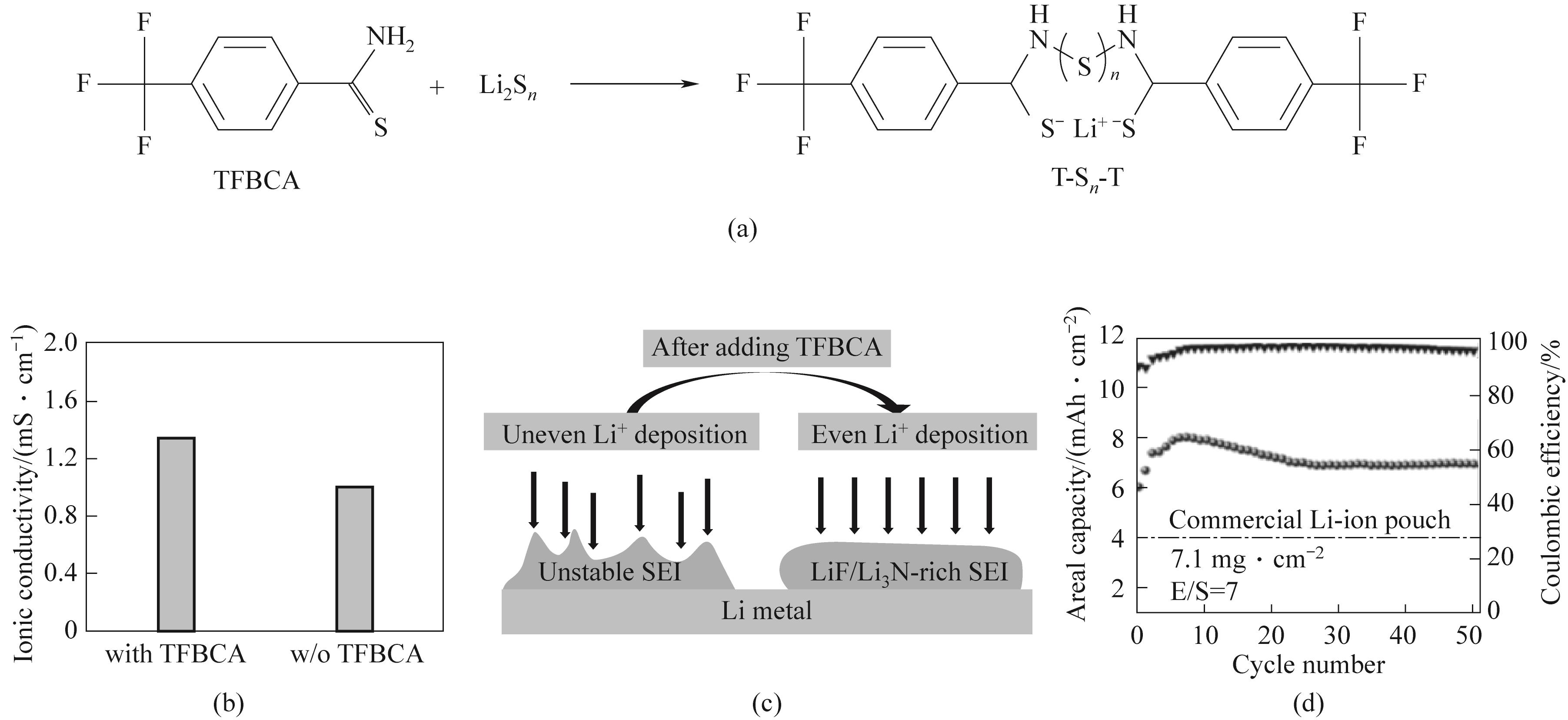
Fig.4 (a) Schematic diagram of the reaction between TFBCA and polysulfides; (b) Ionic conductivity of electrolytes with and without TFBCA; (c) SEI schematic diagram with and without TFBCA; (d) Cycle performance of TFBCA battery under 0.1C, sulfur load of 7.1 mg·cm-2[47]
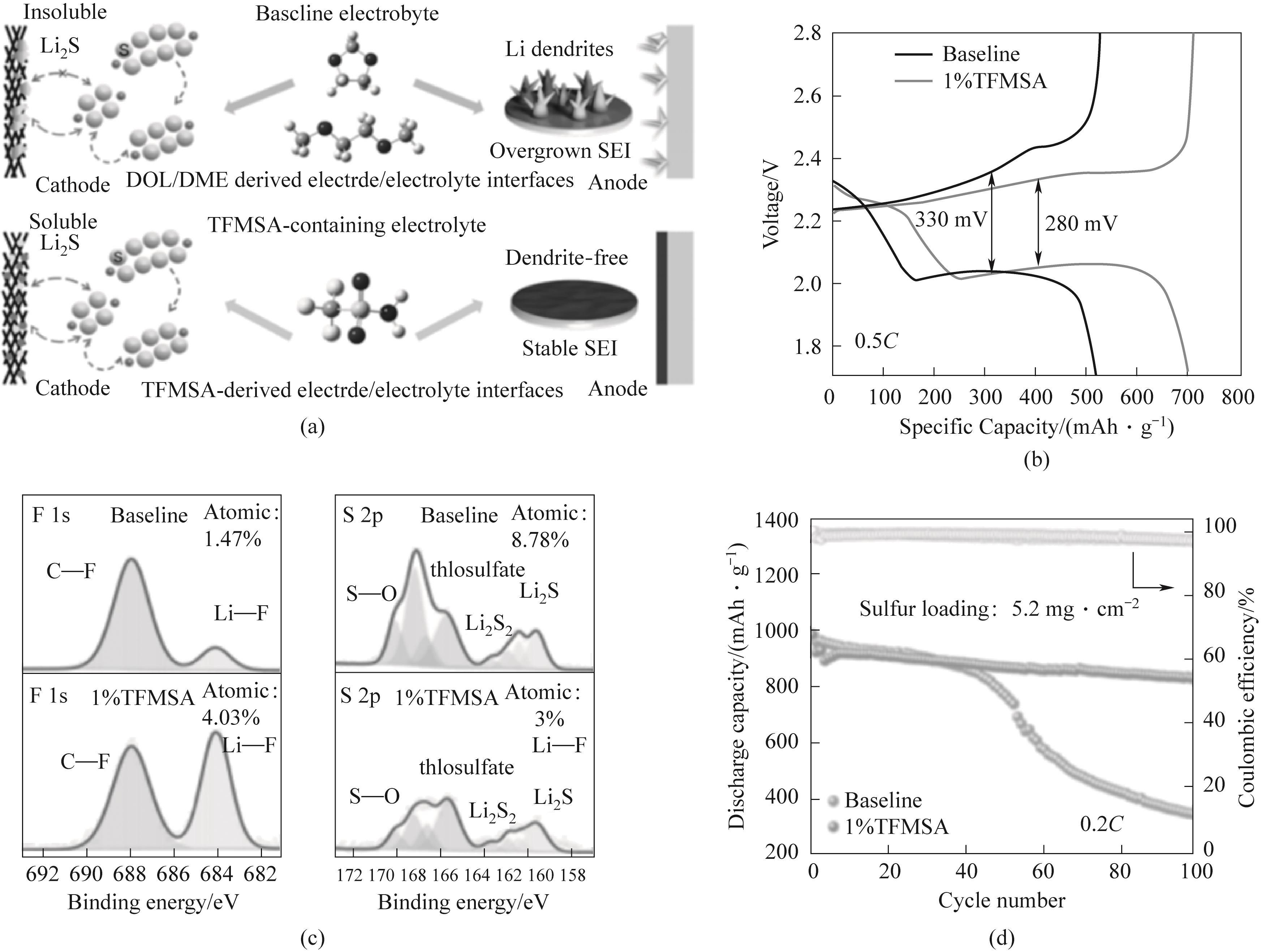
Fig.5 (a) Schematic diagram of the effect of TFMSA additive on the electrode/electrolyte interface of lithium sulfur batteries; (b) Charge discharge curves of batteries containing blank electrolyte and TFMSA electrolyte at 0.5C rate; (c) Surface F 1s and S 2p XPS spectra after cycling with blank electrolyte and TFMSA electrolyte; (d) 0.2C, Cycle performance of lithium sulfur battery with sulfur load of 5.2 mg·cm-2[50]
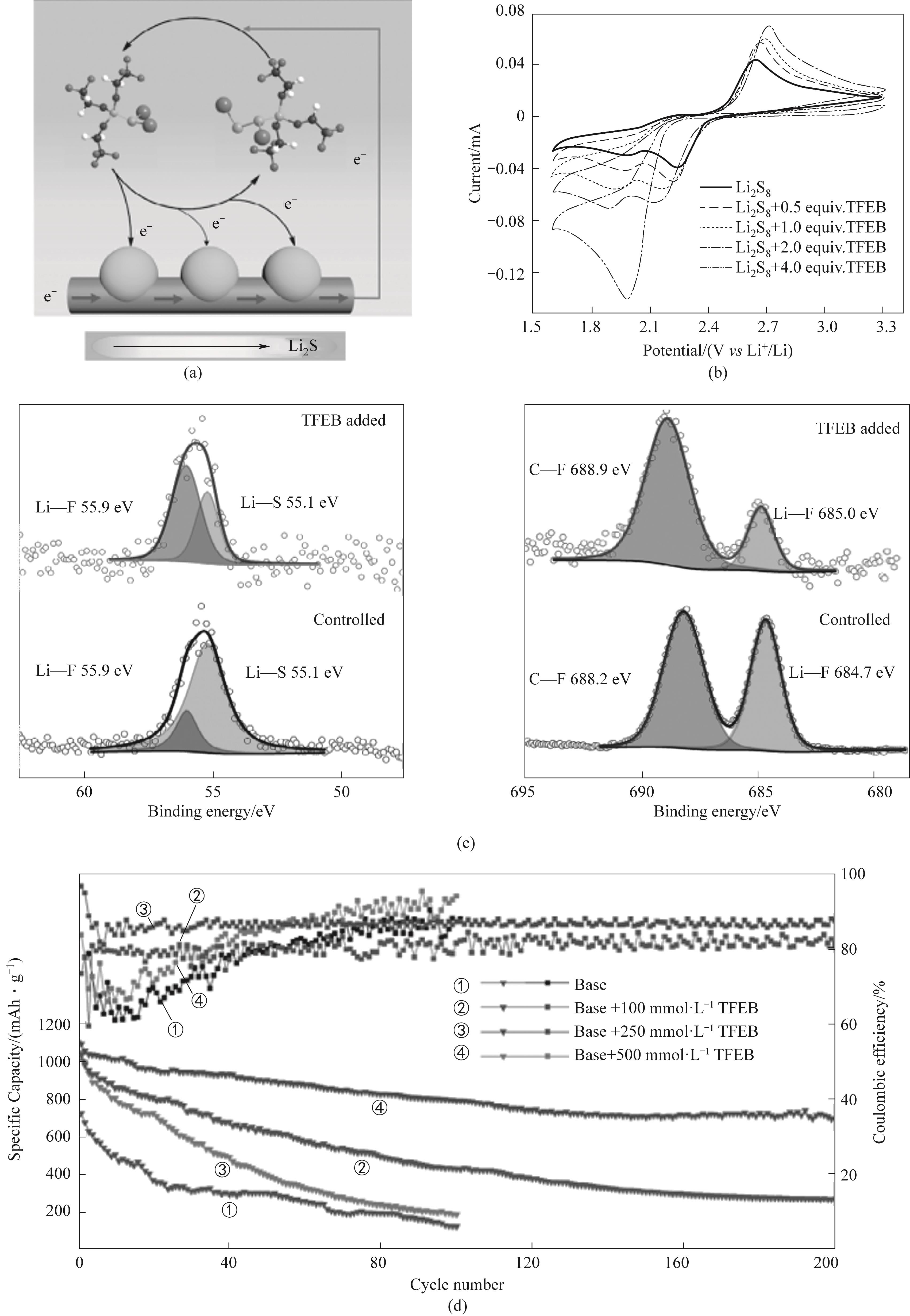
Fig.6 (a) TFEB-Li2S x medium redox catalytic sulfur conversion; (b) Cyclic voltammetry of 2 mmol·L-1 Li2S8 positive electrode electrolyte with different concentrations of TFEB at 200 mV·S-1; (c) XPS spectra of circulating lithium negative electrode with and without TFEB electrolyte; (d) 0.1C, Cycle stability of lithium sulfur batteries with sulfur loading of 5 mg·cm-2 and different TFEB contents[51]
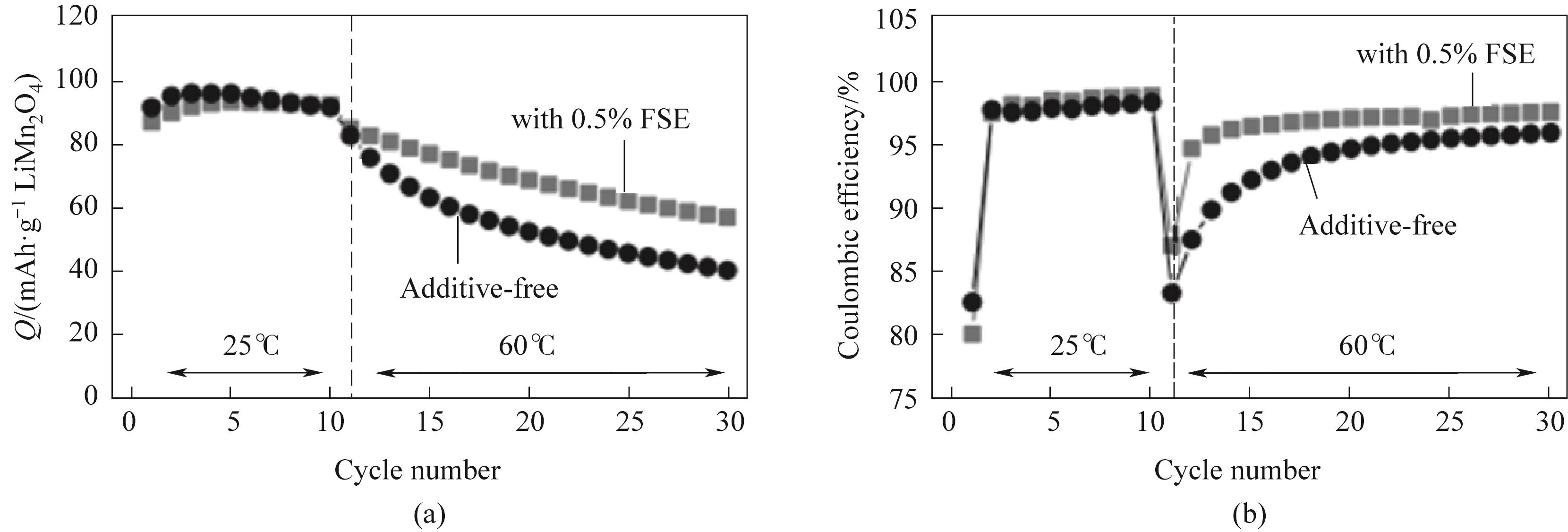
Fig.7 (a) Discharge capacity and (b) Coulombic efficiency (10 mA·g-1 LiMn2O4) of graphite/LiMn2O4 battery under the conditions of no FSE and 0.5% (mass) FSE addition, after 10 initial cycles at room temperature, the 11th to 30th cycles were carried out at 60℃[53]
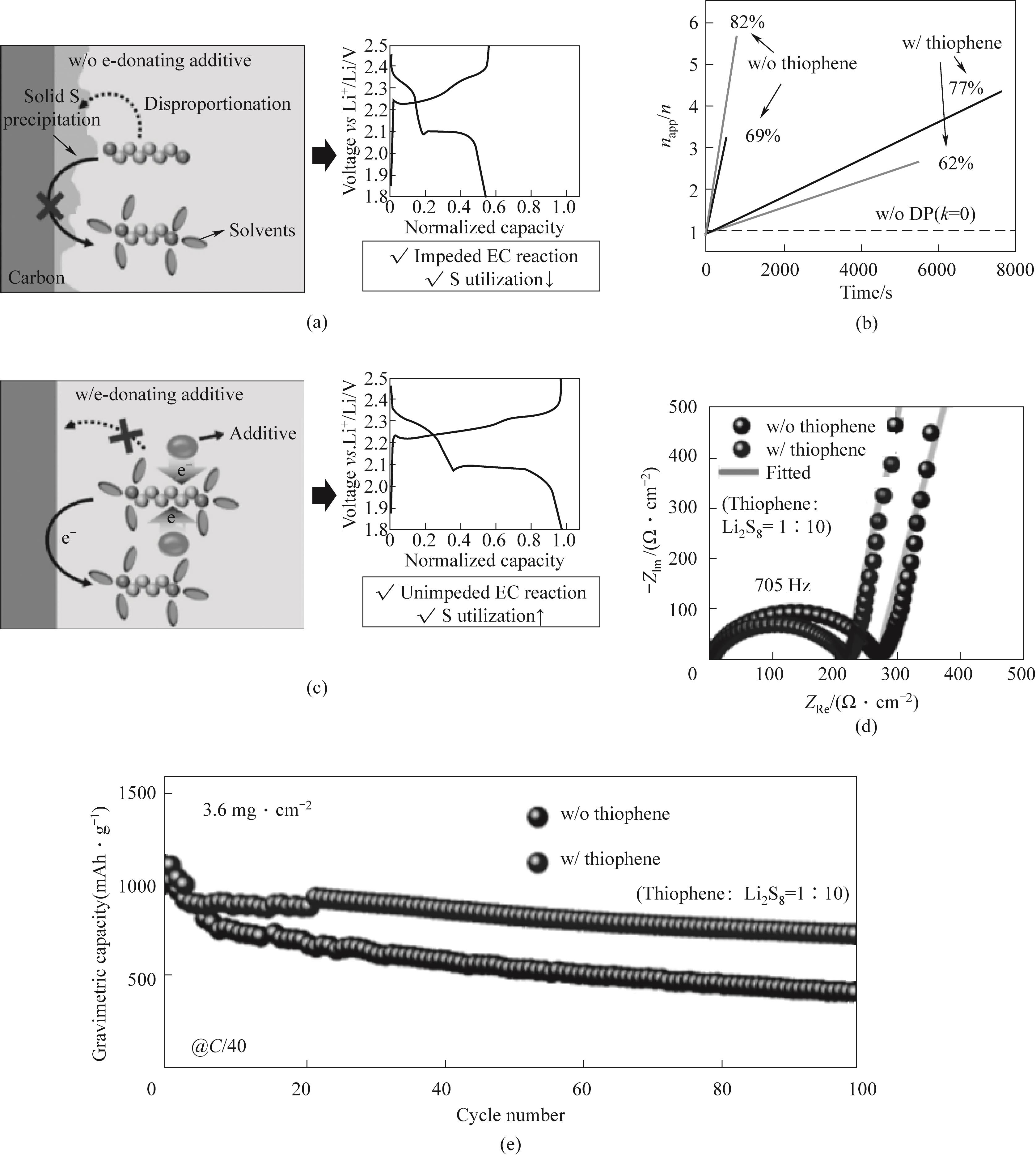
Fig.8 Reaction pathway of lithium polysulfide on the positive electrode and charge discharge curve of Li-S battery (a) without additives and (c) additives; (b) napp/n diagram; (d) Electrochemical impedance spectroscopy (EIS) plots of symmetric sulfur/carbon batteries with and without thiophene batteries exhibiting a 10 mV amplitude perturbation in the frequency range of 105 to 10-2 Hz; (e) C/40, Electrochemical performance of lithium sulfur battery with sulfur loading of 3.6 mg·cm-2[62]
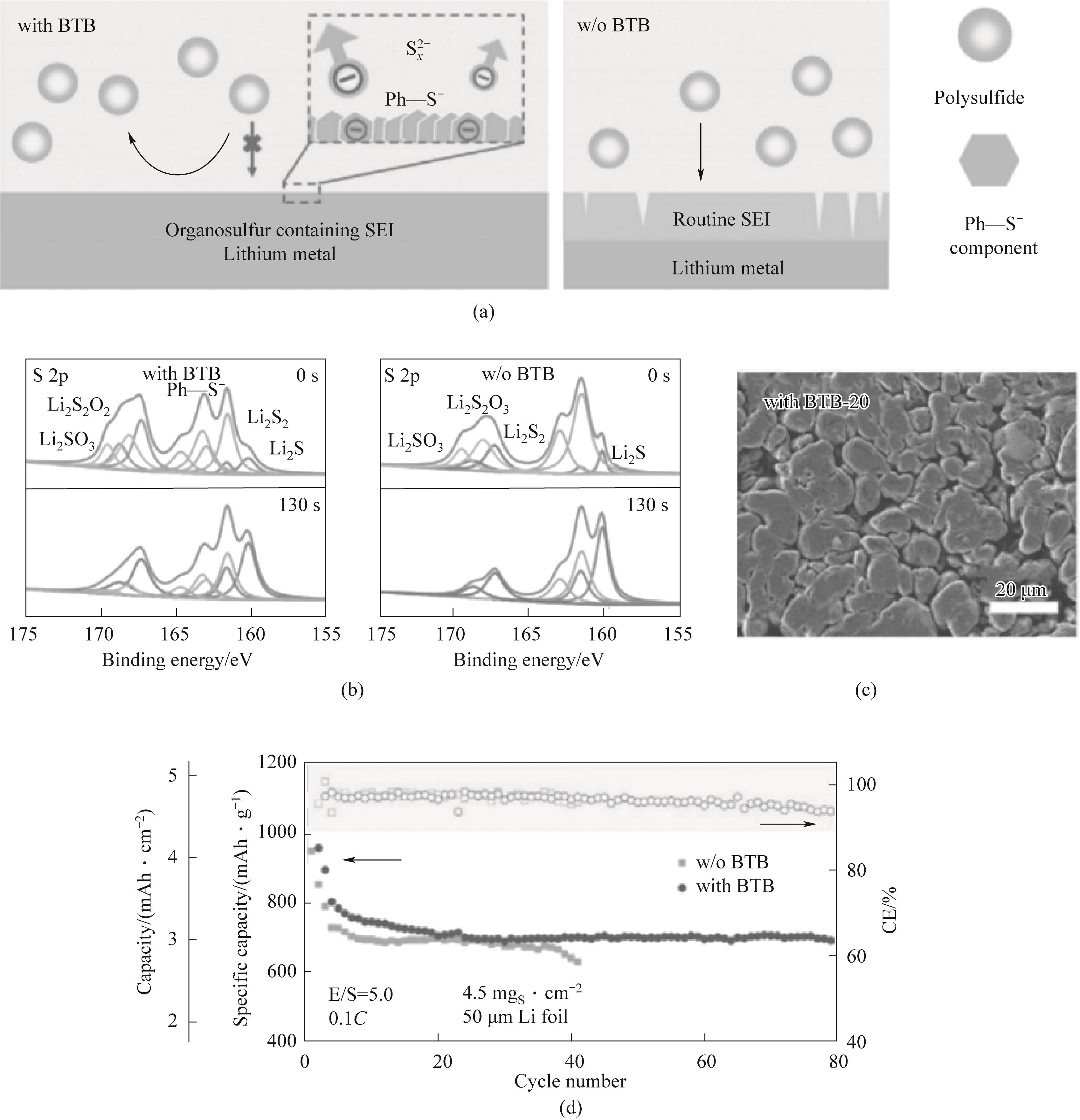
Fig. 9 (a) Schematic diagram of the formation of solid electrolyte interface facial mask; (b) XPS depth profiles of SEI with and without BTB additive for lithium negative electrode after 5 cycles; (c) SEM of cycling Li negative electrode with and without BTB additive in Li-S battery after 20 cycles; (d) 0.1C, Cycle performance of high load S positive electrode, low E/S ratio, and ultra-thin Li negative electrode[63]
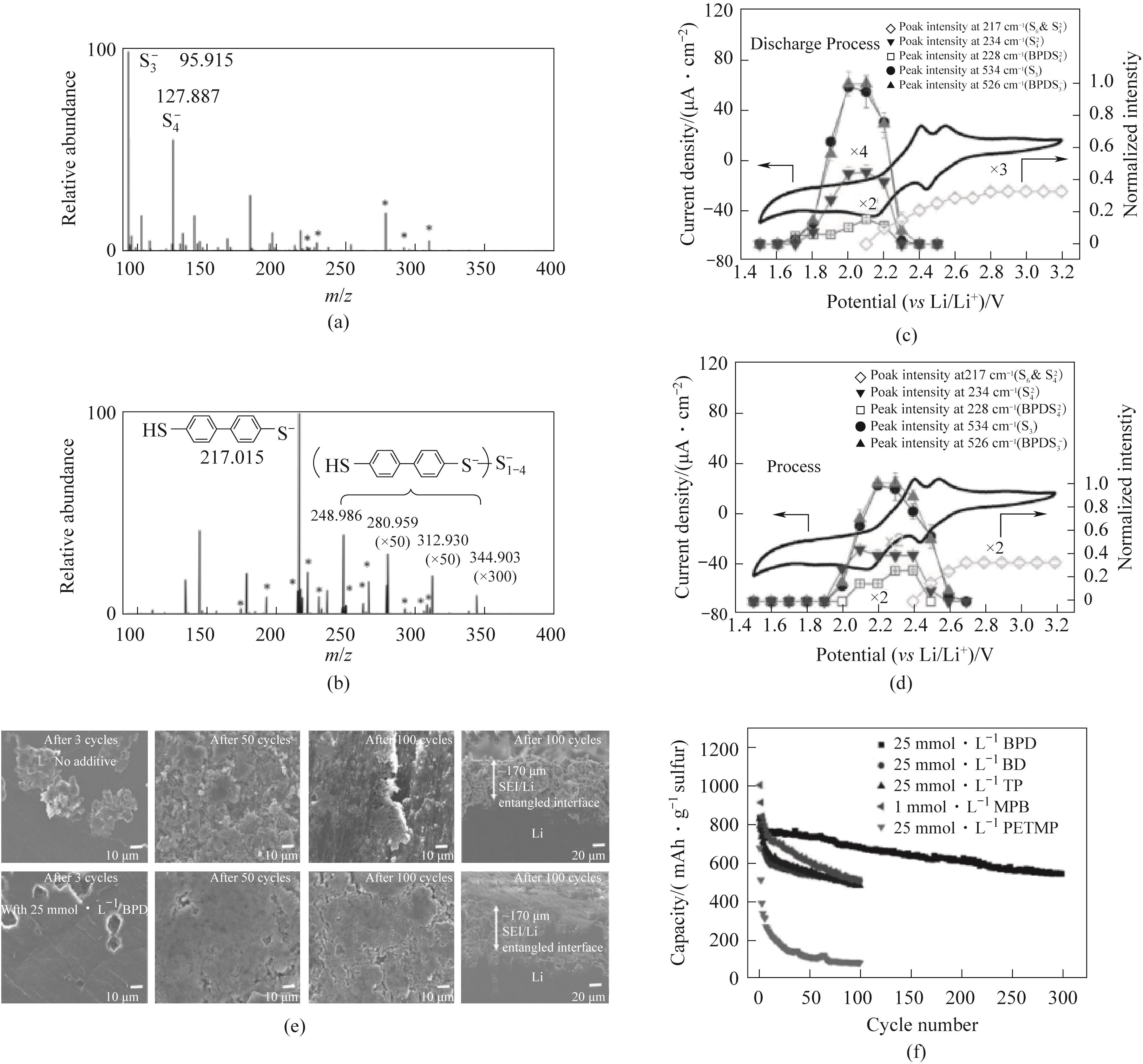
Fig.10 (a) not included; (b) Electrospray ionization mass spectrum of Li2S4 solution added with BPD; The CV and Raman peaks at 217, 228, 234, 526, and 534 cm-1 of the sulfur carbon cathode obtained in alkaline electrolyte with 5 mmol·L-1 BPD during (c) discharge and (d) charge processes; (e) SEM of lithium negative electrode with and without the addition of 25 mmol·L-1 BPD after 0.1C cycling; (f) The cyclic performance of sulfur carbon cathode with different thiol based additives at 0.1C[64]
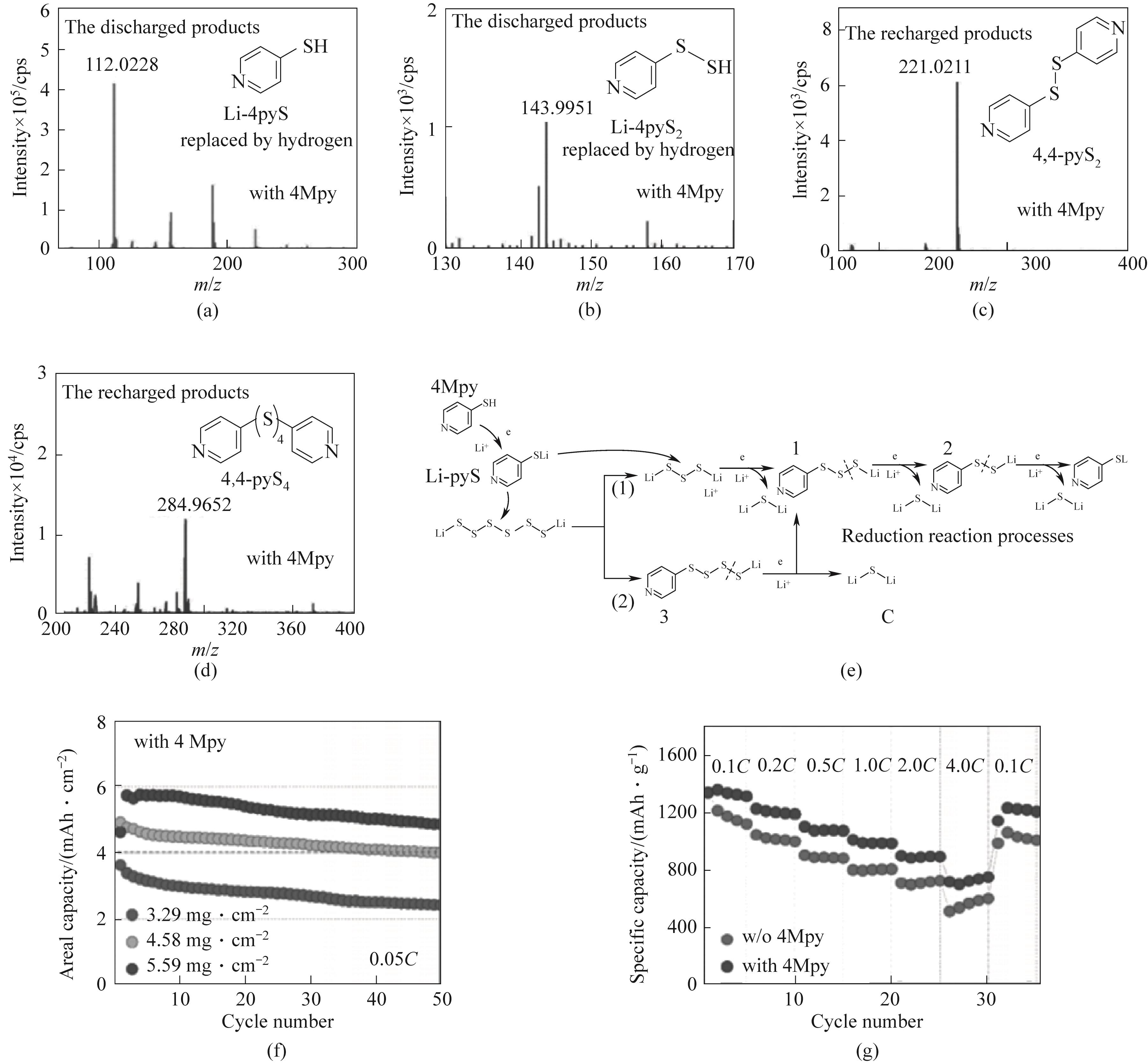
Fig.11 LC-MS spectra of discharged products in Li-S batteries with 4Mpy additive: (a) lithium-pyridinethiolate (Li-pyS) and (b) Li-pyS2; LC-MS spectra of recharged products: (c) 4,4-pyridinedisulfide (4,4-pyS2) and (d) 4,4-pyridinetetrasulfide (4,4-pyS4);(e) Discharge reduction process of Li-S batteries with 4Mpy additive; (f) Cycle performance under high sulfur load of 0.05C; (g) Rate performance at 0.1C to 4C[73]
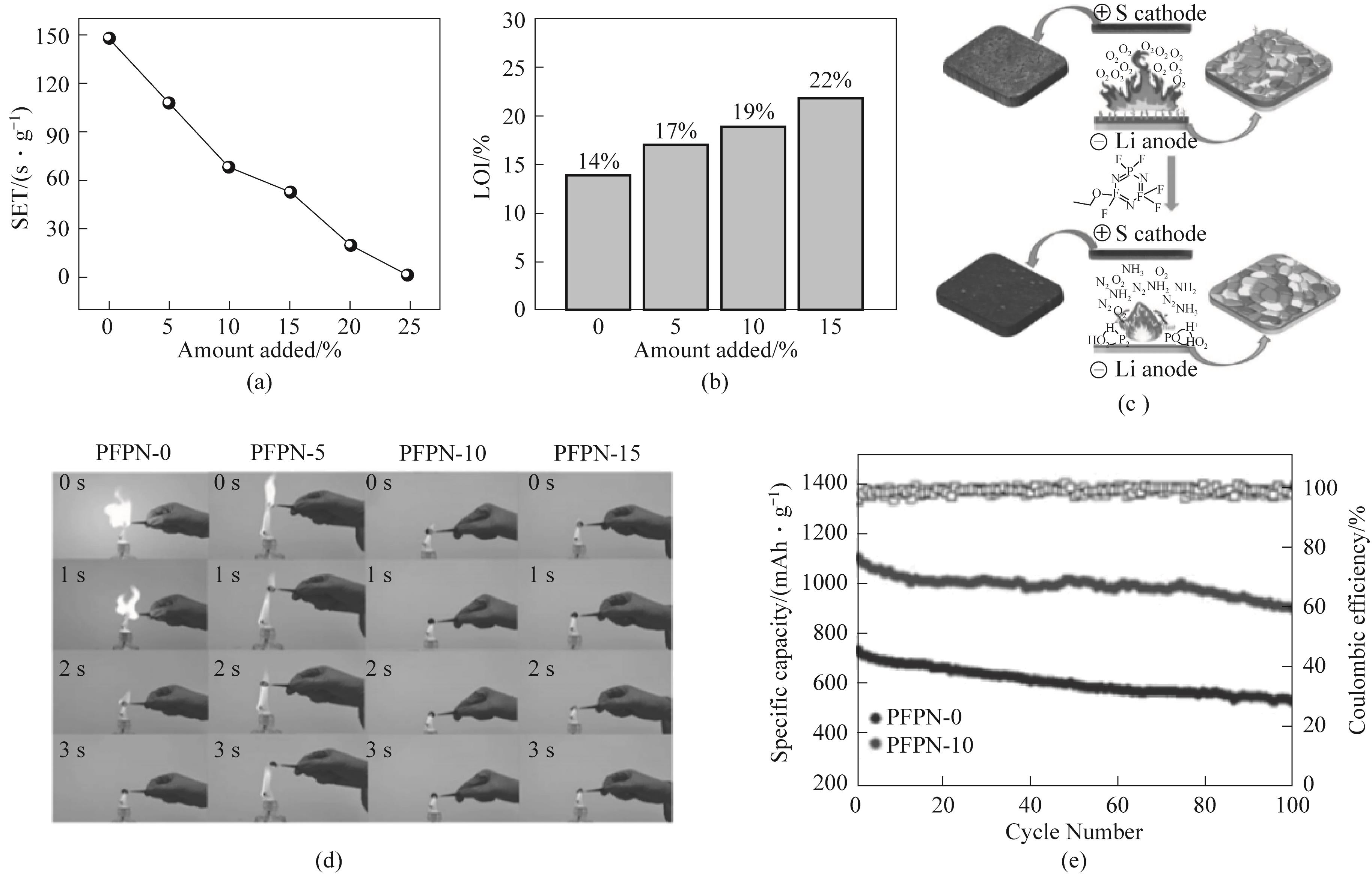
Fig.12 (a) Self extinguishing time test of different electrolytes; (b) Limit oxygen index of different electrolytes; (c) Schematic diagram of PFPN flame retardant mechanism and its influence on electrode surface SEI; (d) Combustion test of cyclic sulfur positive electrode containing PFPN in ether based electrolyte after 50 cycles; (e) 0.2C Cycle performance[74]
