CIESC Journal ›› 2024, Vol. 75 ›› Issue (6): 2243-2251.DOI: 10.11949/0438-1157.20240083
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Guangyu ZHANG1( ), Ranfei FU2, Bing SUN1, Juncong YUAN2, Xiang FENG2(
), Ranfei FU2, Bing SUN1, Juncong YUAN2, Xiang FENG2( ), Chaohe YANG2, Wei XU1(
), Chaohe YANG2, Wei XU1( )
)
Received:2024-01-17
Revised:2024-02-27
Online:2024-07-03
Published:2024-06-25
Contact:
Xiang FENG, Wei XU
张广宇1( ), 付然飞2, 孙冰1, 袁俊聪2, 冯翔2(
), 付然飞2, 孙冰1, 袁俊聪2, 冯翔2( ), 杨朝合2, 徐伟1(
), 杨朝合2, 徐伟1( )
)
通讯作者:
冯翔,徐伟
作者简介:张广宇(1990—),男,博士,副研究员,zhanggy.qday@sinopec.com
基金资助:CLC Number:
Guangyu ZHANG, Ranfei FU, Bing SUN, Juncong YUAN, Xiang FENG, Chaohe YANG, Wei XU. Synthesis of propylene carbonate from CO2 and propylene oxide: hydrogen bond activation strategy[J]. CIESC Journal, 2024, 75(6): 2243-2251.
张广宇, 付然飞, 孙冰, 袁俊聪, 冯翔, 杨朝合, 徐伟. CO2-环氧丙烷合成碳酸丙烯酯:氢键供体效应研究[J]. 化工学报, 2024, 75(6): 2243-2251.
Add to citation manager EndNote|Ris|BibTeX
| 催化剂名称 | 氢键供体类型 | 环氧丙烷 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸 丙烯酯 | 1,2- 丙二醇 | |||
| TBABr | — | 49.82 | 98.68 | 1.32 |
| CH3CH2OH | 53.63 | 100.00 | 0 | |
| CH3CH2CH2OH | 53.89 | 100.00 | 0 | |
| CH3(CH2)3OH | 57.46 | 100.00 | 0 | |
| CH3(CH2)5OH | 97.10 | 98.32 | 1.68 | |
| CH3(CH2)9OH | 94.12 | 98.83 | 1.17 | |
Table 1 Effect of different hydrogen bond donors on propylene oxide
| 催化剂名称 | 氢键供体类型 | 环氧丙烷 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸 丙烯酯 | 1,2- 丙二醇 | |||
| TBABr | — | 49.82 | 98.68 | 1.32 |
| CH3CH2OH | 53.63 | 100.00 | 0 | |
| CH3CH2CH2OH | 53.89 | 100.00 | 0 | |
| CH3(CH2)3OH | 57.46 | 100.00 | 0 | |
| CH3(CH2)5OH | 97.10 | 98.32 | 1.68 | |
| CH3(CH2)9OH | 94.12 | 98.83 | 1.17 | |
