CIESC Journal ›› 2019, Vol. 70 ›› Issue (7): 2574-2583.DOI: 10.11949/0438-1157.20190014
• Separation engineering • Previous Articles Next Articles
Yuanhui TANG1( ),Yang HU1,Zhiqin YAN2,Chunyu LI1
),Yang HU1,Zhiqin YAN2,Chunyu LI1
Received:2019-01-07
Revised:2019-04-15
Online:2019-07-05
Published:2019-07-05
Contact:
Yuanhui TANG
通讯作者:
唐元晖
基金资助:CLC Number:
Yuanhui TANG, Yang HU, Zhiqin YAN, Chunyu LI. Experimental study on nanofiltration separation of high concentrated saline glyphosate solution[J]. CIESC Journal, 2019, 70(7): 2574-2583.
唐元晖, 扈阳, 燕至琴, 李春玉. 高浓度含盐草甘膦溶液的纳滤分离实验研究[J]. 化工学报, 2019, 70(7): 2574-2583.
Add to citation manager EndNote|Ris|BibTeX
| 原料液编号 | 原料液组成/(g/L) | |
|---|---|---|
| 草甘膦 | NaCl | |
| 1 | 60 | |
| 2 | 80 | |
| 3 | 100 | |
| 4 | 1 | |
| 5 | 4 | |
| 6 | 7 | |
| 7 | 1 | 100 |
| 8 | 4 | 100 |
| 9 | 7 | 100 |
Table1 Concentrations of feed solution
| 原料液编号 | 原料液组成/(g/L) | |
|---|---|---|
| 草甘膦 | NaCl | |
| 1 | 60 | |
| 2 | 80 | |
| 3 | 100 | |
| 4 | 1 | |
| 5 | 4 | |
| 6 | 7 | |
| 7 | 1 | 100 |
| 8 | 4 | 100 |
| 9 | 7 | 100 |
| 溶质 | 浓度/(g/L) | σ | P×106/(m/s) |
|---|---|---|---|
| NaCl | 60 | 0.151 | 3.07 |
| 80 | 0.121 | 3.36 | |
| 100 | 0.119 | 3.89 | |
| 草甘膦 | 1 | 0.971 | 0.271 |
| 4 | 0.973 | 0.202 | |
| 7 | 0.985 | 0.198 |
Table 2 Fitting calculation results of membrane characteristic parameters and structural parameters of DK membrane on experimental system
| 溶质 | 浓度/(g/L) | σ | P×106/(m/s) |
|---|---|---|---|
| NaCl | 60 | 0.151 | 3.07 |
| 80 | 0.121 | 3.36 | |
| 100 | 0.119 | 3.89 | |
| 草甘膦 | 1 | 0.971 | 0.271 |
| 4 | 0.973 | 0.202 | |
| 7 | 0.985 | 0.198 |
| 操作压力/MPa | 草甘膦浓度/(g/L) | NaCl浓度/(g/L) | ||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 60 | 80 | 100 | |
| 0.6 | 0.048 | 0.046 | 0.044 | 0.0025 | 0.0015 | 0.00079 |
| 0.9 | 0.067 | 0.065 | 0.064 | 0.0039 | 0.0027 | 0.0017 |
| 1.2 | 0.094 | 0.091 | 0.087 | 0.0059 | 0.0042 | 0.0025 |
| 1.5 | 0.11 | 0.12 | 0.11 | 0.0079 | 0.0050 | 0.0034 |
Table 3 Characterization of concentration polarization degree under different operating conditions
| 操作压力/MPa | 草甘膦浓度/(g/L) | NaCl浓度/(g/L) | ||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 60 | 80 | 100 | |
| 0.6 | 0.048 | 0.046 | 0.044 | 0.0025 | 0.0015 | 0.00079 |
| 0.9 | 0.067 | 0.065 | 0.064 | 0.0039 | 0.0027 | 0.0017 |
| 1.2 | 0.094 | 0.091 | 0.087 | 0.0059 | 0.0042 | 0.0025 |
| 1.5 | 0.11 | 0.12 | 0.11 | 0.0079 | 0.0050 | 0.0034 |

Fig.7 Volumetric flux of DK membrane on glyphosate and NaCl mixed solution and interception rate as a function of operating pressure and concentration
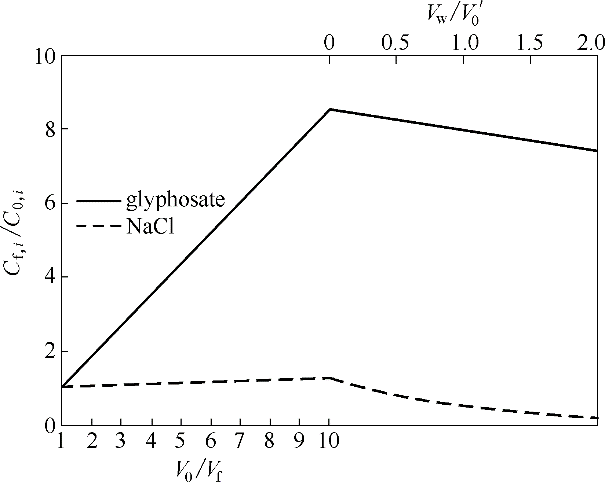
Fig.8 Relationship between concentration ratio of glyphosate and NaCl in feed solution during preconcentration-continuous constant volume percolation with concentration factor (V0/Vf ) and water consumption during percolation process (Vw/V0)
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | ||||
|---|---|---|---|---|---|---|---|
| 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | ||
| 初始 | — | V0 | 1.00 | 100.0 | — | — | — |
| 预浓缩后 | 0 | 1/10 V0 | 8.51 | 125.92 | 9/10 V0 | 0.17 | 97.12 |
| 连续恒容渗滤后 | 1/5 V0 | 1/10 V0 | 7.43 | 20.81 | 11/10 V0 | 0.24 | 89.02 |
Table 4 Variations of water consumption, volume and concentration of feed and permeate during whole concentration-infiltration process
