CIESC Journal ›› 2020, Vol. 71 ›› Issue (11): 5159-5168.DOI: 10.11949/0438-1157.20200252
• Fluid dynamics and transport phenomena • Previous Articles Next Articles
Wanqiang LIU( ),Fan YANG(
),Fan YANG( ),Hua YUAN,Yuanda ZHANG,Pinggui YI,Hu ZHOU(
),Hua YUAN,Yuanda ZHANG,Pinggui YI,Hu ZHOU( )
)
Received:2020-03-12
Revised:2020-06-08
Online:2020-11-05
Published:2020-11-05
Contact:
Hu ZHOU
通讯作者:
周虎
作者简介:刘万强(1971—),男,博士,副教授,基金资助:CLC Number:
Wanqiang LIU,Fan YANG,Hua YUAN,Yuanda ZHANG,Pinggui YI,Hu ZHOU. Molecular dynamics simulation and mechanism study on thermal conductivity of alcohols[J]. CIESC Journal, 2020, 71(11): 5159-5168.
刘万强,杨帆,袁华,张远达,易平贵,周虎. 醇类有机物热传导的分子动力学模拟及微观机理研究[J]. 化工学报, 2020, 71(11): 5159-5168.
Add to citation manager EndNote|Ris|BibTeX
| Method | Compound | Deviation/% | Ref. |
|---|---|---|---|
| NEMD | alcohols | 18—30 | [ |
| BD-NEMD | methanol and ethanol | 5 | [ |
| Green-Kubo | ethanol | 10 | [ |
| Green-Kubo | ethylene glycol | 20—30 | [ |
Table 1 Results of some MD simulation for calculating thermal conductivity of alcohols
| Method | Compound | Deviation/% | Ref. |
|---|---|---|---|
| NEMD | alcohols | 18—30 | [ |
| BD-NEMD | methanol and ethanol | 5 | [ |
| Green-Kubo | ethanol | 10 | [ |
| Green-Kubo | ethylene glycol | 20—30 | [ |
| Compound | λ273K/(W·m-1·K-1) | λ288K/(W·m-1·K-1) | λ298K/(W·m-1·K-1) | λ323K/(W·m-1·K-1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | |
| ethanol | 0.172 | 0.175 | -1.71 | 0.169 | 0.171 | -1.17 | 0.170 | 0.168 | 1.19 | 0.167 | 0.162 | 3.09 |
| propanol | 0.163 | 0.162 | 0.62 | 0.159 | 0.158 | 0.63 | 0.163 | 0.156 | 4.49 | 0.150 | 0.151 | -0.66 |
| butanol | 0.160 | 0.158 | 1.27 | 0.157 | 0.155 | 1.29 | 0.150 | 0.153 | -1.96 | 0.149 | 0.148 | 0.67 |
| pentanol | 0.155 | 0.157 | -1.27 | 0.152 | 0.154 | -1.30 | 0.156 | 0.153 | 1.96 | 0.152 | 0.149 | 2.01 |
| hexanol | 0.164 | 0.159 | 3.14 | 0.151 | 0.156 | -3.21 | 0.154 | 0.154 | 0.00 | 0.146 | 0.148 | -1.35 |
| heptanol | 0.165 | 0.162 | 1.85 | 0.158 | 0.159 | -0.63 | 0.150 | 0.157 | -4.46 | 0.148 | 0.151 | -1.99 |
| octanol | 0.166 | 0.166 | 0.00 | 0.170 | 0.162 | 4.94 | 0.159 | 0.160 | -0.63 | 0.155 | 0.154 | 0.65 |
| nonanol | 0.160 | 0.166 | -3.61 | 0.161 | 0.163 | -1.23 | 0.161 | 0.161 | 0.00 | 0.155 | 0.155 | 0.00 |
Table 2 Comparison of thermal conductivity of experimental values and simulated values of alcohols obtained by NEMD simulation
| Compound | λ273K/(W·m-1·K-1) | λ288K/(W·m-1·K-1) | λ298K/(W·m-1·K-1) | λ323K/(W·m-1·K-1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | Cal. | Exp. | RD/% | |
| ethanol | 0.172 | 0.175 | -1.71 | 0.169 | 0.171 | -1.17 | 0.170 | 0.168 | 1.19 | 0.167 | 0.162 | 3.09 |
| propanol | 0.163 | 0.162 | 0.62 | 0.159 | 0.158 | 0.63 | 0.163 | 0.156 | 4.49 | 0.150 | 0.151 | -0.66 |
| butanol | 0.160 | 0.158 | 1.27 | 0.157 | 0.155 | 1.29 | 0.150 | 0.153 | -1.96 | 0.149 | 0.148 | 0.67 |
