CIESC Journal ›› 2025, Vol. 76 ›› Issue (8): 4284-4296.DOI: 10.11949/0438-1157.20250046
• Energy and environmental engineering • Previous Articles Next Articles
Yuhong TIAN1,2( ), Zhuangzhuang DU1, Huifang XU1, Ziqiang ZHU1, Yucong WANG1
), Zhuangzhuang DU1, Huifang XU1, Ziqiang ZHU1, Yucong WANG1
Received:2025-01-13
Revised:2025-04-30
Online:2025-09-17
Published:2025-08-25
Contact:
Yuhong TIAN
田宇红1,2( ), 杜壮壮1, 徐慧芳1, 祝自强1, 王宇聪1
), 杜壮壮1, 徐慧芳1, 祝自强1, 王宇聪1
通讯作者:
田宇红
作者简介:田宇红(1977—),女,博士,副教授,tiantianyuhong@163.com
基金资助:CLC Number:
Yuhong TIAN, Zhuangzhuang DU, Huifang XU, Ziqiang ZHU, Yucong WANG. Preparation of ZIF-8 based porous liquid and its SO2 adsorption performance[J]. CIESC Journal, 2025, 76(8): 4284-4296.
田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296.
Add to citation manager EndNote|Ris|BibTeX
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 孔径/nm |
|---|---|---|---|
| ZIF-8 | 1031.02 | 0.467 | 0.61 |
| ZIF-8-PLs | 911.83 | 0.383 | 0.60 |
Table 1 Pore structure parameters of ZIF-8 and ZIF-8 in porous liquids
| 材料 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 孔径/nm |
|---|---|---|---|
| ZIF-8 | 1031.02 | 0.467 | 0.61 |
| ZIF-8-PLs | 911.83 | 0.383 | 0.60 |
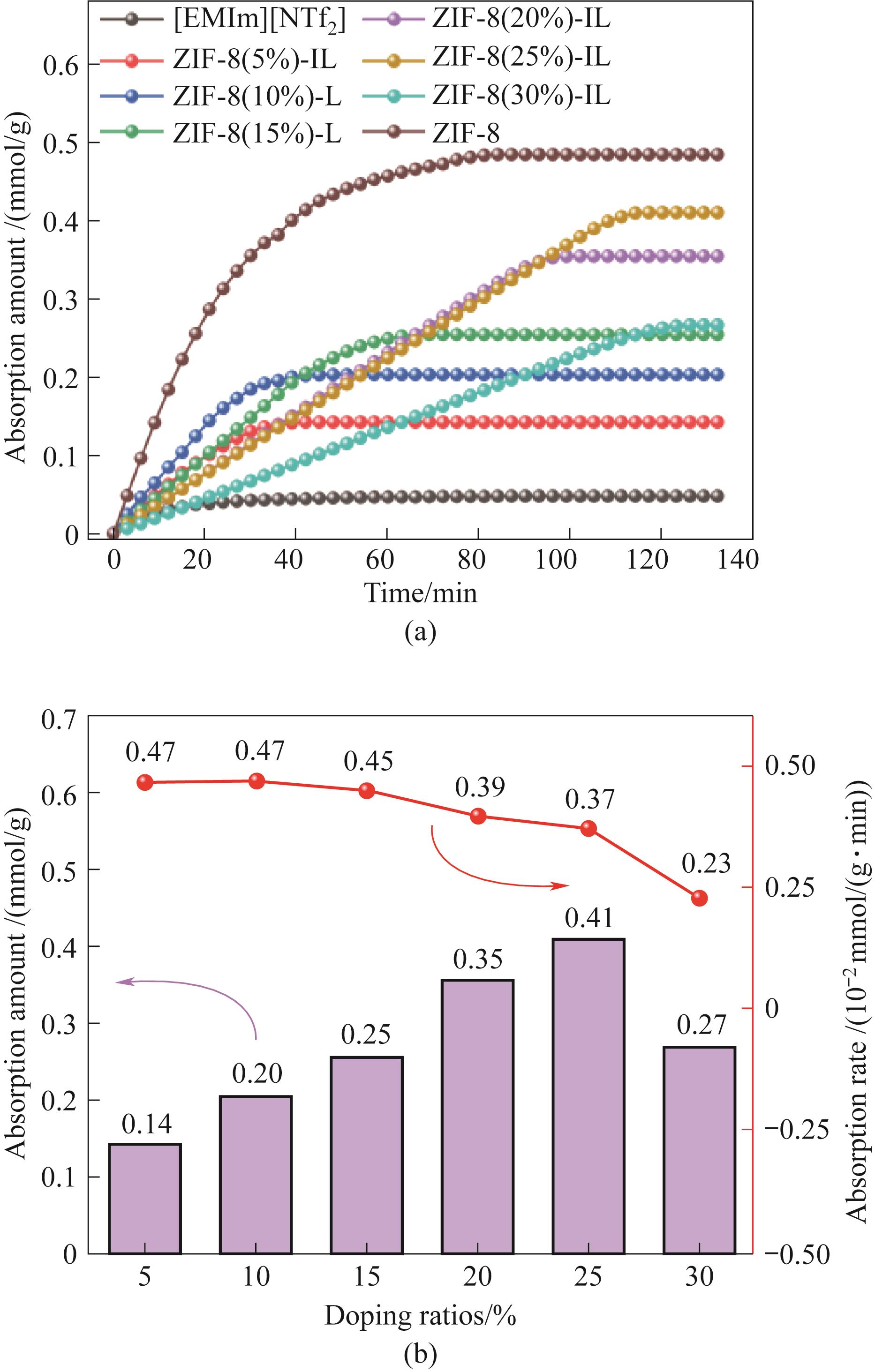
Fig.11 The change curve of adsorption capacity with time (a) and the change relationship between adsorption capacity and adsorption rate (b) of porous liquid with different ZIF-8 loads
| 吸附剂类型 | 吸附温度/K | 饱和吸附量/(mmol/g) | 文献 |
|---|---|---|---|
| UiO-66-甲酸 | 298 | 0.405 | [ |
| Zr-MOF-NH2/CTF-Cu2+ | 298 | 0.614 | [ |
| Zr-MOF-NH2/PAN | 298 | 0.297 | [ |
| MOF-199/PAN | 298 | 0.219 | [ |
| UiO-66-NH2@CNTs/PTFE | 298 | 0.6 | [ |
| ZIF-8(25%)-IL | 303 | 0.41 | 本研究 |
Table 2 Comparison of SO2 adsorption data of different porous materials
| 吸附剂类型 | 吸附温度/K | 饱和吸附量/(mmol/g) | 文献 |
|---|---|---|---|
| UiO-66-甲酸 | 298 | 0.405 | [ |
| Zr-MOF-NH2/CTF-Cu2+ | 298 | 0.614 | [ |
| Zr-MOF-NH2/PAN | 298 | 0.297 | [ |
| MOF-199/PAN | 298 | 0.219 | [ |
| UiO-66-NH2@CNTs/PTFE | 298 | 0.6 | [ |
| ZIF-8(25%)-IL | 303 | 0.41 | 本研究 |
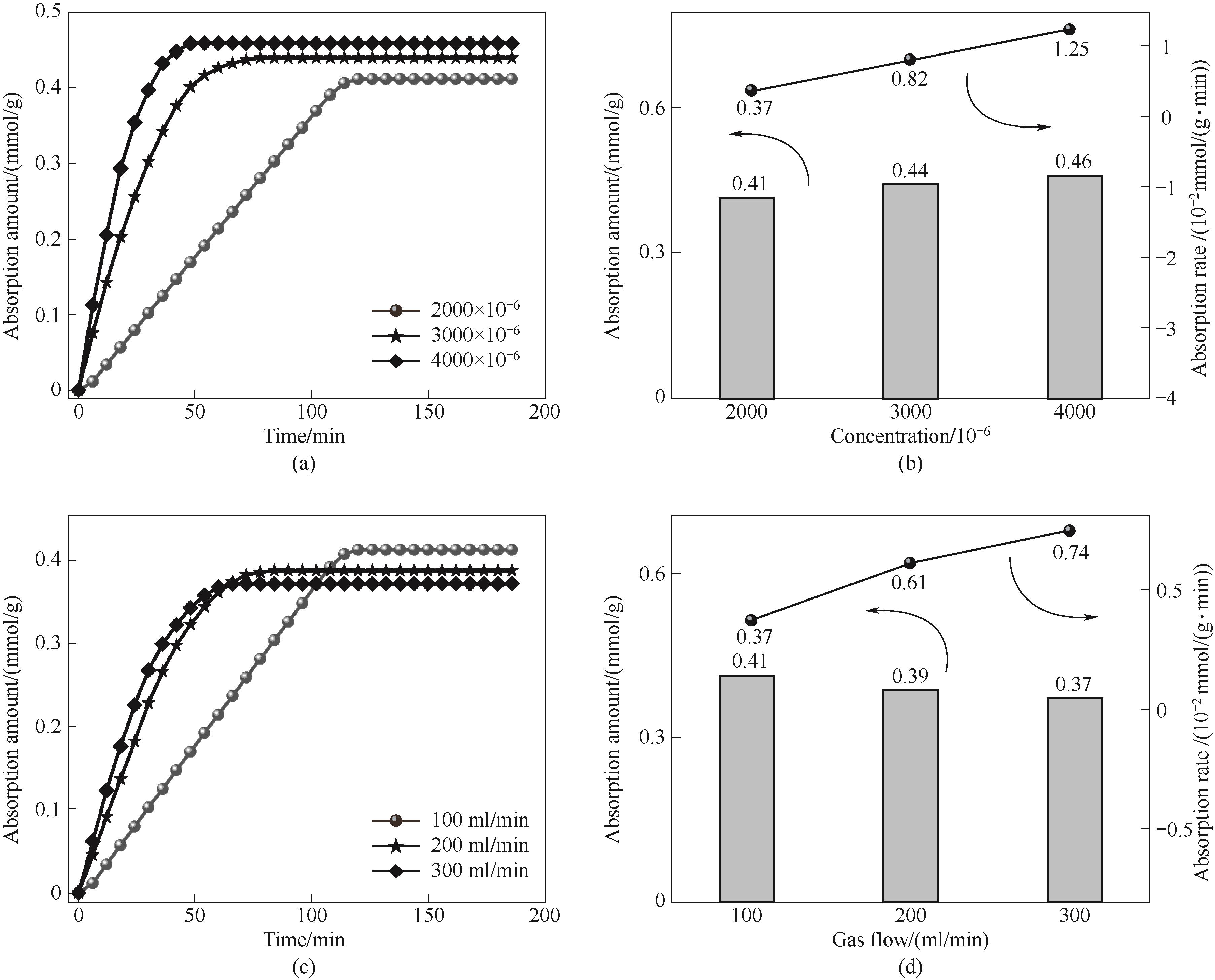