| 渗滤过程 | 水消耗量 | 料液 | 透过液 | ||||
|---|---|---|---|---|---|---|---|
| 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | 体积 | c(草甘膦)/(g/L) | c(NaCl)/(g/L) | ||
| 初始 | — | V0 | 1.00 | 100.0 | — | — | — |
| 预浓缩后 | 0 | 1/10 V0 | 8.51 | 125.92 | 9/10 V0 | 0.17 | 97.12 |
| 连续恒容渗滤后 | 1/5 V0 | 1/10 V0 | 7.43 | 20.81 | 11/10 V0 | 0.24 | 89.02 |
| 1 | ErikssonP. Nanofiltration extends the range of membrane filtration [J]. Environmental Progress, 1988, 7(1): 58-62. |
| 2 | MohammadA W, TeowY H, AngW L, et al. Nanofiltration membranes review: recent advances and future prospects[J]. Desalination, 2015, 356(S1): 226-254. |
| 3 | EsmiaC F, SchriveaL, BarreaY, et al. Using nanofiltration in a “zero-rejection” process: the removal of Ni2+ and Co2+ from salty wastewater[J]. Desalination and Water Treatment, 2013, 51(1/2/3): 476-484. |
| 4 | Perez-GonzalezA, IbanezR, GomezP, et al. Nanofiltration separation of polyvalent and monovalent anions in desalination brines[J]. Journal of Membrane Science, 2015, 473: 16-27. |
| 5 | BennaniC F, M’hiriO. Comparative study of the removal of heavy metals by two nanofiltration membranes[J]. Desalination and Water Treatment, 2015, 53(4): 1024-1030. |
| 6 | WangX L, ZhangC H, OuyangP. The possibility of separating saccharides from a NaCl solution by using nanofiltration in diafiltration mode[J]. Journal of Membrane Science, 2002, 204(1/2): 271-281. |
| 7 | ZhaoH F, HuaX, YangR J, et al. Diafiltration process on xylo-oligosaccharides syrup using nanofiltration and its modeling[J]. International Journal of Food Science and Technology, 2012, 47(1): 32-39. |
| 8 | MikulasekaP, CuhorkaJ. Desalination and concentration of liquid dyes by nanofiltration[J]. Desalination and Water Treatment, 2015, 55(10): 2711-2720. |
| 9 | HeY, LiG, WangH, et al. Diafiltration and water recovery of Reactive Brilliant Blue KN-R solution by two-stage membrane separation process[J]. Chemical Engineering and Processing, 2010, 49(5): 476-483. |
| 10 | TuC H, FangY Y, ZhuJ, et al. Free energies of the ion equilibrium partition of KCl into nanofiltration membranes based on applied electrical potential and rejection[J]. Langmuir, 2011, 27(16): 10274-10281. |
| 11 | TuC H, WangH L, WangX L. Study on transmembrane electrical potential of nanofiltration membranes in KCl and MgCl2 solutions[J]. Langmuir, 2010, 26(22): 17656-17664. |
| 12 | TuC H, WuL, WangD X, et al. Prediction of separation performance of NF membranes for mixed electrolytes solution[J]. Desalination, 2010, 260(1/2/3): 218-224. |
| 13 | WangD X, SuM, YuZ Y, et al. Separation performance of a nanofiltration membrane influenced by species and concentration of ions[J]. Desalination, 2005, 175(2): 219-225. |
| 14 | SuM, WangD X, WangX L, et al. Rejection of ions by NF membranes for binary electrolyte solutions of NaCl, NaNO3, CaCl2 and Ca(NO3)2[J]. Desalination, 2006, 191(1/2/3): 303-308. |
| 15 | MurthyZ V P, ChaudhariL B. Rejection behavior of nickel ions from synthetic wastewater containing Na2SO4, NiSO4, MgCl2 and CaCl2 salts by nanofiltration and characterization of the membrane[J]. Desalination, 2009, 247(1/2/3): 610-622. |
| 16 | WuL, SongL, WangX L, et al. Experimental study on separation performance of nanofiltration membranes for bicarbonate salts solution[J]. Desalination, 2009, 236(1/2/3): 299-305. |