| 特征原子 | 添加剂 | 面载量/ (mg·cm-2) | 放电比容量/面积容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| N | 卤化季铵盐-四丙基溴化铵(T3Br) | 1.5~2.0 | 1132 mAh·g-1(0.1C) | 1C,700次循环后放电比容量590 mAh·g-1 | [ |
| 三唑(Ta)、四唑(Tta) | 3.4、3.7 | 800 mAh·g-1、约790 mAh·g-1(0.1C) | 0.1C,100次循环后容量保持率96.5%(Ta)87.5%(Tta) | [ | |
| 4-(三氟甲基)硫代苯甲酰胺(TFBCA) | 7.1 | 8.1 mAh cm-2 (0.2C) | 0.1C,50次循环后面积容量7 mAh·cm-2 | [ | |
| F | 三氟甲磺酰胺(TFMSA) | 5.2 | 1000 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量828 mAh·g-1 | [ |
| 三(2,2,2-三氟乙基)硼酸酯(TFEB) | 5.0 | 1084 mAh·g-1(0.1C) | 0.1C,200次循环后容量保持率63.3% | [ | |
| 1,2-双(二氟甲基硅基)乙烷(FSE)(锂离子电池) | — | 约90 mAh (g of LiMn2O4) -1 (60℃ 10 mA (g of LiMn2O4) -1) | 60℃,20次循环后放容量保持率超过62%;从室温升到60℃时,电池的库仑效率立即恢复 | [ | |
| S | 噻吩 | 3.6 | 1016 mAh·g-1(C/40) | C/40,100次循环后容量保持率74% | [ |
| 3,5-双(三氟甲基)苯硫酚(BTB) | 4.5 | 950 mAh·g-1(0.1C) | 0.1C,82次循环后放电比容量700 mAh·g-1 | [ | |
| 联苯-4,4′-二硫醇(BPD) | 0.7~1.2 | 900 mAh·g-1(0.1C) | 0.1C,300次循环后放电比容量575 mAh·g-1 | [ | |
| N、S | 4-巯基吡啶(4Mpy) | 5.59 | 6 mAh·cm-2(0.05C) | 0.05C,50次循环后面积容量4.85 mAh·cm-2 | [ |
| N、F | 乙氧基(五氟)环三磷腈(PFPN) | 2.3 | 1103.4 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量904.6 mAh·g-1;使用PFPN电池循环50次后硫正极点燃后迅速熄灭 | [ |
Table 1 Performance parameters of lithium sulfur batteries assembled using different electrolyte additives
| 特征原子 | 添加剂 | 面载量/ (mg·cm-2) | 放电比容量/面积容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| N | 卤化季铵盐-四丙基溴化铵(T3Br) | 1.5~2.0 | 1132 mAh·g-1(0.1C) | 1C,700次循环后放电比容量590 mAh·g-1 | [ |
| 三唑(Ta)、四唑(Tta) | 3.4、3.7 | 800 mAh·g-1、约790 mAh·g-1(0.1C) | 0.1C,100次循环后容量保持率96.5%(Ta)87.5%(Tta) | [ | |
| 4-(三氟甲基)硫代苯甲酰胺(TFBCA) | 7.1 | 8.1 mAh cm-2 (0.2C) | 0.1C,50次循环后面积容量7 mAh·cm-2 | [ | |
| F | 三氟甲磺酰胺(TFMSA) | 5.2 | 1000 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量828 mAh·g-1 | [ |
| 三(2,2,2-三氟乙基)硼酸酯(TFEB) | 5.0 | 1084 mAh·g-1(0.1C) | 0.1C,200次循环后容量保持率63.3% | [ | |
| 1,2-双(二氟甲基硅基)乙烷(FSE)(锂离子电池) | — | 约90 mAh (g of LiMn2O4) -1 (60℃ 10 mA (g of LiMn2O4) -1) | 60℃,20次循环后放容量保持率超过62%;从室温升到60℃时,电池的库仑效率立即恢复 | [ | |
| S | 噻吩 | 3.6 | 1016 mAh·g-1(C/40) | C/40,100次循环后容量保持率74% | [ |
| 3,5-双(三氟甲基)苯硫酚(BTB) | 4.5 | 950 mAh·g-1(0.1C) | 0.1C,82次循环后放电比容量700 mAh·g-1 | [ | |
| 联苯-4,4′-二硫醇(BPD) | 0.7~1.2 | 900 mAh·g-1(0.1C) | 0.1C,300次循环后放电比容量575 mAh·g-1 | [ | |
| N、S | 4-巯基吡啶(4Mpy) | 5.59 | 6 mAh·cm-2(0.05C) | 0.05C,50次循环后面积容量4.85 mAh·cm-2 | [ |
| N、F | 乙氧基(五氟)环三磷腈(PFPN) | 2.3 | 1103.4 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量904.6 mAh·g-1;使用PFPN电池循环50次后硫正极点燃后迅速熄灭 | [ |
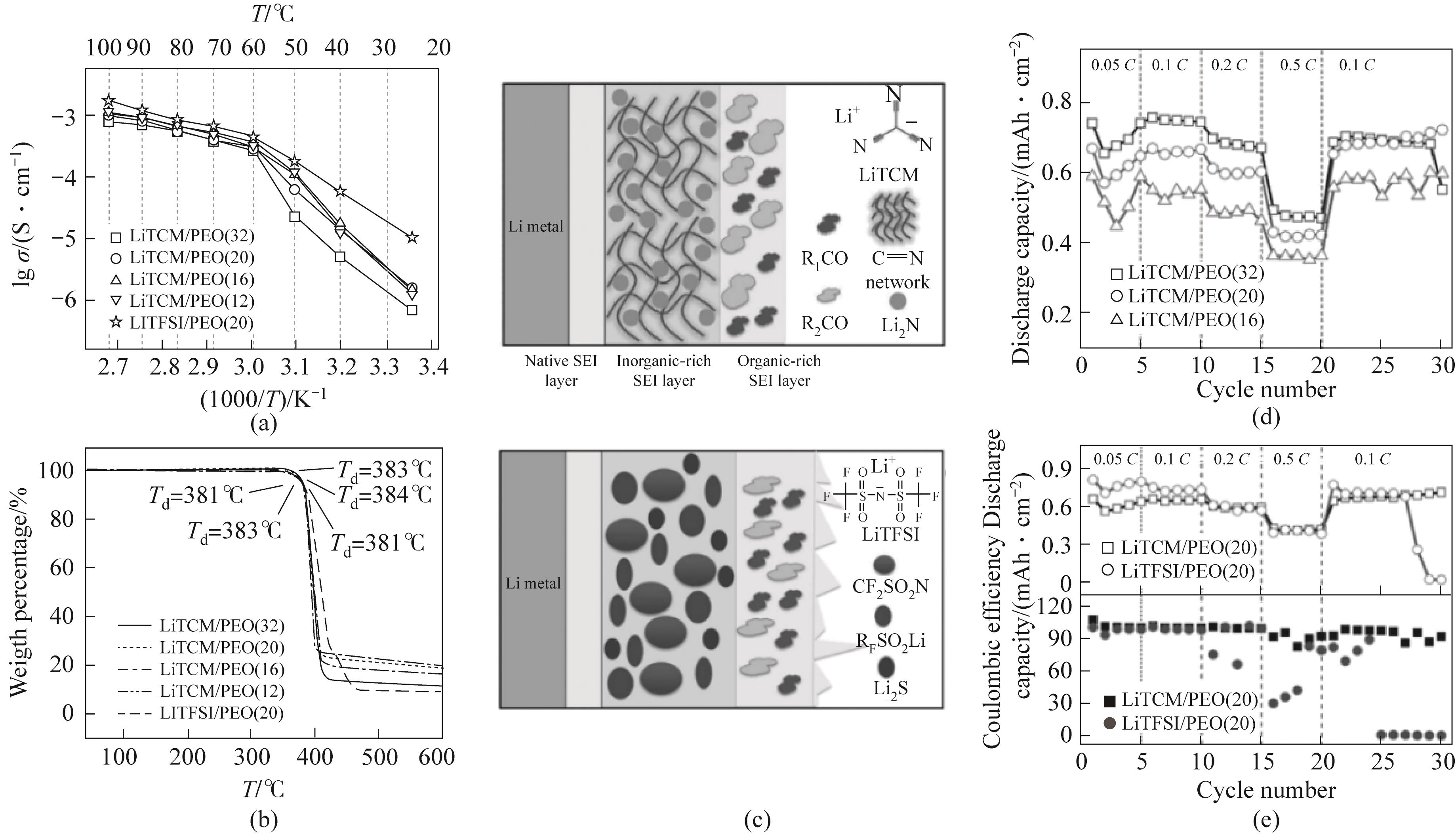
Fig.13 (a) Arrhenius plot of ion conductivity; (b) Thermogravimetric analysis (TGA) curve of electrolyte; (c) Schematic diagram of solid electrolyte interface facial mask formed on lithium cathode in LiTCM and LiTFSI batteries; (d) The rate performance of LiTCM/PEO electrolyte under different salt contents; (e) Comparison of cycling performance of lithium sulfur batteries using Li X /PEO (X=TCM- or TFSI-) electrolyte under the same ethylene oxide unit (EO) / Li ratio of 20[88]
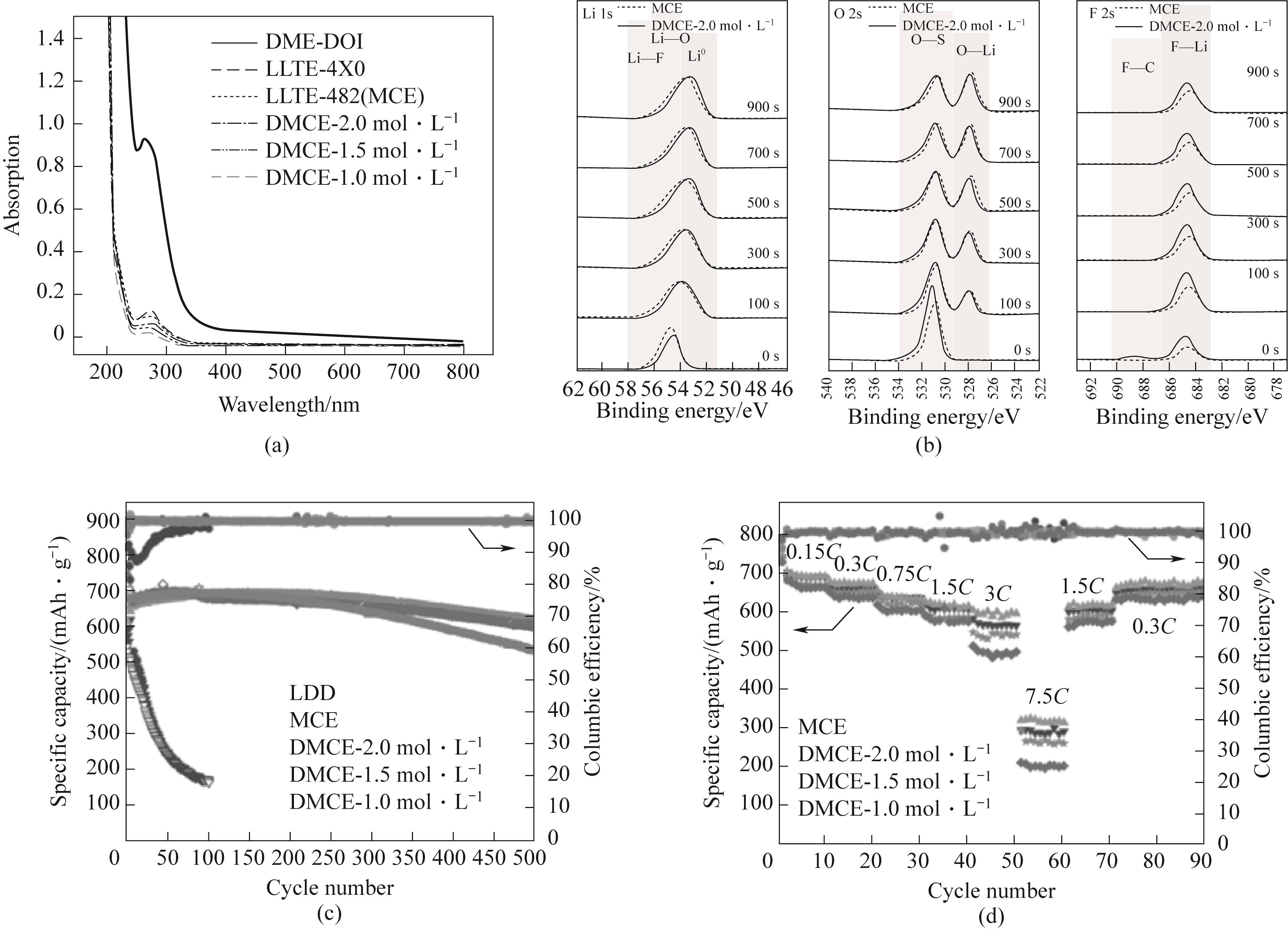
Figure 14 (a) UV visible absorption spectrum; High resolution spectra of (b) Li 1s、O 2s and F 2s XPS on the surface of lithium electrode; (c) 0.3C, Long term cycling performance of Li-S batteries under different electrolytes; (d) Rate performance of Li-S batteries using dual salt medium concentration electrolyte (MCE) and a novel diluted medium concentration electrolyte (DMCE) electrolyte[89]
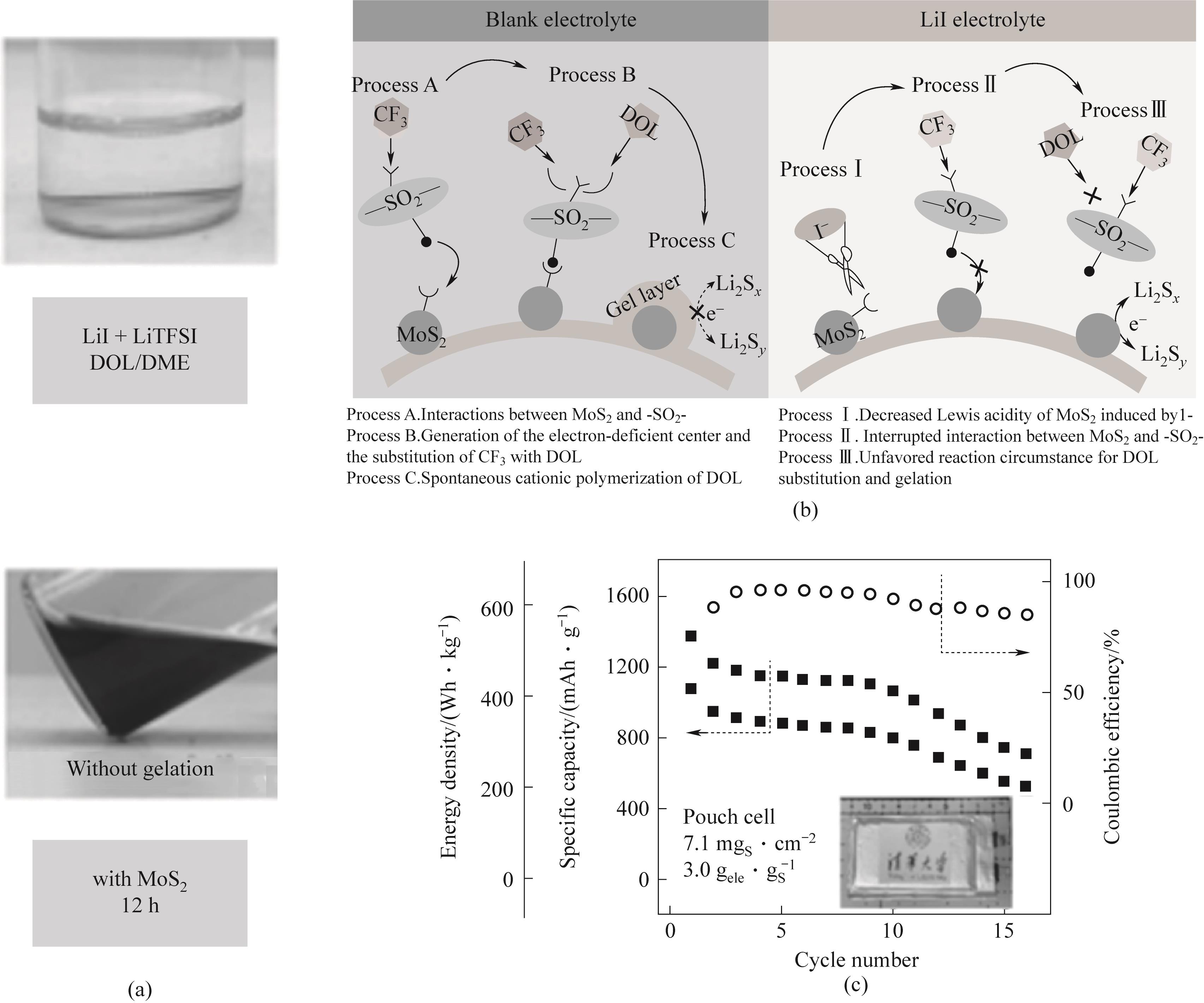
Fig.15 (a) Optical image of LiI gel suppression phenomenon; (b) Schematic diagram of reaction pathways of Li-S battery in blank electrolyte and LiI electrolyte; (c) 0.05C, Cycle performance of 400 Wh·kg-1 soft pack battery[90]