Fig.13 The change curve of adsorption capacity with time (a) and the change relationship between adsorption capacity and adsorption rate (b) of ZIF-8(25%)-IL at different SO2 concentrations; adsorption curve of ZIF-8(25%)-IL with time (c) and relationship between adsorption amount and adsorption rate (d) at different gas flow rates
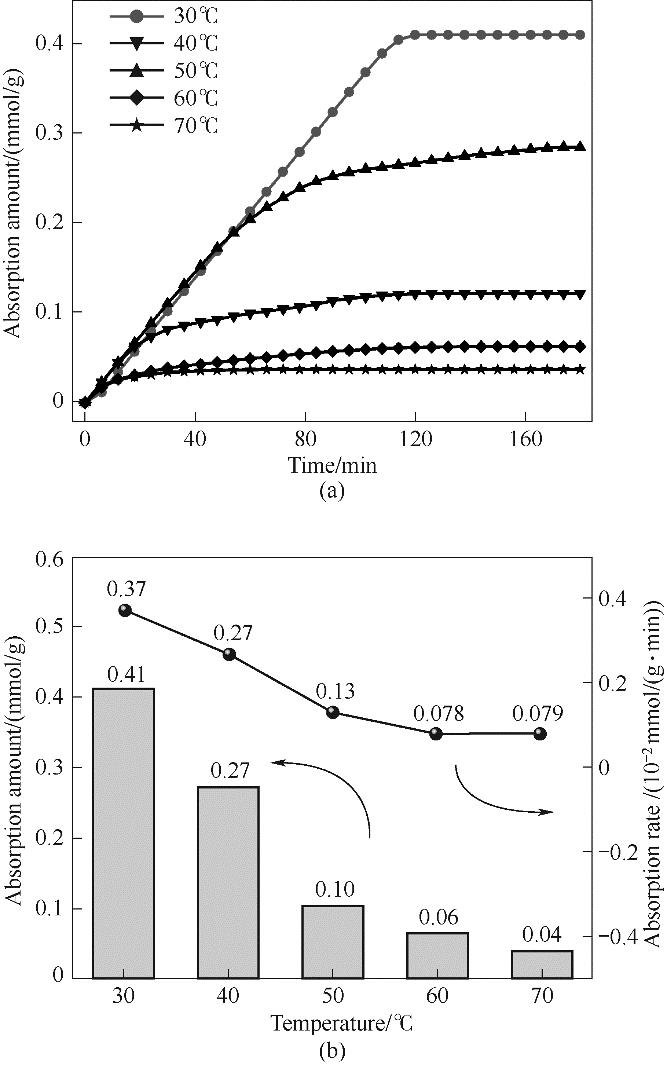
Fig.14 Change curve of adsorption capacity with time (a) and change relationship between adsorption capacity and adsorption rate (b) of ZIF-8(25%)-IL at different temperatures
| 样品 | 伪一级动力学模型 | 伪二级动力学模型 | Avrami 吸附动力学模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe/(mmol/g) | k1/min-1 | R2 | qe/(mmol/g) | k2/(g/(min·mmol)) | R2 | qe/(mmol/g) | kA/min-1 | nA | R2 | |
| [EMIm][NTf2] | 0.0471 | 0.0832 | 0.991 | 0.0518 | 2.4151 | 0.991 | 0.0475 | 0.0090 | 0.8580 | 0.997 |
| ZIF-8(5%)-IL | 0.1450 | 0.0597 | 0.976 | 0.1661 | 0.4637 | 0.928 | 0.1428 | 0.0158 | 1.4573 | 0.997 |
| ZIF-8(10%)-IL | 0.2073 | 0.0579 | 0.975 | 0.2383 | 0.3085 | 0.928 | 0.2039 | 0.0143 | 1.4764 | 0.997 |
| ZIF-8(15%)-IL | 0.2732 | 0.0297 | 0.967 | 0.3544 | 0.0775 | 0.943 | 0.2571 | 0.0044 | 1.5796 | 0.996 |
| ZIF-8(20%)-IL | 0.5645 | 0.0089 | 0.979 | 0.9439 | 0.0058 | 0.977 | 0.3858 | 0.0020 | 1.5110 | 0.993 |
| ZIF-8(25%)-IL | 1.1751 | 0.0036 | 0.993 | 2.1766 | 0.0009 | 0.993 | 0.5799 | 0.0029 | 1.2668 | 0.996 |
| ZIF-8(30%)-IL | 1.3981 | 0.0017 | 0.997 | 2.7028 | 0.0003 | 0.997 | 0.4914 | 0.0026 | 1.1860 | 0.998 |
| ZIF-8 | 0.4941 | 0.0418 | 0.998 | 0.5939 | 0.0796 | 0.980 | 0.4882 | 0.303 | 1.1056 | 0.999 |
Table 3 Kinetic model fitting parameters for adsorption of SO2 by porous liquids with different ZIF-8 loads
| 样品 | 伪一级动力学模型 | 伪二级动力学模型 | Avrami 吸附动力学模型 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe/(mmol/g) | k1/min-1 | R2 | qe/(mmol/g) | k2/(g/(min·mmol)) | R2 | qe/(mmol/g) | kA/min-1 | nA | R2 | |
| [EMIm][NTf2] | 0.0471 | 0.0832 | 0.991 | 0.0518 | 2.4151 | 0.991 | 0.0475 | 0.0090 | 0.8580 | 0.997 |
| ZIF-8(5%)-IL | 0.1450 | 0.0597 | 0.976 | 0.1661 | 0.4637 | 0.928 | 0.1428 | 0.0158 | 1.4573 | 0.997 |
| ZIF-8(10%)-IL | 0.2073 | 0.0579 | 0.975 | 0.2383 | 0.3085 | 0.928 | 0.2039 | 0.0143 | 1.4764 | 0.997 |
| ZIF-8(15%)-IL | 0.2732 | 0.0297 | 0.967 | 0.3544 | 0.0775 | 0.943 | 0.2571 | 0.0044 | 1.5796 | 0.996 |