| 17 | TanninenJ, ManttariM, NystromM. Effect of salt mixture concentration on fractionation with NF membranes[J]. Journal of Membrane Science, 2006, 283(1/2): 57-64. |
| 18 | Al-ZoubiH, OmarW. Rejection of salt mixtures from high saline by nanofiltration membranes[J]. Korean Journal of Chemical Engineering, 2009, 26(3): 799-805. |
| 19 | SilvaV, GeraldesV, Brites AlvesA M, et al. Multi-ionic nanofiltration of highly concentrated salt mixtures in the seawater range[J]. Desalination, 2011, 277(1/2/3): 29-39. |
| 20 | HilalN, KochkodanV, Al AbdulgaderH, et al. A combined ion exchange–nanofiltration process for water desalination(Ⅱ): Membrane selection[J]. Desalination, 2015, 363(S1): 51-57. |
| 21 | YaroshchukA E. Non-steric mechanisms of nanofiltration: superposition of Donnan and dielectric exclusion[J]. Separation and Purification Technology, 2001, 22/22/23(S1): 143-158. |
| 22 | 吴哲峰. 草甘膦生产线上副产固体盐精制的研究[D]. 浙江: 浙江理工大学, 2015. |
| WuZ F. Study on the purification of the buproduct salt from the glyphosate production process[D]. Zhejiang: Zhejiang Sci-tech University, 2015. | |
| 23 | XieM, LiuZ Y, XuY H, Removal of glyphosate in neutralization liquor from the glycine-dimethylphosphit process by nanofiltration[J]. Journal of Hazardous Materials, 2010, 181(1/2/3): 975-980. |
| 24 | 谢明, 刘志英, 赵贤广, 等. 草甘膦模拟废水的纳滤分离过程研究[J]. 环境工程学报, 2010, 4(7): 1483-1487. |
| XieM, LiuZ Y, ZhaoX G, et al. Nanofiltration separation process of model glyphosate wastewater[J]. Chinese Journal of Environmental Engineering, 2010, 4(7): 1483-1487. | |
| 25 | 黄华, 李雪梅, 潘成, 等. SPEEK涂层纳滤膜处理草甘膦废水[J]. 环境工程学报, 2012, 6(8): 2489-2494. |
| HuangH, LiX M, PanC, et al. Treatment of glyphosate containing wastewater using SPEEK coated nanofiltration membranes[J]. Chinese Journal of Environmental Engineering, 2012, 6(8): 2489-2494. | |
| 26 | SongJ F, LiX M, AlbetoF, et al. Composite hollow fiber nanofiltration membranes for recovery of glyphosate from saline wastewater[J]. Water Research, 2013, 47(6): 2065-2074. |
| 27 | YuanJ, DuanJ M, SaintC P. Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration[J]. Environmental Technology, 2018, 39(11): 1384-1392. |
| 28 | 徐演初, 吴礼光, 沈江南, 等. 一种集成膜分离浓缩草甘膦母液方法: 1775786B [P]. 2011. |
| XuY C, WuL G, ShenJ N, et al. A method for separating and separating glyphosate mother liquor by integrated membrane: 1775786B [P]. 2011. | |
| 29 | ShenJ N, HuangJ, RuanH M, et al. Techno-economic analysis of resource recovery of glyphosate liquor by membrane technology[J]. Desalination, 2014, 342(S1): 118-125. |
| 30 | ShenJ N, HuangJ, LiuL F, et al. The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge[J]. Journal of Hazardous Materials, 2013, 260(18): 660-667. |
| 31 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 草甘膦原药: GB/T 12686—2017[S]. 北京: 中国标准出版社, 2017. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Glyphosate technical: GB/T 12686—2017[S]. Beijing: Standards Press of China, 2017. | |
| 32 | SpieglerK S, KedemO. Thermodynamics of hyperfiltration (reverse osmosis): criteria for efficient membranes[J]. Desalination 1966, 1(S1): 311-326. |
| 33 | SchockG, MiquelA. Mass transfer and pressure loss in spiral wound modules[J]. Desalination, 1987, 64(S1): 339-352. |