| pentanol | 0.155 | 0.157 | -1.27 | 0.152 | 0.154 | -1.30 | 0.156 | 0.153 | 1.96 | 0.152 | 0.149 | 2.01 |
| hexanol | 0.164 | 0.159 | 3.14 | 0.151 | 0.156 | -3.21 | 0.154 | 0.154 | 0.00 | 0.146 | 0.148 | -1.35 |
| heptanol | 0.165 | 0.162 | 1.85 | 0.158 | 0.159 | -0.63 | 0.150 | 0.157 | -4.46 | 0.148 | 0.151 | -1.99 |
| octanol | 0.166 | 0.166 | 0.00 | 0.170 | 0.162 | 4.94 | 0.159 | 0.160 | -0.63 | 0.155 | 0.154 | 0.65 |
| nonanol | 0.160 | 0.166 | -3.61 | 0.161 | 0.163 | -1.23 | 0.161 | 0.161 | 0.00 | 0.155 | 0.155 | 0.00 |
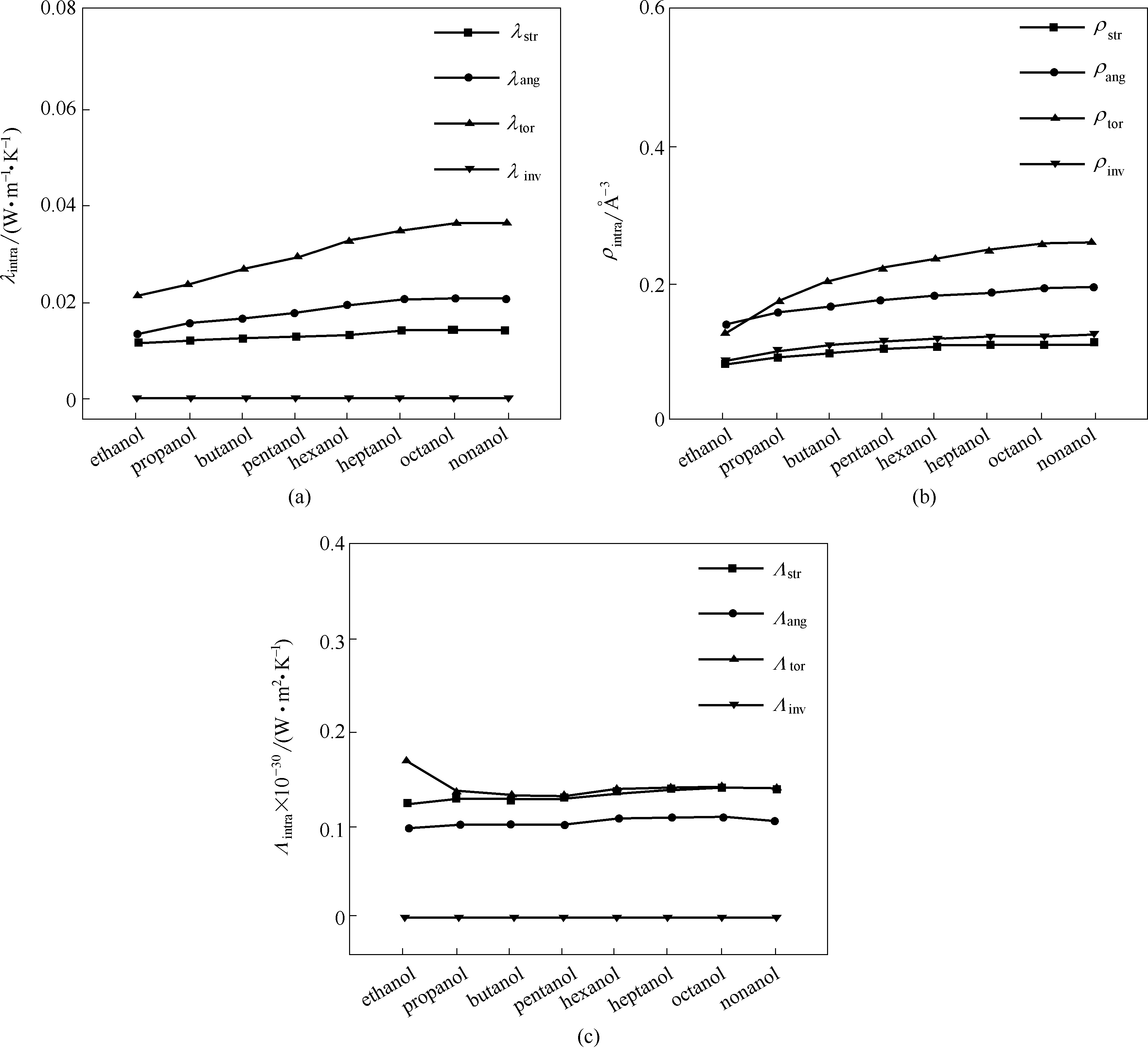
Fig.4 The partial thermal conductivity λintra(a), the density ρintra(b), and the efficiency Λintra(c) of intramolecular interaction paths for alcohols at 273 K, respectively
| Compound | ρ273K/(g?cm-3) | ρ288K/(g?cm-3) | ρ298K/(g?cm-3) | ρ323K/(g?cm-3) | ||||
|---|---|---|---|---|---|---|---|---|
| Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | |
| ethanol | 0.808 | 0.751 | 0.795 | 0.741 | 0.786 | 0.722 | 0.763 | 0.706 |
| propanol | 0.822 | 0.755 | 0.809 | 0.744 | 0.800 | 0.736 | 0.778 | 0.709 |
| butanol | 0.826 | 0.763 | 0.814 | 0.745 | 0.806 | 0.747 | 0.785 | 0.713 |
| pentanol | 0.831 | 0.768 | 0.820 | 0.757 | 0.812 | 0.747 | 0.792 | 0.724 |
| hexanol | 0.834 | 0.772 | 0.823 | 0.757 | 0.816 | 0.749 | 0.798 | 0.727 |
| heptanol | 0.838 | 0.772 | 0.827 | 0.759 | 0.820 | 0.746 | 0.801 | 0.726 |
| octanol | 0.841 | 0.770 | 0.830 | 0.762 | 0.823 | 0.752 | 0.804 | 0.734 |
| nonanol | 0.841 | 0.769 | 0.831 | 0.762 | 0.825 | 0.751 | 0.808 | 0.738 |
Table A1 Experimental and calculated values of mass density of simulated systems at different temperatures
| Compound | ρ273K/(g?cm-3) | ρ288K/(g?cm-3) | ρ298K/(g?cm-3) | ρ323K/(g?cm-3) | ||||
|---|---|---|---|---|---|---|---|---|
| Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | Exp. | Cal. | |
| ethanol | 0.808 | 0.751 | 0.795 | 0.741 | 0.786 | 0.722 | 0.763 | 0.706 |
| propanol | 0.822 | 0.755 | 0.809 | 0.744 | 0.800 | 0.736 | 0.778 | 0.709 |
| butanol | 0.826 | 0.763 | 0.814 | 0.745 | 0.806 | 0.747 | 0.785 | 0.713 |
| pentanol | 0.831 | 0.768 | 0.820 | 0.757 | 0.812 | 0.747 | 0.792 | 0.724 |
| hexanol | 0.834 | 0.772 | 0.823 | 0.757 | 0.816 | 0.749 | 0.798 | 0.727 |
| heptanol | 0.838 | 0.772 | 0.827 | 0.759 | 0.820 | 0.746 | 0.801 | 0.726 |
| octanol | 0.841 | 0.770 | 0.830 | 0.762 | 0.823 | 0.752 | 0.804 | 0.734 |
| nonanol | 0.841 | 0.769 | 0.831 | 0.762 | 0.825 | 0.751 | 0.808 | 0.738 |
| Compound | Jstr/ (MW·m-2) | Jang/ (MW·m-2) | Jtor/ (MW·m-2) | Jinv/ (MW·m-2) | JvdW/ (MW·m-2) | JCl/ (MW·m-2) | Jpot/ (MW·m-2) | Jkin/ (MW·m-2) |
|---|---|---|---|---|---|---|---|---|
| 273 K | ||||||||
| ethanol | 82.35061 | 105.5749 | 173.2012 | 1.166184 | 93.33839 | 286.4405 | 371.678 | 248.0089 |
| propanol | 95.427 | 130.7635 | 196.4533 | 0.585375 | 93.64934 | 412.9896 | 140.9174 | 280.7405 |