| ZIF-8(20%)-IL | 0.5645 | 0.0089 | 0.979 | 0.9439 | 0.0058 | 0.977 | 0.3858 | 0.0020 | 1.5110 | 0.993 |
| ZIF-8(25%)-IL | 1.1751 | 0.0036 | 0.993 | 2.1766 | 0.0009 | 0.993 | 0.5799 | 0.0029 | 1.2668 | 0.996 |
| ZIF-8(30%)-IL | 1.3981 | 0.0017 | 0.997 | 2.7028 | 0.0003 | 0.997 | 0.4914 | 0.0026 | 1.1860 | 0.998 |
| ZIF-8 | 0.4941 | 0.0418 | 0.998 | 0.5939 | 0.0796 | 0.980 | 0.4882 | 0.303 | 1.1056 | 0.999 |
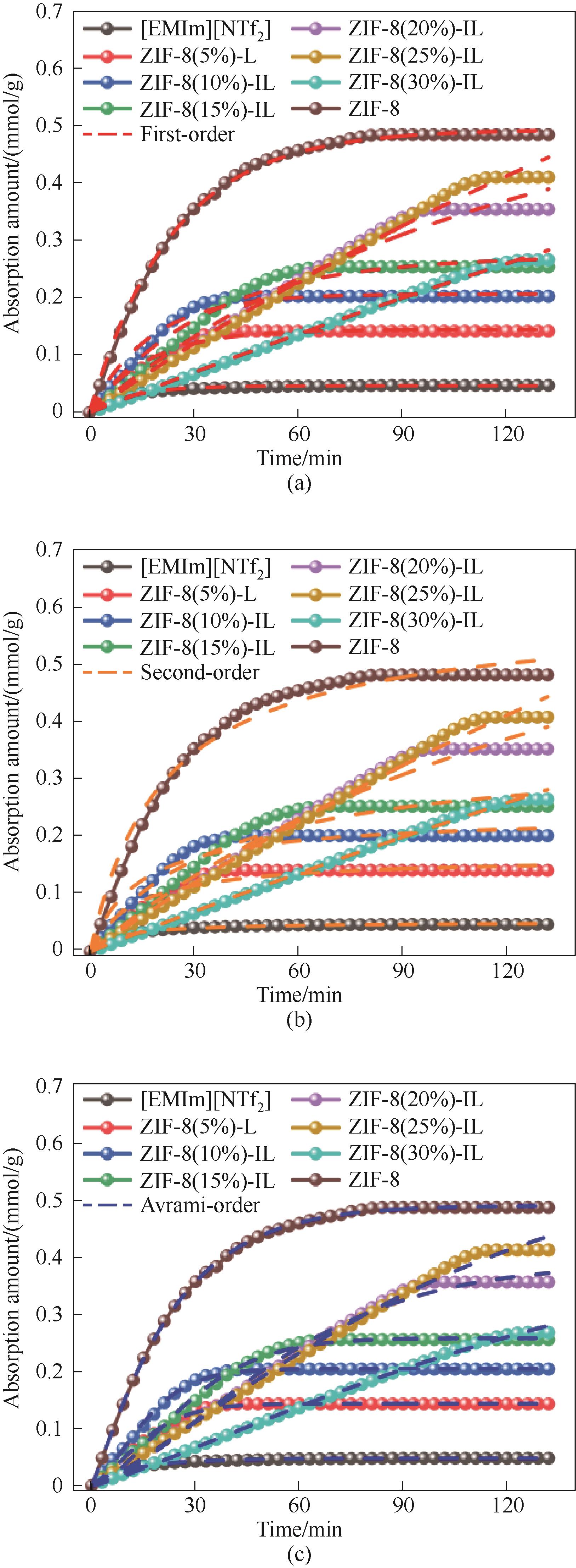
Fig.15 Pseudo-first-order adsorption kinetics model (a), pseudo-second-order adsorption kinetics model (b) and Avrami adsorption kinetics model (c) for different ZIF-8 loads of porous liquids
| [1] | He Z H, Wang Y, Liu Y X, et al. Recent advances in sulfur poisoning of selective catalytic reduction (SCR) denitration catalysts[J]. Fuel, 2024, 365: 131126. |
| [2] | Hou Y H, Chen Y H, He X H, et al. Insights into the adsorption of CO2, SO2 and NO x in flue gas by carbon materials: a critical review[J]. Chemical Engineering Journal, 2024, 490: 151424. |
| [3] | Kong M, Song L J, Liao H P, et al. A review on development of post-combustion CO2 capture technologies: performance of carbon-based, zeolites and MOFs adsorbents[J]. Fuel, 2024, 371: 132103. |
| [4] | Zhang X M, Zhang Z H, Zhang B H, et al. Synergistic effect of Zr-MOF on phosphomolybdic acid promotes efficient oxidative desulfurization[J]. Applied Catalysis B: Environmental, 2019, 256: 117804. |
| [5] | McLinden C A, Fioletov V, Shephard M W, et al. Space-based detection of missing sulfur dioxide sources of global air pollution[J]. Nature Geoscience, 2016, 9: 496-500. |
| [6] | Liu X P, Zhang Y L, Li M Z, et al. The effect of ZIF-67 nanoparticles on the desulfurization performance of deep eutectic solvent based nanofluid system[J]. Journal of Hazardous Materials, 2022, 426: 128098. |
| [7] | Hou P F, Bai J Y, Yin J. On-line monitoring and optimization of performance indexes for limestone wet desulfurization technology[J]. Applied Mechanics and Materials, 2013, 295/296/297/298: 1020-1028. |
| [8] | Ng K H, Lai S Y, Jamaludin N F M, et al. A review on dry-based and wet-based catalytic sulphur dioxide (SO2) reduction technologies[J]. Journal of Hazardous Materials, 2022, 423: 127061. |