| 34 | HilalN, Al-zoubiH, MohammadA W, et al. Nanofiltration of highly concentrated salt solutions up to seawater salinity [J]. Desalination, 2005, 184(1/2/3): 315-326. |
| 35 | 燕至琴. 高浓一二价盐溶液的纳滤膜分离性能研究[D]. 北京: 清华大学, 2016. |
| YanZ Q. Research on separation performance of mono and divalent salt solution with high concentration by nanofiltration membrane [D]. Beijing: Tsinghua University, 2016. |
| [1] | Baiyu YANG, Yue KOU, Juntao JIANG, Yali ZHAN, Qinghong WANG, Chunmao CHEN. Chemical conversion of dissolved organic matter in petrochemical spent caustic along a wet air oxidation pretreatment process [J]. CIESC Journal, 2023, 74(9): 3912-3920. |
| [2] | Yaxin ZHAO, Xueqin ZHANG, Rongzhu WANG, Guo SUN, Shanjing YAO, Dongqiang LIN. Removal of monoclonal antibody aggregates with ion exchange chromatography by flow-through mode [J]. CIESC Journal, 2023, 74(9): 3879-3887. |
| [3] | Lei XING, Chunyu MIAO, Minghu JIANG, Lixin ZHAO, Xinya LI. Optimal design and performance analysis of downhole micro gas-liquid hydrocyclone [J]. CIESC Journal, 2023, 74(8): 3394-3406. |
| [4] | Jiayi ZHANG, Jiali HE, Jiangpeng XIE, Jian WANG, Yu ZHAO, Dongqiang ZHANG. Research progress of pervaporation technology for N-methylpyrrolidone recovery in lithium battery production [J]. CIESC Journal, 2023, 74(8): 3203-3215. |
| [5] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [6] | Xin YANG, Xiao PENG, Kairu XUE, Mengwei SU, Yan WU. Preparation of molecularly imprinted-TiO2 and its properties of photoelectrocatalytic degradation of solubilized PHE [J]. CIESC Journal, 2023, 74(8): 3564-3571. |
| [7] | Shuang LIU, Linzhou ZHANG, Zhiming XU, Suoqi ZHAO. Study on molecular level composition correlation of viscosity of residual oil and its components [J]. CIESC Journal, 2023, 74(8): 3226-3241. |
| [8] | Yuanliang ZHANG, Xinqi LUAN, Weige SU, Changhao LI, Zhongxing ZHAO, Liqin ZHOU, Jianmin CHEN, Yan HUANG, Zhenxia ZHAO. Study on selective extraction of nicotine by ionic liquids composite extractant and DFT calculation [J]. CIESC Journal, 2023, 74(7): 2947-2956. |
| [9] | Jinming GAO, Yujiao GUO, Chenglin E, Chunxi LU. Study on the separation characteristics of a downstream gas-liquid vortex separator in a closed hood [J]. CIESC Journal, 2023, 74(7): 2957-2966. |
| [10] | Zhaolun WEN, Peirui LI, Zhonglin ZHANG, Xiao DU, Qiwang HOU, Yegang LIU, Xiaogang HAO, Guoqing GUAN. Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration [J]. CIESC Journal, 2023, 74(7): 2988-2998. |
| [11] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| [12] | Xingchi ZHU, Zhiyuan GUO, Zhiyong JI, Jing WANG, Panpan ZHANG, Jie LIU, Yingying ZHAO, Junsheng YUAN. Simulation and optimization of selective electrodialysis magnesium and lithium separation process [J]. CIESC Journal, 2023, 74(6): 2477-2485. |
| [13] | Yanmei ZHANG, Tao YUAN, Jiang LI, Yajie LIU, Zhanxue SUN. Study on the construction of high-efficient SRB mixed microflora and its performance under acid stress [J]. CIESC Journal, 2023, 74(6): 2599-2610. |
| [14] | Lei WANG, Lei WANG, Yunlong BAI, Liuliu HE. Preparation of SA lithium ion sieve membrane and its adsorptive properties [J]. CIESC Journal, 2023, 74(5): 2046-2056. |
| [15] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||