催化剂 名称 | 氢键供体类型 | 环氧丙烷转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸丙烯酯 | 1,2-丙二醇 | |||
| TBABr | 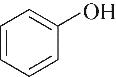 | 99.30 | 97.93 | 2.07 |
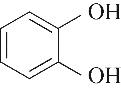 | 99.80 | 99.51 | 0.49 | |
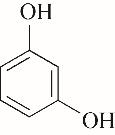 | 96.68 | 99.52 | 0.48 | |
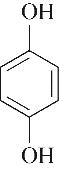 | 98.19 | 97.00 | 3.00 | |
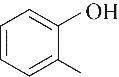 | 95.86 | 97.72 | 2.28 | |
Table 2 Effect of different hydrogen bond donors on propylene oxide
催化剂 名称 | 氢键供体类型 | 环氧丙烷转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸丙烯酯 | 1,2-丙二醇 | |||
| TBABr |  | 99.30 | 97.93 | 2.07 |
 | 99.80 | 99.51 | 0.49 | |
 | 96.68 | 99.52 | 0.48 | |
 | 98.19 | 97.00 | 3.00 | |
 | 95.86 | 97.72 | 2.28 | |
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
| CH3CH2OH | 51.90 | 0.66 | 0.86 | 0.75 | 0.54 | 53.63 |
| CH3CH2CH2OH | 50.70 | 0.62 | 0.84 | 0.90 | 0.52 | 53.89 |
| CH3(CH2)3OH | 50.20 | 0.60 | 0.84 | 0.84 | 0.47 | 57.46 |
| CH3(CH2)5OH | 48.80 | 0.56 | 0.80 | 0.84 | 0.40 | 97.10 |
| CH3(CH2)9OH | 47.60 | 0.53 | 0.70 | 0.82 | 0.45 | 94.12 |
Table 3 Kamlet-Taft parameters of alcohol[28-30]
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
| CH3CH2OH | 51.90 | 0.66 | 0.86 | 0.75 | 0.54 | 53.63 |
| CH3CH2CH2OH | 50.70 | 0.62 | 0.84 | 0.90 | 0.52 | 53.89 |
| CH3(CH2)3OH | 50.20 | 0.60 | 0.84 | 0.84 | 0.47 | 57.46 |
| CH3(CH2)5OH | 48.80 | 0.56 | 0.80 | 0.84 | 0.40 | 97.10 |
| CH3(CH2)9OH | 47.60 | 0.53 | 0.70 | 0.82 | 0.45 | 94.12 |
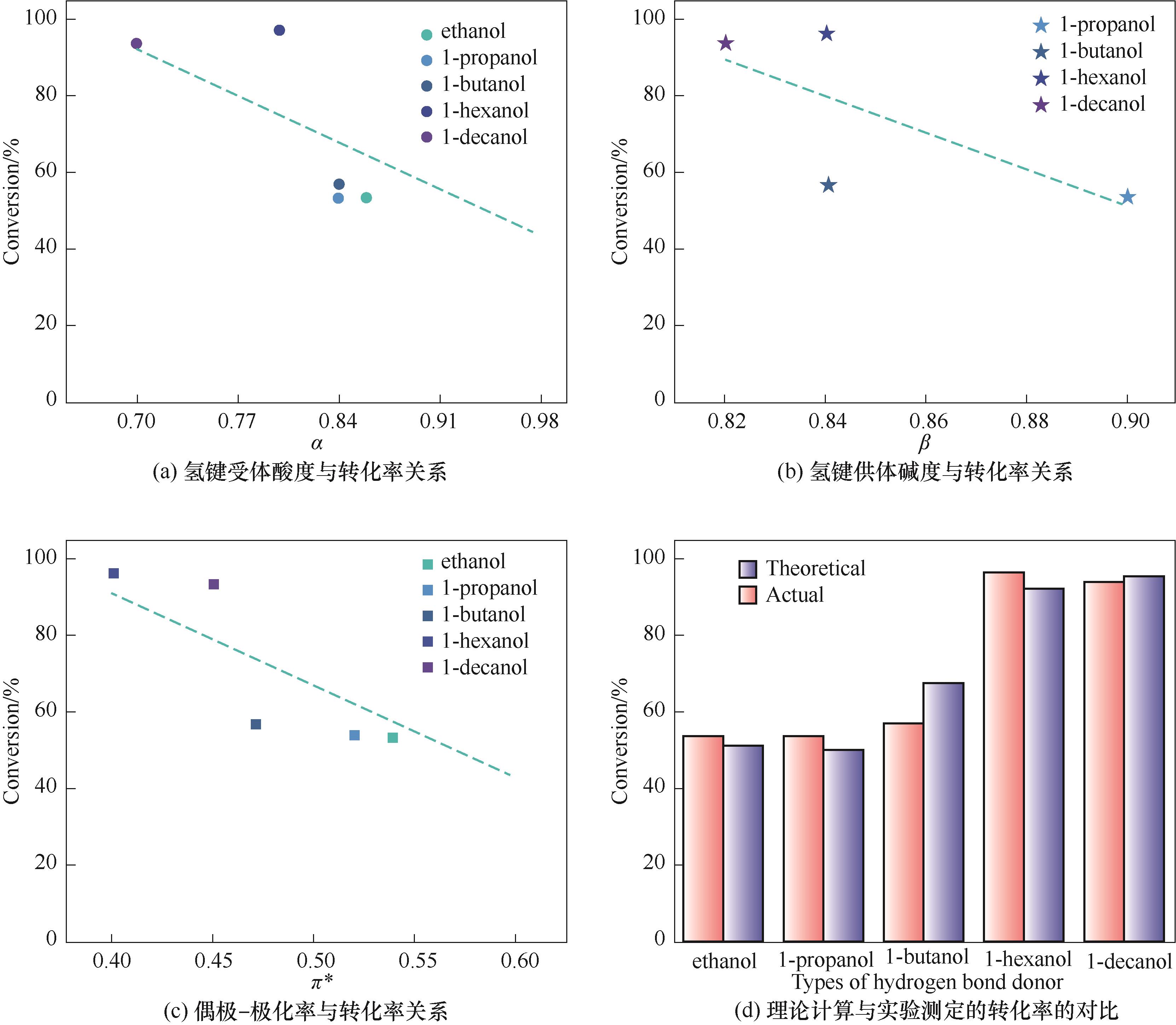
Fig.2 The relationship between Kamlet-Taft parameters of alcohols and conversion of PO and the comparison between theoretical calculation and experimental conversion
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