| [9] | Zhao K, Sun X, Wang C, et al. Supported catalysts for simultaneous removal of SO2, NO x, and Hg0 from industrial exhaust gases: a review[J]. Chinese Chemical Letters, 2021, 32(10): 2963-2974. |
| [10] | 武传朋, 李传坤, 杨哲, 等. 固体吸附材料脱除SO2研究进展[J]. 化工进展, 2022, 41(7): 3840-3854. |
| Wu C P, Li C K, Yang Z, et al. Research progress of SO2 removal by solid adsorbents[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3840-3854. | |
| [11] | Chang C W, Borne I, Lawler R M, et al. Accelerating solvent selection for type Ⅱ porous liquids[J]. Journal of the American Chemical Society, 2022, 144(9): 4071-4079. |
| [12] | James S L. The dam bursts for porous liquids[J]. Advanced Materials, 2016, 28(27): 5712-5716. |
| [13] | 宇国佳, 靳冬玉, 周智勇, 等. 多孔液体的设计合成与应用研究进展[J]. 化工学报, 2023, 74(1): 257-275. |
| Yu G J, Jin D Y, Zhou Z Y, et al. Advances in the design, synthesis and application of porous liquids[J]. CIESC Journal, 2023, 74(1): 257-275. | |
| [14] | O'Reilly N, Giri N, James S L. Porous liquids[J]. Chemistry–A European Journal, 2007, 13(11): 3020-3025. |
| [15] | Horike S, Kitagawa S. Unveiling liquid MOFs[J]. Nature Materials, 2017, 16(11): 1054-1055. |
| [16] | Rimsza J M, Nenoff T M. Porous liquids: computational design for targeted gas adsorption[J]. ACS Applied Materials & Interfaces, 2022, 14(16): 18005-18015. |
| [17] | Wang D C, Xin Y Y, Yao D D, et al. Shining light on porous liquids: from fundamentals to syntheses, applications and future challenges[J]. Advanced Functional Materials, 2022, 32(1): 2104162. |
| [18] | Liu S J, Liu J D, Hou X D, et al. Porous liquid: a stable ZIF-8 colloid in ionic liquid with permanent porosity[J]. Langmuir, 2018, 34(12): 3654-3660. |
| [19] | Costa Gomes M, Pison L, Červinka C, et al. Porous ionic liquids or liquid metal–organic frameworks?[J]. Angewandte Chemie, 2018, 130(37): 12085-12088. |
| [20] | Shan W D, Fulvio P F, Kong L Y, et al. New class of type Ⅲ porous liquids: a promising platform for rational adjustment of gas sorption behavior[J]. ACS Applied Materials & Interfaces, 2018, 10(1): 32-36. |