| butanol | 102.2969 | 144.5943 | 230.6405 | 0.25144 | 90.01795 | 331.163 | 160.8052 | 295.7485 |
| pentanol | 107.868 | 154.3851 | 253.6832 | 0.000517 | 87.71279 | 291.5588 | 152.6921 | 307.3169 |
| hexanol | 111.1385 | 161.5822 | 272.2318 | 0.192211 | 85.97666 | 262.5442 | 149.1838 | 314.8754 |
| heptanol | 114.1706 | 166.809 | 285.5701 | 0.332879 | 83.79046 | 238.7217 | 143.6668 | 320.7745 |
| octanol | 116.1588 | 170.8392 | 295.6281 | 0.456438 | 81.58639 | 220.2334 | 138.452 | 324.2486 |
| nonanol | 118.2226 | 174.2786 | 304.655 | 0.546975 | 80.09264 | 204.8891 | 135.421 | 328.5367 |
| 288 K | ||||||||
| ethanol | 88.59901 | 113.6205 | 174.6725 | 1.23144 | 93.83133 | 267.6399 | 356.5573 | 267.6399 |
| propanol | 99.90495 | 136.3767 | 194.1579 | 0.605893 | 91.07394 | 417.0604 | 118.3188 | 294.1697 |
| butanol | 107.0843 | 150.4192 | 226.3646 | 0.262161 | 85.5488 | 333.7362 | 133.8917 | 309.7504 |
| pentanol | 113.2669 | 161.7038 | 250.8701 | 0.006098 | 83.09045 | 296.5954 | 125.7172 | 322.7532 |
| hexanol | 116.3273 | 168.5521 | 268.0233 | 0.19607 | 81.54108 | 265.5234 | 122.2882 | 329.8472 |
| heptanol | 119.7637 | 174.7064 | 281.0965 | 0.356169 | 79.75569 | 241.4785 | 116.8366 | 336.7934 |
| octanol | 118.6948 | 174.2466 | 293.9069 | 0.474525 | 80.82509 | 221.6068 | 128.2734 | 331.6131 |
| nonanol | 123.9406 | 182.1128 | 301.084 | 0.569318 | 77.058 | 207.7246 | 111.1315 | 344.7189 |
| 298 K | ||||||||
| ethanol | 87.98672 | 112.9572 | 168.2807 | 1.206443 | 88.32364 | 293.7441 | 331.1771 | 266.7173 |
| propanol | 102.5047 | 139.5892 | 192.5958 | 0.615713 | 89.51541 | 419.0179 | 105.4844 | 301.6295 |
| butanol | 110.7437 | 155.6268 | 226.0706 | 0.264945 | 86.09783 | 338.6185 | 121.7853 | 320.4123 |
| pentanol | 112.9239 | 160.9453 | 241.8836 | 0.004015 | 79.95448 | 323.2599 | 109.6565 | 323.2599 |
| hexanol | 119.3759 | 172.3581 | 265.5312 | 0.207462 | 79.42465 | 267.028 | 107.6302 | 338.3892 |
| heptanol | 122.6607 | 177.7654 | 278.0849 | 0.36209 | 76.12581 | 243.1349 | 100.1216 | 344.334 |
| octanol | 125.058 | 182.0887 | 289.8456 | 0.483914 | 75.27087 | 224.3338 | 99.52106 | 348.9841 |
| nonanol | 126.9109 | 186.5117 | 297.3773 | 0.587973 | 73.19684 | 208.9527 | 93.39459 | 354.1787 |
| 323 K | ||||||||
| ethanol | 94.44256 | 121.7495 | 164.777 | 1.270205 | 86.1226 | 304.3557 | 294.614 | 289.2095 |
| propanol | 109.4977 | 148.8176 | 187.5502 | 0.645945 | 83.78129 | 425.1214 | 64.5793 | 323.3033 |
| butanol | 117.2486 | 164.5488 | 218.7043 | 0.269378 | 78.09459 | 343.464 | 76.82576 | 340.5539 |
| pentanol | 123.3485 | 175.3366 | 242.1652 | 0.007284 | 75.84128 | 303.4792 | 71.65321 | 353.422 |
| hexanol | 128.1472 | 183.9092 | 258.5265 | 0.214408 | 72.87701 | 272.8254 | 63.01978 | 363.5166 |
| heptanol | 131.455 | 190.117 | 270.929 | 0.387389 | 69.7516 | 248.777 | 55.96572 | 370.4024 |