 | 53.40 | 0.70 | 1.10 | 0.30 | 0.67 | 99.30 |
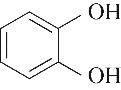 | 52.10 | 0.66 | 0.85 | 0.58 | 1.07 | 99.80 |
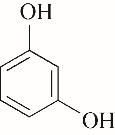 | 53.60 | 0.71 | 0.61 | 0.60 | 1.00 | 96.68 |
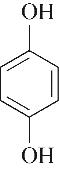 | 54.40 | 0.73 | 1.16 | 0.52 | 1.00 | 98.19 |
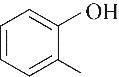 | 51.90 | 0.66 | 0.52 | 0.34 | 0.69 | 95.86 |
Table 4 Kamlet-Taft parameters and conversion of phenol[31-33]
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
 | 53.40 | 0.70 | 1.10 | 0.30 | 0.67 | 99.30 |
 | 52.10 | 0.66 | 0.85 | 0.58 | 1.07 | 99.80 |
 | 53.60 | 0.71 | 0.61 | 0.60 | 1.00 | 96.68 |
 | 54.40 | 0.73 | 1.16 | 0.52 | 1.00 | 98.19 |
 | 51.90 | 0.66 | 0.52 | 0.34 | 0.69 | 95.86 |
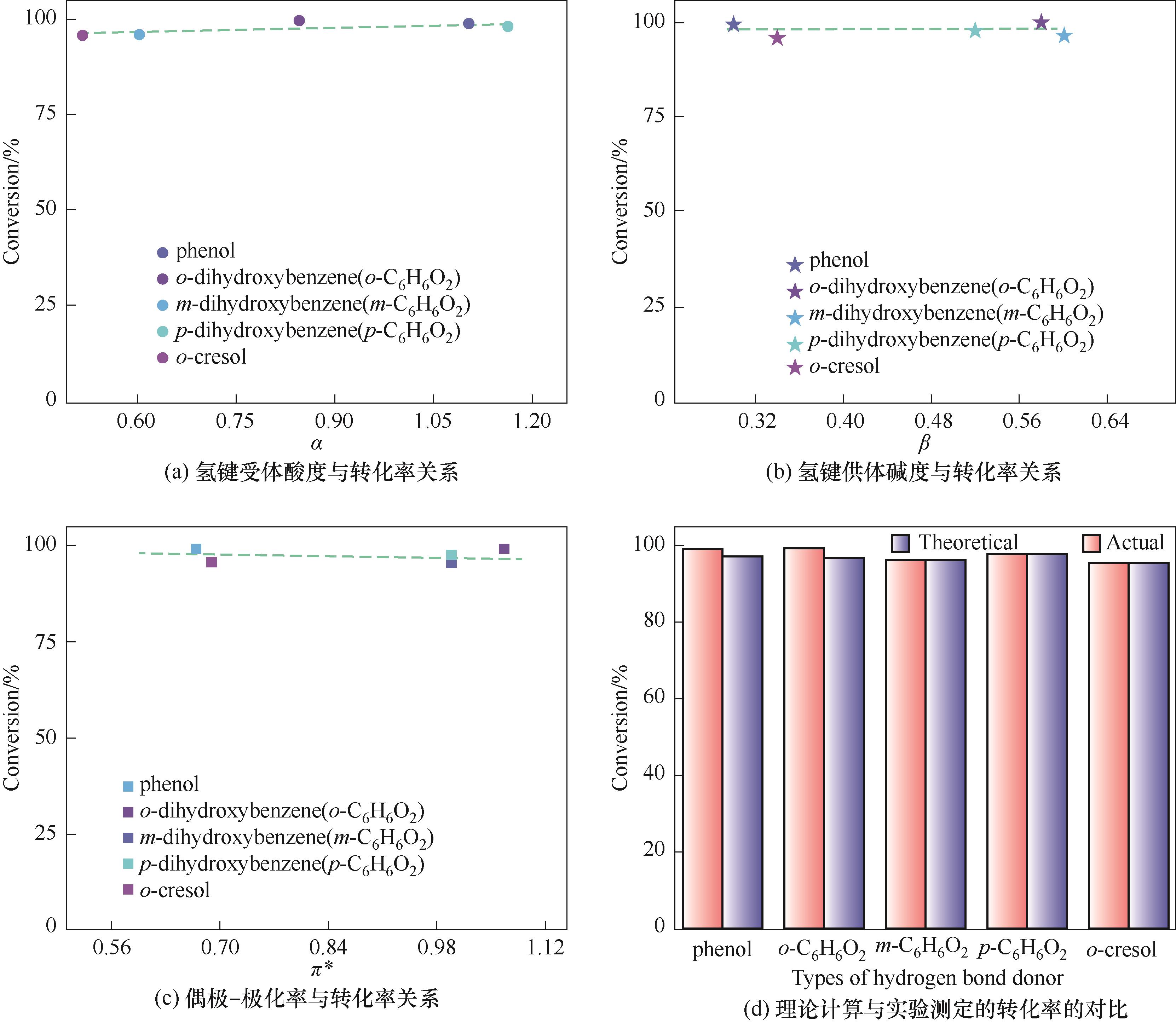
Fig.3 The relationship between Kamlet-Taft parameters of phenols and conversion of PO and the comparison between theoretical calculation and experimental conversion
| 1 | Nemirowsky J. Ueber die einwirkung von chlorkohlenoxyd auf aethylenglycol; vorläufige mittheilung[J]. Journal Für Praktische Chemie, 1883, 28(1): 439-440. |
| 2 | Khokarale S G, Mikkola J P. Metal free synthesis of ethylene and propylene carbonate from alkylene halohydrin and CO2 at room temperature[J]. RSC Advances, 2019, 9(58): 34023-34031. |
| 3 | Deng L L, Sun W Z, Shi Z J, et al. Highly synergistic effect of ionic liquids and Zn-based catalysts for synthesis of cyclic carbonates from urea and diols[J]. Journal of Molecular Liquids, 2020, 316: 113883. |
| 4 | Zhang Z F, Liu Z W, Lu J, et al. Synthesis of dimethyl carbonate from carbon dioxide and methanol over Ce x Zr1- x O2 and [EMIM]Br/Ce0.5Zr0.5O2 [J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 1981-1988. |