| [21] | Troyano J, Carné-Sánchez A, Avci C, et al. Colloidal metal-organic framework particles: the pioneering case of ZIF-8[J]. Chemical Society Reviews, 2019, 48(23): 5534-5546. |
| [22] | Dinker M K, Zhao K, Dai Z X, et al. Porous liquids responsive to light[J]. Angewandte Chemie International Edition, 2022, 61(50): e202212326. |
| [23] | Giri N, Del Pópolo M G, Melaugh G, et al. Liquids with permanent porosity[J]. Nature, 2015, 527(7577): 216-220. |
| [24] | Li X Q, Ding Y D, Guo L H, et al. Non-aqueous energy-efficient absorbents for CO2 capture based on porous silica nanospheres impregnated with amine[J]. Energy, 2019, 171: 109-119. |
| [25] | Liu D F, Wu Y B, Xia Q B, et al. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8[J]. Adsorption, 2013, 19(1): 25-37. |
| [26] | Zhang K, Lively R P, Zhang C, et al. Exploring the framework hydrophobicity and flexibility of ZIF-8: from biofuel recovery to hydrocarbon separations[J]. The Journal of Physical Chemistry Letters, 2013, 4(21): 3618-3622. |
| [27] | Bhattacharyya S, Pang S H, Dutzer M R, et al. Interactions of SO2-containing acid gases with ZIF-8: structural changes and mechanistic investigations[J]. The Journal of Physical Chemistry C, 2016, 120(48): 27221-27229. |
| [28] | Feng C B, Chen L, Yan Z C. Phase behavior and aggregation property of polyglyceryl-modified silicone surfactant in [EMIM][NTf2][J]. Journal of Molecular Liquids, 2016, 222: 133-137. |
| [29] | Makino T, Kanakubo M, Masuda Y, et al. CO2 absorption properties, densities, viscosities, and electrical conductivities of ethylimidazolium and 1-ethyl-3-methylimidazolium ionic liquids[J]. Fluid Phase Equilibria, 2014, 362: 300-306. |
| [30] | Zhao X X, Ding Y D, Ma L J, et al. An enhancement of CO2 capture in a type-Ⅲ porous liquid by 2-methylimidazole zinc salt (ZIF-8)[J]. Journal of Molecular Liquids, 2022, 367: 120523. |
| [31] | Peng J Y, Li Y, Sun X L, et al. Controlled manipulation of metal-organic framework layers to nanometer precision inside large mesochannels of ordered mesoporous silica for enhanced removal of bisphenol A from water[J]. ACS Applied Materials & Interfaces, 2019, 11(4): 4328-4337. |