| 体系 | 锂盐 | 面载量/(mg·cm-2) | 放电比容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| 单盐体系 | 三氰甲烷化物(LiTCM)① | 0.8~1.1 | ≈0.7 mAh·cm -2(0.1C) | 0.2C,库仑效率约100%,保持30次循环 | [ |
| 双盐体系 | LiTFSI-LiFSI② | 2.0 | 682 mAh·g-1(0.3C) | 0.3C,500次循环放电比容量626 mAh·g-1 | [ |
| LiTFSI-LiI③ | 7.1(单面) | 416 Wh·kg-1(0.05C) | 0.05C,16次循环保持稳定的库仑效率 | [ |
Table 2 Performance parameters of lithium-sulfur batteries assembled with different lithium salts
| 体系 | 锂盐 | 面载量/(mg·cm-2) | 放电比容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| 单盐体系 | 三氰甲烷化物(LiTCM)① | 0.8~1.1 | ≈0.7 mAh·cm -2(0.1C) | 0.2C,库仑效率约100%,保持30次循环 | [ |
| 双盐体系 | LiTFSI-LiFSI② | 2.0 | 682 mAh·g-1(0.3C) | 0.3C,500次循环放电比容量626 mAh·g-1 | [ |
| LiTFSI-LiI③ | 7.1(单面) | 416 Wh·kg-1(0.05C) | 0.05C,16次循环保持稳定的库仑效率 | [ |
| [1] | Nasajpour-Esfahani N, Garmestani H, Bagheritabar M, et al. Comprehensive review of lithium-ion battery materials and development challenges[J]. Renewable and Sustainable Energy Reviews, 2024, 203: 114783. |
| [2] | Zhang C F, Chou S L, Guo Z P, et al. Beyond lithium-ion batteries[J]. Advanced Functional Materials, 2024, 34(5): 2308001. |
| [3] | Xue J X, Jia S X, Xiang T Q, et al. Cross-linkable binders for Si anodes in high-energy-density lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2024, 16(29): 38458-38465. |
| [4] | Janek J, Zeier W G. A solid future for battery development[J]. Nature Energy, 2016, 1(9): 16141. |
| [5] | Ji L, Jia Y F, Wang X, et al. Strong adsorption, catalysis and lithiophilic modulation of carbon nitride for lithium/sulfur battery[J]. Nanotechnology, 2021, 32(19): 192002. |
| [6] | Peng Y Q, Zhao M, Chen Z X, et al. Boosting sulfur redox kinetics by a pentacenetetrone redox mediator for high-energy-density lithium-sulfur batteries[J]. Nano Research, 2023, 16(6): 8253-8259. |
| [7] | Du M, Shi J K, Geng P B, et al. Nitrogen and sulfur co-doped MXene@FeCoNiP as an efficient catalyst for enhanced lithium-sulfur batteries[J]. Materials Today Chemistry, 2024, 41: 102289. |
| [8] | Chung W J, Griebel J J, Kim E T, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials[J]. Nature Chemistry, 2013, 5(6): 518-524. |
| [9] | Yang S B, Wang B, Lv Q, et al. Recent advances in cathodes for all-solid-state lithium-sulfur batteries[J]. Chinese Chemical Letters, 2023, 34(7): 107783. |
| [10] | Tan J, Liu D N, Xu X, et al. In situ/operando characterization techniques for rechargeable lithium-sulfur batteries: a review[J]. Nanoscale, 2017, 9(48): 19001-19016. |
| [11] | Li Z, Li B Q, Bi C X, et al. A review of lithium-sulfur batteries at different working conditions: the role of ambient temperature, external force, and electromagnetic field[J]. Materials Science and Engineering: R: Reports, 2025, 164: 100955. |
| [12] | Zhang Y Q, Yuan H M, Guo E Y, et al. Facilitating sulfur species capture and bi-directional redox in Li-S batteries with single-atomic Co-O2N2 coordination structure[J]. Journal of Energy Chemistry, 2024, 99: 604-614. |
| [13] | Jeong Y C, Kim J H, Nam S, et al. Rational design of nanostructured functional interlayer/separator for advanced Li-S batteries[J]. Advanced Functional Materials, 2018, 28(38): 1707411. |
| [14] | Lin B X, Zhang Y Y, Li W F, et al. Recent advances in rare earth compounds for lithium-sulfur batteries[J]. eScience, 2024, 4(3): 100180. |
| [15] | Zhang L L, Wang Y J, Niu Z Q, et al. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries[J]. Carbon, 2019, 141: 400-416. |
| [16] | Zhang S S. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions[J]. Journal of Power Sources, 2013, 231: 153-162. |
| [17] | Zhao S L, Hao Q Y, Qian X Y, et al. Application of Y-Zn-MOF derived Y2O3/ZnO@C in modification of lithium-sulfur battery separator[J]. Journal of Energy Storage, 2024, 101: 113833. |
| [18] | Tan J, Li X Y, Fang Z, et al. Designing a stable solid electrolyte interphase on lithium metal anodes by tailoring a Mg atom center and the inner Helmholtz plane for lithium-sulfur batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(14): 17893-17903. |