| octanol | 134.7473 | 195.9925 | 282.6226 | 0.526943 | 69.26389 | 230.4936 | 53.36009 | 377.35 |
| nonanol | 136.9088 | 199.7251 | 291.0947 | 0.631816 | 67.9927 | 214.095 | 51.09756 | 381.8325 |
Table A2 Proportions of each type of heat flux of alcohol organics at different temperatures obtained by heat flux decomposition, respectively
| Compound | Jstr/ (MW·m-2) | Jang/ (MW·m-2) | Jtor/ (MW·m-2) | Jinv/ (MW·m-2) | JvdW/ (MW·m-2) | JCl/ (MW·m-2) | Jpot/ (MW·m-2) | Jkin/ (MW·m-2) |
|---|---|---|---|---|---|---|---|---|
| 273 K | ||||||||
| ethanol | 82.35061 | 105.5749 | 173.2012 | 1.166184 | 93.33839 | 286.4405 | 371.678 | 248.0089 |
| propanol | 95.427 | 130.7635 | 196.4533 | 0.585375 | 93.64934 | 412.9896 | 140.9174 | 280.7405 |
| butanol | 102.2969 | 144.5943 | 230.6405 | 0.25144 | 90.01795 | 331.163 | 160.8052 | 295.7485 |
| pentanol | 107.868 | 154.3851 | 253.6832 | 0.000517 | 87.71279 | 291.5588 | 152.6921 | 307.3169 |
| hexanol | 111.1385 | 161.5822 | 272.2318 | 0.192211 | 85.97666 | 262.5442 | 149.1838 | 314.8754 |
| heptanol | 114.1706 | 166.809 | 285.5701 | 0.332879 | 83.79046 | 238.7217 | 143.6668 | 320.7745 |
| octanol | 116.1588 | 170.8392 | 295.6281 | 0.456438 | 81.58639 | 220.2334 | 138.452 | 324.2486 |
| nonanol | 118.2226 | 174.2786 | 304.655 | 0.546975 | 80.09264 | 204.8891 | 135.421 | 328.5367 |
| 288 K | ||||||||
| ethanol | 88.59901 | 113.6205 | 174.6725 | 1.23144 | 93.83133 | 267.6399 | 356.5573 | 267.6399 |
| propanol | 99.90495 | 136.3767 | 194.1579 | 0.605893 | 91.07394 | 417.0604 | 118.3188 | 294.1697 |
| butanol | 107.0843 | 150.4192 | 226.3646 | 0.262161 | 85.5488 | 333.7362 | 133.8917 | 309.7504 |
| pentanol | 113.2669 | 161.7038 | 250.8701 | 0.006098 | 83.09045 | 296.5954 | 125.7172 | 322.7532 |
| hexanol | 116.3273 | 168.5521 | 268.0233 | 0.19607 | 81.54108 | 265.5234 | 122.2882 | 329.8472 |
| heptanol | 119.7637 | 174.7064 | 281.0965 | 0.356169 | 79.75569 | 241.4785 | 116.8366 | 336.7934 |
| octanol | 118.6948 | 174.2466 | 293.9069 | 0.474525 | 80.82509 | 221.6068 | 128.2734 | 331.6131 |
| nonanol | 123.9406 | 182.1128 | 301.084 | 0.569318 | 77.058 | 207.7246 | 111.1315 | 344.7189 |
| 298 K | ||||||||
| ethanol | 87.98672 | 112.9572 | 168.2807 | 1.206443 | 88.32364 | 293.7441 | 331.1771 | 266.7173 |
| propanol | 102.5047 | 139.5892 | 192.5958 | 0.615713 | 89.51541 | 419.0179 | 105.4844 | 301.6295 |
| butanol | 110.7437 | 155.6268 | 226.0706 | 0.264945 | 86.09783 | 338.6185 | 121.7853 | 320.4123 |
| pentanol | 112.9239 | 160.9453 | 241.8836 | 0.004015 | 79.95448 | 323.2599 | 109.6565 | 323.2599 |
| hexanol | 119.3759 | 172.3581 | 265.5312 | 0.207462 | 79.42465 | 267.028 | 107.6302 | 338.3892 |
| heptanol | 122.6607 | 177.7654 | 278.0849 | 0.36209 | 76.12581 | 243.1349 | 100.1216 | 344.334 |
| octanol | 125.058 | 182.0887 | 289.8456 | 0.483914 | 75.27087 | 224.3338 | 99.52106 | 348.9841 |