| [32] | Wu C, Shi L Z, Xue S G, et al. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils[J]. Science of the Total Environment, 2019, 647: 1158-1168. |
| [33] | Leng L J, Liu R F, Xu S Y, et al. An overview of sulfur-functional groups in biochar from pyrolysis of biomass[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107185. |
| [34] | Zhu Q, Wang C, Yin J, et al. Efficient and remarkable SO2 capture: a discovery of imidazole-based ternary deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 330: 115595. |
| [35] | Xu X Q, Wu P, Li C Y, et al. Reversible removal of SO2 with amine-functionalized ZIF8 dispersed in n-heptanol[J]. Energy & Fuels, 2021, 35(6): 5110-5121. |
| [36] | Sasikumar B, Bisht S, Arthanareeswaran G, et al. Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation[J]. Separation and Purification Technology, 2021, 264: 118471. |
| [37] | Pan Y C, Liu W, Zhao Y J, et al. Improved ZIF-8 membrane: effect of activation procedure and determination of diffusivities of light hydrocarbons[J]. Journal of Membrane Science, 2015, 493: 88-96. |
| [38] | Thommes M, Kaneko K, Neimark A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| [39] | Lai B B, Crawford D E, Wu H C, et al. Using porous liquids to perform liquid-liquid separations[J]. Angewandte Chemie International Edition, 2024, 63(41): e202409894. |
| [40] | Wu J, Mu L W, Feng X, et al. Poly(alkylimidazolium bis(trifluoromethylsulfonyl)imide)-based polymerized ionic liquids: a potential high-performance lubricating grease[J]. Advanced Materials Interfaces, 2019, 6(5): 1801796. |
| [41] | Dou S Y, Liu K, Feng Y R, et al. A new strategy to effectively capture and recover SO2 based on the functional porous liquids[J]. Journal of Cleaner Production, 2024, 467: 143006. |
| [42] | Li X Q, Yao D D, Wang D C, et al. Amino-functionalized ZIFs-based porous liquids with low viscosity for efficient low-pressure CO2 capture and CO2/N2 separation[J]. Chemical Engineering Journal, 2022, 429: 132296. |
| [43] | Ma Y L, Li A R, Wang C. Experimental study on adsorption removal of SO2 in flue gas by defective UiO-66[J]. Chemical Engineering Journal, 2023, 455: 140687. |