| [19] | Wang L, Wang R R, Liu Q Q, et al. WN nanocubes embedded on carbon mesh towards high-performance Li-S batteries: balancing physical capture, chemical adsorption and catalysis capability[J]. Journal of Energy Storage, 2024, 100: 113591. |
| [20] | Akhtar N, Sun X, Akram M Y, et al. A gelatin-based artificial SEI for lithium deposition regulation and polysulfide shuttle suppression in lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2021, 52: 310-317. |
| [21] | Eshetu D G G, Judez X, Li D C, et al. Lithium azide as an electrolyte additive for all-olid-state lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2017, 56(48): 15368-15372. |
| [22] | 贾铭勋, 吴桐, 杨道通, 等. 锂硫电池电解液多功能添加剂:作用机制及先进表征[J]. 储能科学与技术, 2024, 13(1): 36-47. |
| Jia M X, Wu T, Yang D T, et al. Electrolyte multifunctional additives of lithium-sulfur battery: mechanism of action and advanced characterization[J]. Energy Storage Science and Technology, 2024, 13(1): 36-47. | |
| [23] | Ma X K, Song X M, Wang Y D, et al. Polyhalogenated heterocycle additive induced in situ 3D gelatinous polymerization with polysulfides for shuttle effect inhibited lithium-sulfur batteries[J]. Chemical Engineering Journal, 2025, 513: 162921. |
| [24] | Jan W, Khan A D, Iftikhar F J, et al. Electrolyte design for lithium-sulfur batteries: progress and challenges[J]. Renewable and Sustainable Energy Reviews, 2025, 221: 115916. |
| [25] | Yu Z H, Huang X H, Zheng M T, et al. Self-assembled macrocyclic copper complex enables homogeneous catalysis for high-loading lithium-sulfur batteries[J]. Advanced Materials, 2023, 35(26): 2300861. |
| [26] | Kautz D J, Cao X, Gao P Y, et al. Designing moderately-solvating electrolytes for high-performance lithium-sulfur batteries[J]. Advanced Materials, 2025: 2503365. |
| [27] | Zhang J H, Fan X Z, Zhou X H, et al. Charging lithium polysulfides by cationic lithium nitrate species for low-temperature lithium-sulfur batteries[J]. Energy Storage Materials, 2024, 73: 103786. |
| [28] | Aurbach D, Pollak E, Elazari R, et al. On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries[J]. Journal of the Electrochemical Society, 2009, 156 (8): A694. |
| [29] | Xiong S Z, Regula M, Wang D H, et al. Toward better lithium-sulfur batteries: functional non-aqueous liquid electrolytes[J]. Electrochemical Energy Reviews, 2018, 1(3): 388-402. |
| [30] | Song Y W, Shen L, Yao N, et al. Anion-involved solvation structure of lithium polysulfides in lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2024, 63(19): e202400343. |
| [31] | Cai L C, Ying H J, He C W, et al. Polymorph interface strategy presents avenues for kinetics-enhanced and dendrite-free lithium sulfur batteries[J]. Chemical Engineering Journal, 2024, 498: 155856. |
| [32] | Li X Y, Feng S, Song Y W, et al. Kinetic Evaluation on lithium polysulfide in weakly solvating electrolyte toward practical lithium-sulfur batteries[J]. Journal of the American Chemical Society, 2024, 146(21): 14754-14764. |
| [33] | Kilic A, Abdelaty O, Zeeshan M, et al. Selection of ionic liquid electrolytes for high-performing lithium-sulfur batteries: an experiment-guided high-throughput machine learning analysis[J]. Chemical Engineering Journal, 2024, 490: 151562. |
| [34] | Guo J L, Yang Q, Dou Y, et al. Shelf life of lithium-sulfur batteries under lean electrolytes: status and challenges[J]. Energy & Environmental Science, 2024, 17(5): 1695-1724. |
| [35] | Zhou T, Liang J N, Ye S H, et al. Fundamental, application and opportunities of single atom catalysts for Li-S batteries[J]. Energy Storage Materials, 2023, 55: 322-355. |
| [36] | Cui M N, Zheng Z H, Wang J C, et al. Rational design of lithium-sulfur battery cathodes based on differential Atom Electronegativity[J]. Energy Storage Materials, 2021, 35: 577-585. |