| nonanol | 126.9109 | 186.5117 | 297.3773 | 0.587973 | 73.19684 | 208.9527 | 93.39459 | 354.1787 |
| 323 K | ||||||||
| ethanol | 94.44256 | 121.7495 | 164.777 | 1.270205 | 86.1226 | 304.3557 | 294.614 | 289.2095 |
| propanol | 109.4977 | 148.8176 | 187.5502 | 0.645945 | 83.78129 | 425.1214 | 64.5793 | 323.3033 |
| butanol | 117.2486 | 164.5488 | 218.7043 | 0.269378 | 78.09459 | 343.464 | 76.82576 | 340.5539 |
| pentanol | 123.3485 | 175.3366 | 242.1652 | 0.007284 | 75.84128 | 303.4792 | 71.65321 | 353.422 |
| hexanol | 128.1472 | 183.9092 | 258.5265 | 0.214408 | 72.87701 | 272.8254 | 63.01978 | 363.5166 |
| heptanol | 131.455 | 190.117 | 270.929 | 0.387389 | 69.7516 | 248.777 | 55.96572 | 370.4024 |
| octanol | 134.7473 | 195.9925 | 282.6226 | 0.526943 | 69.26389 | 230.4936 | 53.36009 | 377.35 |
| nonanol | 136.9088 | 199.7251 | 291.0947 | 0.631816 | 67.9927 | 214.095 | 51.09756 | 381.8325 |
| 1 | Vargaftik N B. Handbook of Thermal Conductivity of Liquids and Gases[M]. Boca Raton FL: CRC press, 1994. |
| 2 | Algaer E A, Müller-Plathe F. Molecular dynamics calculations of the thermal conductivity of molecular liquids, polymers, and carbon nanotubes[J]. Soft Materials, 2012, 10(1/2/3): 42-80. |
| 3 | Bao H, Chen J, Gu X, et al. A review of simulation methods in micro/nanoscale heat conduction[J]. ES Energy & Environment, 2018, 1: 16-55. |
| 4 | Assael M J, Antoniadis K D, Wakeham W A. Historical evolution of the transient hot-wire technique[J]. International Journal of Thermophysics, 2010, 31(6): 1051-1072. |
| 5 | Nagvekar M, Daubert T E. A group contribution method for liquid thermal conductivity[J]. Industrial & Engineering Chemistry Research, 1987, 26(7): 1362-1365. |
| 6 | Rodenbush C M, Viswanath D S, Hsieh F. A group contribution method for the prediction of thermal conductivity of liquids and its application to the prandtl number for vegetable oils[J]. Industrial & Engineering Chemistry Research, 1999, 38(11): 4513-4519. |
| 7 | Tsotsas E, Schluender E U. Numerical calculation of the thermal conductivity of two regular bidispersed beds of spherical particles[J]. Computers & Chemical Engineering, 1990, 14(9): 1031-1038. |
| 8 | Liu W, Lu H, Cao C, et al. An improved quantitative structure property relationship model for predicting thermal conductivity of liquid aliphatic alcohols[J]. Journal of Chemical and Engineering Data, 2018, 63(12): 4735-4740. |
| 9 | Lu H, Yang F, Liu W, et al. A robust model for estimating thermal conductivity of liquid alkyl halides[J]. SAR and QSAR in Environmental Research, 2020, 31(2): 73-85. |
| 10 | Ohara T. Contribution of intermolecular energy transfer to heat conduction in a simple liquid[J]. Journal of Chemical Physics, 1999, 111(21): 9667-9672. |
| 11 | Ohara T. Intermolecular energy transfer in liquid water and its contribution to heat conduction: a molecular dynamics study[J]. Journal of Chemical Physics, 1999, 111(14): 6492-6500. |