| [44] | Li S M, Dai Y W, Ye P W, et al. Hierarchical porous MOF/CTF hybrid frameworks used as protection against acidic harmful gases[J]. Chemical Engineering Journal, 2024, 491: 152035. |
| [45] | Zhang Y Y, Yuan S, Feng X, et al. Preparation of nanofibrous metal-organic framework filters for efficient air pollution control[J]. Journal of the American Chemical Society, 2016, 138(18): 5785-5788. |
| [46] | Feng S S, Li X Y, Zhao S F, et al. Multifunctional metal organic framework and carbon nanotube-modified filter for combined ultrafine dust capture and SO2 dynamic adsorption[J]. Environmental Science: Nano, 2018, 5(12): 3023-3031. |
| [47] | Zhao X X, Ding Y D, Ma L J, et al. An amine-functionalized strategy to enhance the CO2 absorption of type Ⅲ porous liquids[J]. Energy, 2023, 279: 127975. |
| [48] | Wen S Y, Wang T, Zhang X M, et al. Novel amino acid ionic liquids prepared via one-step lactam hydrolysis for the highly efficient capture of CO2 [J]. AIChE Journal, 2023, 69(11): e18206. |
| [49] | Serna-Guerrero R, Sayari A. Modeling adsorption of CO2 on amine-functionalized mesoporous silica(2): Kinetics and breakthrough curves[J]. Chemical Engineering Journal, 2010, 161(1/2): 182-190. |
| [1] | Xin WU, Jianying GONG, Xiangyu LI, Yutao WANG, Xiaolong YANG, Zhen JIANG. Experimental study on the droplet motion on the hydrophobic surface under ultrasonic excitation [J]. CIESC Journal, 2025, 76(S1): 133-139. |
| [2] | Xingliang PEI, Cuiping YE, Yingli PEI, Wenying LI. Selective adsorption and separation of xylene isomers by alkali-modified MIL-53(Cr) [J]. CIESC Journal, 2025, 76(S1): 258-267. |
| [3] | Zihang WU, Zhenyuan XU, Jinfang YOU, Quanwen PAN, Ruzhu WANG. Cooling system for deep well drilling equipment based on adsorption cold storage technology [J]. CIESC Journal, 2025, 76(S1): 309-317. |
| [4] | Guorui HUANG, Yao ZHAO, Mingxi XIE, Erjian CHEN, Yanjun DAI. Experimental study on a novel waste heat recovery system based on desiccant coated exchanger in data center [J]. CIESC Journal, 2025, 76(S1): 409-417. |
| [5] | Yunhao LI, Chungang XU, Xiaosen LI, Jun FU, Yi WANG, Zhaoyang CHEN. Study on the effect of solid-liquid blended promoters on the formation of CO2 hydrates in saline water system [J]. CIESC Journal, 2025, 76(8): 4228-4238. |