| [37] | Jia M X, Li T N, Yang D T, et al. Polymer electrolytes for lithium-sulfur batteries: progress and challenges[J]. Batteries, 2023, 9(10): 488. |
| [38] | Li S, Luo Z, Tu H Y, et al. N, S-codoped carbon dots as deposition regulating electrolyte additive for stable lithium metal anode[J]. Energy Storage Materials, 2021, 42: 679-686. |
| [39] | Wang Y M, Zhang X X, Liu C S, et al. Remarkable improvement of MOF-based triboelectric nanogenerators with strong electron-withdrawing groups[J]. Nano Energy, 2023, 107: 108149. |
| [40] | Wu P, Dong M X, Tan J, et al. Revamping lithium-sulfur batteries for high cell-level energy density by synergistic utilization of polysulfide additives and artificial solid-electrolyte interphase layers[J]. Advanced Materials, 2021, 33(48): 2104246. |
| [41] | Santos É A, Policano M C, Pinzón M J, et al. operando FTIR study on water additive in lithium-sulfur batteries to mitigate shuttle effect[J]. Journal of Energy Chemistry, 2024, 98: 702-713. |
| [42] | Zhong J, Ren L L, Ying C H, et al. Amino acid as a multifunctional electrolyte additive for enhancing Li-S battery performance[J]. Journal of Energy Storage, 2025, 109: 115251. |
| [43] | Zhang W, Ma F F, Wu Q, et al. Bifunctional fluorinated anthraquinone additive for improving kinetics and interfacial chemistry in rechargeable Li-S batteries[J]. ACS Applied Energy Materials, 2022, 5(12): 15719-15728. |
| [44] | Lin L P, Yang K, Tan R, et al. Effect of sulfur-containing additives on the formation of a solid-electrolyte interphase evaluated by in situ AFM and ex situ characterizations[J]. Journal of Materials Chemistry A, 2017, 5(36): 19364-19370. |
| [45] | Meng D R, He D X, Ong D S J H, et al. A radical pathway and stabilized Li anode enabled by halide quaternary ammonium electrolyte additives for lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2023, 62(38): e202309046. |
| [46] | Wang D Y, Wang W M, Li F L, et al. Nitrogen-rich azoles as trifunctional electrolyte additives for high-performance lithium-sulfur battery[J]. Journal of Energy Chemistry, 2022, 71: 572-579. |
| [47] | Rao J P, Yu T, Zhou Y S, et al. A bifunctional thiobenzamide additive for improvement of cathode kinetics and anode stability in lithium-sulfur batteries[J]. Chemical Engineering Journal, 2024, 498: 155807. |
| [48] | Wu T, Jia M X, Lu Y, et al. Fluorinated imine modulating efficient sulfur redox kinetics and a stable solid electrolyte interphase in lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2025, 13(10): 7196-7206. |
| [49] | Chen X, Ji H, Chen W, et al. In situ protection of a sulfur cathode and a lithium anode via adopting a fluorinated electrolyte for stable lithium-sulfur batteries[J]. Science China-Materials, 2021, 64(9): 2127-2138. |
| [50] | Liu F Y, Zong C X, He L, et al. Improving the electrochemical performance of lithium-sulfur batteries by interface modification with a bifunctional electrolyte additive[J]. Chemical Engineering Journal, 2022, 443: 136489. |
| [51] | Gao S Y, Li B M, Zhu Q J, et al. A fluorinated lewis acidic organoboron tunes polysulfide complex structure for high-performance lithium-sulfur batteries[J]. Advanced Energy Materials, 2025, 15(6): 2403439. |
| [52] | Chen Y Q, Kang Y Q, Zhao Y, et al. A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards[J]. Journal of Energy Chemistry, 2021, 59: 83-99. |
| [53] | Yamagiwa K, Morita D, Yabuuchi N, et al. Improved high-temperature performance and surface chemistry of graphite/LiMn2O4 Li-ion cells by fluorosilane-based electrolyte additive[J]. Electrochimica Acta, 2015, 160: 347-356. |
| [54] | Ming J, Cao Z, Wu Y Q, et al. New insight on the role of electrolyte additives in rechargeable lithium ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2613-2622. |