| 12 | Zhang M, Lussetti E, de Souza L E S, et al. Thermal conductivities of molecular liquids by reverse nonequilibrium molecular dynamics[J]. Journal of Physical Chemistry B, 2005, 109(31): 15060-15067. |
| 13 | 李嘉辰, 俞斌, 王琦, 等. 分子模拟研究壳聚糖-氮化硼纳米管封装及输运阿霉素[J]. 化工学报, 2020, 71(1): 354-360. |
| Li J C, Yu B, Wang Q, et al. Molecular simulation on doxorubicin encapsulation and transport by chitosan-boron nitride nanotubes[J]. CIESC Journal, 2020, 71(1): 354-360. | |
| 14 | 王韬, 刘向阳, 何茂刚. 离子液体[bmim][Tf2N]的分子动力学模拟[J]. 化工学报, 2019, 70: 258-264. |
| Wang T, Liu X Y, He M G. Molecular dynamics simulation of ionic liquid [bmim][Tf2N][J]. CIESC Journal, 2019, 70: 258-264. | |
| 15 | 杨慧芳, 关海莲, 李平, 等. 煤颗粒燃烧过程氧化机理及有机氮转化的分子模拟: 以宁东红石湾煤为例[J]. 化工学报, 2020, 71(2): 799-810. |
| Yang H F, Guan H L, Li P, et al. Molecular modeling of oxidation mechanism and organic nitrogen conversion in coal particle combustion: a case study on HSW coal of Ningdong[J]. CIESC Journal, 2020, 71(2): 799-810. | |
| 16 | 张博, 何依然, 刘迎春, 等. 异喹啉类生物碱和G-四链体结合的分子动力学研究[J]. 化工学报, 2020, 71(1): 344-353. |
| Zhang B, He Y R, Liu Y C, et al. Molecular dynamics study of binding of isoquinoline alkaloids to G-quadruplex[J]. CIESC Journal, 2020, 71(1): 344-353. | |
| 17 | 邹瀚影, 冯妍卉, 邱琳, 等. 十八烷酸热传导机制的尺度效应研究[J]. 化工学报, 2019, 70: 155-160. |
| Zou H Y, Feng Y H, Qiu L, et al. Size effect of heat conduction mechanism on stearic acid[J]. CIESC Journal, 2019, 70: 155-160. | |
| 18 | Matsubara H, Kikugawa G, Bessho T, et al. Understanding the chain length dependence of thermal conductivity of liquid alcohols at 298 K on the basis of molecular-scale energy transfer[J]. Fluid Phase Equilibria, 2017, 441: 24-32. |
| 19 | Guevara-Carrion G, Nieto-Draghi C, Vrabec J, et al. Prediction of transport properties by molecular simulation: methanol and ethanol and their mixture[J]. Journal of Physical Chemistry B, 2008, 112(51): 16664-16674. |
| 20 | Petravic J. Thermal conductivity of ethanol[J]. Journal of Chemical Physics, 2005, 123: 174503. |
| 21 | Lin Y, Hsiao P, Chieng C. Constructing a force interaction model for thermal conductivity computation using molecular dynamics simulation: ethylene glycol as an example[J]. Journal of Chemical Physics, 2011, 134(15): 154509. |
| 22 | Fan Z, Pereira L F C, Wang H, et al. Force and heat current formulas for many-body potentials in molecular dynamics simulations with applications to thermal conductivity calculations[J]. Physical Review B, 2015, 92(9): 94301. |
| 23 | Torii D, Nakano T, Ohara T. Contribution of inter- and intramolecular energy transfers to heat conduction in liquids[J]. Journal of Chemical Physics, 2008, 128(4): 44504. |
| 24 | Matsubara H, Kikugawa G, Bessho T, et al. Effects of molecular structure on microscopic heat transport in chain polymer liquids[J]. Journal of Chemical Physics, 2015, 142(16): 164509. |