| [6] | Songwei SHI, Cheng ZHAO, Shuai LIU, Yuxuan YING, Mi YAN. Removal of biogas H2S using iron-rich fly ash coupled with Fe-Zn/Al2O3 [J]. CIESC Journal, 2025, 76(8): 4239-4247. |
| [7] | Liang QIAO, Shang LI, Xinliang LIU, Ming WANG, Pei ZHANG, Yingfei HOU. Synthesis and molecular simulation of terpolymer viscosity reducer for heavy oil [J]. CIESC Journal, 2025, 76(7): 3686-3695. |
| [8] | Pengguo XU, Ziheng MENG, Ganyu ZHU, Huiquan LI, Chenye WANG, Zhenhua SUN, Guocai TIAN. Study on deep carbonization process and kinetics of crude lithium carbonate with CO2 microbubbles [J]. CIESC Journal, 2025, 76(7): 3325-3338. |
| [9] | Yufeng TANG, Chunhui TAO, Yongzheng WANG, Yinhui LI, Ran DUAN, Zeyi ZHAO, Heping MA. Preparation of carbon based porous adsorbent with ultra high specific surface area and its Kr gas storage performance [J]. CIESC Journal, 2025, 76(7): 3339-3349. |
| [10] | Zhaoming MAI, Yingtao WU, Wei WANG, Haibao MU, Zuohua HUANG, Chenglong TANG. Study on nonlinear ignition characteristics and dilution gas effect of n-dodecane methane dual fuel [J]. CIESC Journal, 2025, 76(6): 3115-3124. |
| [11] | Xinyan PENG, Yunhong LIU, Lingyu CHEN, Yuelan WEI, Shuqin CHEN, Zhudong HU. Preparation of hypercrosslinked polystyrene hemosorbents based on small-molecule external cross-linkers [J]. CIESC Journal, 2025, 76(6): 3093-3103. |
| [12] | Qingping ZHAO, Min ZHANG, Hui ZHAO, Gang WANG, Yongfu QIU. Hydrogen bond effect and kinetic studies on hydroesterification of ethylene to methyl propionate [J]. CIESC Journal, 2025, 76(6): 2701-2713. |
| [13] | Jun HE, Yong LI, Nan ZHAO, Xiaojun HE. Study on the properties of carbon with Se doping cobalt sulfide in lithium-sulfur batteries [J]. CIESC Journal, 2025, 76(6): 2995-3008. |
| [14] | Shenghua YANG, Yangjie SUN, Xiaojun XUE, Jie MI, Jiancheng WANG, Yu FENG. Research progress on gas pollutants removal by defective metal oxides [J]. CIESC Journal, 2025, 76(6): 2469-2482. |
| [15] | Pengtao GUO, Ting WANG, Bo XUE, Yunpan YING, Dahuan LIU. Ultramicroporous MOF with multiple adsorption sites for CH4/N2 separation [J]. CIESC Journal, 2025, 76(5): 2304-2312. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||