| 5 | Han F, Li H, Zhuang H F, et al. Direct synthesis of cyclic carbonates from olefins and CO2: single- or multi-component catalytic systems via epoxide or halohydrin intermediate[J]. Journal of CO2 Utilization, 2021, 53: 101742. |
| 6 | Fierro F, Lamparelli D H, Genga A, et al. I-LDH as a heterogeneous bifunctional catalyst for the conversion of CO2 into cyclic organic carbonates[J]. Molecular Catalysis, 2023, 538: 112994. |
| 7 | Zhang F, Wang Y Y, Zhang X C, et al. Recent advances in the coupling of CO2 and epoxides into cyclic carbonates under halogen-free condition[J]. Green Chemical Engineering, 2020, 1(2): 82-93. |
| 8 | Wang J L, Wang J Q, He L N, et al. A CO2/H2O2-tunable reaction: direct conversion of styrene into styrene carbonate catalyzed by sodium phosphotungstate/n-Bu4NBr[J]. Green Chemistry, 2008, 10(11): 1218-1223. |
| 9 | Li J X, Yue C G, Ji W H, et al. Recent advances in cycloaddition of CO2 with epoxides: halogen-free catalysis and mechanistic insights[J]. Frontiers of Chemical Science and Engineering, 2023, 17(12): 1879-1894. |
| 10 | Chen Y L, Xu P, Arai M, et al. Cycloaddition of carbon dioxide to epoxides for the synthesis of cyclic carbonates with a mixed catalyst of layered double hydroxide and tetrabutylammonium bromide at ambient temperature[J]. Advanced Synthesis & Catalysis, 2019, 361(2): 335-344. |
| 11 | Isaeva V I, Timofeeva M N, Lukoyanov I A, et al. Novel MOF catalysts based on calix[4]arene for the synthesis of propylene carbonate from propylene oxide and CO2 [J]. Journal of CO2 Utilization, 2022, 66: 102262. |
| 12 | Alassmy Y A, Pescarmona P P. The role of water revisited and enhanced: a sustainable catalytic system for the conversion of CO2 into cyclic carbonates under mild conditions[J]. ChemSusChem, 2019, 12(16): 3856-3863. |