| 25 | Lv W, Henry A. Direct calculation of modal contributions to thermal conductivity via Green-Kubo modal analysis[J]. New Journal of Physics, 2016, 18(1): 13028. |
| 26 | Sun H, Mumby S J, Maple J R, et al. An ab initio CFF93 all-atom force field for polycarbonates[J]. Journal of the American Chemical Society, 1994, 116(7): 2978-2987. |
| 27 | Zhu W, Wang X, Xiao J, et al. Molecular dynamics simulations of AP/HMX composite with a modified force field[J]. Journal of Hazardous Materials, 2009, 167(1): 810-816. |
| 28 | Sun Y, Chen L, Cui L, et al. Molecular dynamics simulation of cross-linked epoxy resin and its interaction energy with graphene under two typical force fields[J]. Computational Materials Science, 2018, 143: 240-247. |
| 29 | Ikeshoji T, Hafskjold B. Non-equilibrium molecular dynamics calculation of heat conduction in liquid and through liquid-gas interface[J]. Molecular Physics, 1994, 81(2): 251-261. |
| 30 | Jund P, Jullien R. Molecular-dynamics calculation of the thermal conductivity of vitreous silica[J]. Physical Review B, 1999, 59(21): 13707. |
| 31 | Brown W M, Yamada M. Implementing molecular dynamics on hybrid high performance computers—three-body potentials[J]. Computer Physics Communications, 2013, 184(12): 2785-2793. |
| 32 | Brown W M, Kohlmeyer A, Plimpton S J, et al. Implementing molecular dynamics on hybrid high performance computers—particle-particle particle-mesh[J]. Computer Physics Communications, 2012, 183(3): 449-459. |
| 33 | Brown W M, Wang P, Plimpton S J, et al. Implementing molecular dynamics on hybrid high performance computers—short range forces[J]. Computer Physics Communications, 2011, 182(4): 898-911. |
| 34 | Nguyen T D. GPU-accelerated Tersoff potentials for massively parallel molecular dynamics simulations[J]. Computer Physics Communications, 2017, 212: 113-122. |
| 35 | Nguyen T D, Plimpton S J. Accelerating dissipative particle dynamics simulations for soft matter systems[J]. Computational Materials Science, 2015, 100: 173-180. |
| 36 | Plimpton S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| 37 | Boone P, Babaei H, Wilmer C E. Heat flux for many-body interactions: corrections to LAMMPS[J]. Journal of Chemical Theory and Computation, 2019, 15(10): 5579-5587. |
| 38 | Wirnsberger P, Frenkel D, Dellago C. An enhanced version of the heat exchange algorithm with excellent energy conservation properties[J]. Journal of Chemical Physics, 2015, 143(12): 124104. |
| 39 | Hoover W G. Canonical dynamics: equilibrium phase-space distributions[J]. Physical Review A, 1985, 31(3): 1695-1697. |
| 40 | Ohara T, Chia Yuan T, Torii D, et al. Heat conduction in chain polymer liquids: molecular dynamics study on the contributions of inter- and intramolecular energy transfer[J]. Journal of Chemical Physics, 2011, 135(3): 34507. |