| [55] | Wu T, Sun G R, Lu W, et al. A polypyrrole/black-TiO2/S double-shelled composite fixing polysulfides for lithium-sulfur batteries[J]. Electrochimica Acta, 2020, 353: 136529. |
| [56] | Liu G, Cao Z, Zhou L, et al. Additives engineered nonflammable electrolyte for safer potassium ion batteries[J]. Advanced Functional Materials, 2020, 30(43): 2001934. |
| [57] | Li G R, Chen Z W, Lu J. Lithium-sulfur batteries for commercial applications[J]. Chem, 2018, 4(1): 3-7. |
| [58] | Song Y W, Shen L, Li X Y, et al. Phase equilibrium thermodynamics of lithium-sulfur batteries[J]. Nature Chemical Engineering, 2024, 1: 588-596. |
| [59] | Fan L L, Deng N P, Yan J, et al. The recent research status quo and the prospect of electrolytes for lithium sulfur batteries[J]. Chemical Engineering Journal, 2019, 369: 874-897. |
| [60] | Li J Y, Gao L, Pan F Y, et al. Engineering strategies for suppressing the shuttle effect in lithium-sulfur batteries[J]. Nano-Micro Letters, 2023, 16(1): 12. |
| [61] | Liu X, Sun X R, Yan R, et al. Structural and functional optimization of lithium‐sulfur battery separators by sulfur‐containing of covalent organic frameworks[J]. Advanced Functional Materials, 2025: 2505986. |
| [62] | Lee S, Sim K, Cho K Y, et al. Regulating the interfacial reaction pathway by controlling the disproportionation of lithium polysulfides to improve the performance of lithium-sulfur batteries[J]. Journal of Power Sources, 2023, 582: 233517. |
| [63] | Wei J Y, Zhang X Q, Hou L P, et al. Shielding polysulfide intermediates by an organosulfur‐containing solid electrolyte interphase on the lithium anode in lithium-sulfur batteries[J]. Advanced Materials, 2020, 32(37): 2003012. |
| [64] | Wu H L, Shin M, Liu Y M, et al. Thiol-based electrolyte additives for high-performance lithium-sulfur batteries[J]. Nano Energy, 2017, 32: 50-58. |
| [65] | Ding Y, Li X, Chen Y M, et al. Hit two birds with one stone: a bi-functional selenium-substituted organosulfur polymer additive for high-performance lithium-sulfur batteries[J]. Chemical Engineering Journal, 2024, 482: 148803. |
| [66] | Geng C N, Qu W J, Han Z Y, et al. Superhigh coulombic efficiency lithium-sulfur batteries enabled by in situ coating lithium sulfide with polymerizable electrolyte additive[J]. Advanced Energy Materials, 2023, 13(15): 2204246. |
| [67] | Nie K L, Fu Q Q, Gao R L, et al. Dual-functional chloropyrazine additives for enhanced performance of lithium-sulfur batteries[J]. Energy Storage Materials, 2023, 63: 103011. |
| [68] | Wang Z, Al Alwan B, Simon Ng K Y. Multi-functions of amino thiophenol additives for shielding lithium metal anode in advanced Li-S battery[J]. Materials Science and Engineering: B, 2023, 297: 116727. |
| [69] | Li J, He L, Qin F R, et al. Dual-enhancement on electrochemical performance with thioacetamide as an electrolyte additive for lithium-sulfur batteries[J]. Electrochimica Acta, 2021, 376: 138041. |
| [70] | Gu J H, Yang D, Wang X Y, et al. Ammonium benzenesulfonate as an electrolyte additive to relieve the irreversible accumulation of lithium sulfide for high-energy density lithium-sulfur battery[J]. Journal of Colloid and Interface Science, 2023, 629: 368-376. |
| [71] | Lu H, Liu M, Zhang X L, et al. Catalytic effect of ammonium thiosulfate as a bifunctional electrolyte additive for regulating redox kinetics in lithium-sulfur batteries by altering the reaction pathway[J]. ACS Applied Materials & Interfaces, 2024, 16(11): 13640-13650. |
| [72] | Han W C, Hou J Y, Wang F, et al. Dual functional coordination interactions enable fast polysulfide conversion and robust interphase for high-loading lithium-sulfur batteries[J]. Materials Horizons, 2025, 12(5): 1473-1485. |
| [73] | Deng T, Wang J, Zhao H Y, et al. Dynamically regulating polysulfide degradation via organic sulfur electrolyte additives in lithium-sulfur batteries[J]. Advanced Energy Materials, 2024, 14(47): 2402319. |
| [74] | Li N, Zhang Y, Zhang S, et al. Insight into the probability of ethoxy(pentafluoro) cyclotriphosphazene (PFPN) as the functional electrolyte additive in lithium-sulfur batteries[J]. RSC Advances, 2024, 14(18): 12754-12761. |
| [75] | Li J, Hu H, Fang W, et al. Impact of fluorine‐based lithium salts on SEI for all‐solid‐state PEO‐based lithium metal batteries[J]. Advanced Functional Materials, 2023, 33(38): 2303718. |
| [76] | Liu Y H, Chang W, Qu J, et al. A polymer organosulfur redox mediator for high-performance lithium-sulfur batteries[J]. Energy Storage Materials, 2022, 46: 313-321. |
| [77] | Kuai D C, Wang S, Beltran S P, et al. Interfacial electrochemical lithiation and dissolution mechanisms at a sulfurized polyacrylonitrile cathode surface[J]. ACS Energy Letters, 2024, 9(3): 810-818. |
| [78] | Dong Y Y, Wu M Q, Cai D, et al. Confined biomimetic catalysts boost LiNO3-free lithium-sulfur batteries via enhanced LiTFSI decomposition[J]. Energy Storage Materials, 2025, 74: 103937. |
| [79] | Schedlbauer T, Rodehorst U C, Schreiner C, et al. Blends of lithium bis(oxalato)borate and lithium tetrafluoroborate: useful substitutes for lithium difluoro(oxalato)borate in electrolytes for lithium metal based secondary batteries[J]. Electrochimica Acta, 2013, 107: 26-32. |
| [80] | Wu F, Chen R J, Wu F, et al. Binary room-temperature complex electrolytes based on LiClO4 and organic compounds with acylamino group and its characterization for electric double layer capacitors[J]. Journal of Power Sources, 2008, 184(2): 402-407. |
| [81] | Chu H, Noh H, Kim Y J, et al. Achieving three-dimensional lithium sulfide growth in lithium-sulfur batteries using high-donor-number anions[J]. Nature Communications, 2019, 10(1): 188. |
| [82] | Xiang H F, Shi P C, Bhattacharya P, et al. Enhanced charging capability of lithium metal batteries based on lithium bis(trifluoromethanesulfonyl)imide-lithium bis(oxalate)borate dual-salt electrolytes[J]. Journal of Power Sources, 2016, 318: 170-177. |
| [83] | Park H, Kang H, Kim H, et al. Strategy for high-energy Li-S battery coupling with a Li metal anode and a sulfurized polyacrylonitrile cathode[J]. ACS Applied Materials & Interfaces, 2023, 15(39): 45876-45885. |
| [84] | Liu J, Zhang J P, Zhu J, et al. A lithium sulfonylimide COF-modified separator for high-performance Li-S batteries[J]. Nano Research, 2023, 16(11): 12601-12607. |
| [85] | Song Z Y, Zheng L P, Cheng P F, et al. Taming the chemical instability of lithium hexafluorophosphate-based electrolyte with lithium fluorosulfonimide salts[J]. Journal of Power Sources, 2022, 526: 231105. |
| [86] | Rohde D M, Eiden D P, Leppert V, et al. Li[B(OCH2CF3)4]: synthesis, characterization and electrochemical application as a conducting salt for Li-S batteries[J]. ChemPhysChem, 2015, 16(3): 666-675. |
| [87] | Zhu Z, Li X, Qi X, et al. Demystifying the salt-induced Li loss: a universal procedure for the electrolyte design of lithium-metal batteries[J]. Nano-Micro Letters, 2023, 15(1): 234. |
| [88] | Zhang H, Judez X, Santiago A, et al. Fluorine-free noble salt anion for high-performance all-solid-state lithium-sulfur batteries[J]. Advanced Energy Materials, 2019, 9(25): 1900763. |
| [89] | Kong X R, Kong Y C, Zheng Y Y, et al. Hydrofluoroether diluted dual-salts-based electrolytes for lithium-sulfur batteries with enhanced lithium anode protection[J]. Small, 2022, 18(52): 2205017. |
| [90] | Wang J N, Yi S S, Liu J W, et al. Suppressing the shuttle effect and dendrite growth in lithium-sulfur batteries[J]. ACS Nano, 2020, 14(8): 9819-9831. |
| [91] | Zhou L, Danilov D L, Qiao F, et al. Sulfur reduction reaction in lithium-sulfur batteries: mechanisms, catalysts, and characterization[J]. Advanced Energy Materials, 2022, 12(44): 2202094. |