| [1] | Shuangxing ZHANG, Fangchen LIU, Yifei ZHANG, Wenjing DU. Experimental study on phase change heat storage and release performance of R-134a pulsating heat pipe [J]. CIESC Journal, 2023, 74(S1): 165-171. |
| [2] | Yifei ZHANG, Fangchen LIU, Shuangxing ZHANG, Wenjing DU. Performance analysis of printed circuit heat exchanger for supercritical carbon dioxide [J]. CIESC Journal, 2023, 74(S1): 183-190. |
| [3] | Aiqiang CHEN, Yanqi DAI, Yue LIU, Bin LIU, Hanming WU. Influence of substrate temperature on HFE7100 droplet evaporation process [J]. CIESC Journal, 2023, 74(S1): 191-197. |
| [4] | Mingxi LIU, Yanpeng WU. Simulation analysis of effect of diameter and length of light pipes on heat transfer [J]. CIESC Journal, 2023, 74(S1): 206-212. |
| [5] | Zhiguo WANG, Meng XUE, Yushuang DONG, Tianzhen ZHANG, Xiaokai QIN, Qiang HAN. Numerical simulation and analysis of geothermal rock mass heat flow coupling based on fracture roughness characterization method [J]. CIESC Journal, 2023, 74(S1): 223-234. |
| [6] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [7] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [8] | Yubing WANG, Jie LI, Hongbo ZHAN, Guangya ZHU, Dalin ZHANG. Experimental study on flow boiling heat transfer of R134a in mini channel with diamond pin fin array [J]. CIESC Journal, 2023, 74(9): 3797-3806. |
| [9] | Cong QI, Zi DING, Jie YU, Maoqing TANG, Lin LIANG. Study on solar thermoelectric power generation characteristics based on selective absorption nanofilm [J]. CIESC Journal, 2023, 74(9): 3921-3930. |
| [10] | Ke LI, Jian WEN, Biping XIN. Study on influence mechanism of vacuum multi-layer insulation coupled with vapor-cooled shield on self-pressurization process of liquid hydrogen storage tank [J]. CIESC Journal, 2023, 74(9): 3786-3796. |
| [11] | Tianhua CHEN, Zhaoxuan LIU, Qun HAN, Chengbin ZHANG, Wenming LI. Research progress and influencing factors of the heat transfer enhancement of spray cooling [J]. CIESC Journal, 2023, 74(8): 3149-3170. |
| [12] | Rubin ZENG, Zhongjie SHEN, Qinfeng LIANG, Jianliang XU, Zhenghua DAI, Haifeng LIU. Study of the sintering mechanism of Fe2O3 nanoparticles based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3353-3365. |
| [13] | Rui HONG, Baoqiang YUAN, Wenjing DU. Analysis on mechanism of heat transfer deterioration of supercritical carbon dioxide in vertical upward tube [J]. CIESC Journal, 2023, 74(8): 3309-3319. |
| [14] | Yue YANG, Dan ZHANG, Jugan ZHENG, Maoping TU, Qingzhong YANG. Experimental study on flash and mixing evaporation of aqueous NaCl solution [J]. CIESC Journal, 2023, 74(8): 3279-3291. |
| [15] | Hai WANG, Hong LIN, Chen WANG, Haojie XU, Lei ZUO, Junfeng WANG. Investigation of enhanced boiling heat transfer on porous structural surfaces by high voltage electric field [J]. CIESC Journal, 2023, 74(7): 2869-2879. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||