| [92] | Li X Y, Feng S, Zhao C X, et al. Regulating lithium salt to inhibit surface gelation on an electrocatalyst for high-energy-density lithium-sulfur batteries[J]. Journal of the American Chemical Society, 2022, 144(32): 14638-14646. |
| [1] | Xin WU, Jianying GONG, Xiangyu LI, Yutao WANG, Xiaolong YANG, Zhen JIANG. Experimental study on the droplet motion on the hydrophobic surface under ultrasonic excitation [J]. CIESC Journal, 2025, 76(S1): 133-139. |
| [2] | Xianghai LI, Delin LAI, Gang KONG, Jian ZHOU. Molecular dynamics simulations on synergistic underwater oleophobicity mechanism of dual-biomimic surfaces [J]. CIESC Journal, 2025, 76(9): 4551-4562. |
| [3] | Jiaqi XU, Wenjun ZHANG, Yanping YU, Baogen SU, Qilong REN, Qiwei YANG. Numerical simulation and experimental study of the conversion of refinery gas to syngas via thermal plasma [J]. CIESC Journal, 2025, 76(9): 4462-4473. |
| [4] | Zhuolong LIU, Yunhua GAN, Keyang QU, Ningguang CHEN, Minghui PAN. Research on the effect of uniform electric field on characteristics of biodiesel small-scale jet diffusion combustion [J]. CIESC Journal, 2025, 76(9): 4800-4808. |
| [5] | Yuhong TIAN, Zhuangzhuang DU, Huifang XU, Ziqiang ZHU, Yucong WANG. Preparation of ZIF-8 based porous liquid and its SO2 adsorption performance [J]. CIESC Journal, 2025, 76(8): 4284-4296. |
| [6] | Yunhao LI, Chungang XU, Xiaosen LI, Jun FU, Yi WANG, Zhaoyang CHEN. Study on the effect of solid-liquid blended promoters on the formation of CO2 hydrates in saline water system [J]. CIESC Journal, 2025, 76(8): 4228-4238. |
| [7] | Yufeng WANG, Xiaoxue LUO, Hongliang FAN, Baijing WU, Cunpu LI, Zidong WEI. Green organic electrosynthesis coupled with water electrolysis to produce hydrogen—overview of electrode interface regulation strategies [J]. CIESC Journal, 2025, 76(8): 3753-3771. |
| [8] | Zhengzheng GUO, Yidan ZHAO, Fuqiang WANG, Lu PEI, Yanling JIN, Fang REN, Penggang REN. Construction and electromagnetic wave absorption properties of MoS2/RGO/NiFe2O4 composites with heterogeneous architecture [J]. CIESC Journal, 2025, 76(7): 3719-3732. |
| [9] | Liang QIAO, Shang LI, Xinliang LIU, Ming WANG, Pei ZHANG, Yingfei HOU. Synthesis and molecular simulation of terpolymer viscosity reducer for heavy oil [J]. CIESC Journal, 2025, 76(7): 3686-3695. |
| [10] | Liang GUO, Ye CHEN, Qiming JIA, Xiujuan XIE. Simulated and experimental investigations on self-pressurization of liquid helium storage tank [J]. CIESC Journal, 2025, 76(7): 3561-3571. |
| [11] | Pengguo XU, Ziheng MENG, Ganyu ZHU, Huiquan LI, Chenye WANG, Zhenhua SUN, Guocai TIAN. Study on deep carbonization process and kinetics of crude lithium carbonate with CO2 microbubbles [J]. CIESC Journal, 2025, 76(7): 3325-3338. |
| [12] | Feng LIU, Chunshuo HAN, Yi ZHANG, Yancheng LIU, Linjun YU, Jiawei SHEN, Xiaoquan GAO, Kai YANG. Micro-mechanism study on the effect of single and double hydrocarbon chain surfactants on oil-water interface properties under high temperature and high salt reservoir [J]. CIESC Journal, 2025, 76(6): 2939-2957. |
| [13] | Qingping ZHAO, Min ZHANG, Hui ZHAO, Gang WANG, Yongfu QIU. Hydrogen bond effect and kinetic studies on hydroesterification of ethylene to methyl propionate [J]. CIESC Journal, 2025, 76(6): 2701-2713. |
| [14] | Changyu LI, Qiang ZENG, Jie XIAO, Yangjie ZHANG, zheng ZHANG, Yuanhua LIN. Study on interface modification of LATP-based solid electrolyte membrane by PVDF [J]. CIESC Journal, 2025, 76(6): 2974-2982. |
| [15] | Zhaoming MAI, Yingtao WU, Wei WANG, Haibao MU, Zuohua HUANG, Chenglong TANG. Study on nonlinear ignition characteristics and dilution gas effect of n-dodecane methane dual fuel [J]. CIESC Journal, 2025, 76(6): 3115-3124. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||