| 13 | Roy S, Das K, Halder S. Development of suitable hydrogen bond donor (HBD) catalysts for the synthesis of cyclic carbonates and dithiocarbonates from epoxide[J]. Catalysis Letters, 2023, 154: 2243-2254. |
| 14 | Liu X J, Yan P, Han Y. H2O-polyaluminium chloride-TBAB as synergistic catalysts for the synthesis of cyclic carbonate[J]. IOP Conference Series: Materials Science and Engineering, 2018, 292: 012118. |
| 15 | Yue S, Qu H L, Song X X, et al. Novel hydroxyl-functionalized ionic liquids as efficient catalysts for the conversion of CO2 into cyclic carbonates under metal/halogen/cocatalyst/solvent-free conditions[J]. New Journal of Chemistry, 2022, 46(12): 5881-5888. |
| 16 | Wen Q, Yuan X X, Zhou Q Q, et al. Functionalized β-cyclodextrins catalyzed environment-friendly cycloaddition of carbon dioxide and epoxides[J]. Materials, 2022, 16(1): 53. |
| 17 | Liang S G, Liu H Z, Jiang T, et al. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI[J]. Chemical Communications, 2011, 47(7): 2131-2133. |
| 18 | Emenike B U, Sevimler A, Farshadmand A, et al. Rationalizing hydrogen bond solvation with Kamlet-Taft LSER and molecular torsion balances[J]. Physical Chemistry Chemical Physics: PCCP, 2023, 25(27): 17808-17814. |
| 19 | Kim Y, Oh H. Comparison between multiple regression analysis, polynomial regression analysis, and an artificial neural network for tensile strength prediction of BFRP and GFRP[J]. Materials, 2021, 14(17): 4861. |
| 20 | Wang X Y, Rinaldi R. Solvent effects on the hydrogenolysis of diphenyl ether with Raney nickel and their implications for the conversion of lignin[J]. ChemSusChem, 2012, 5(8): 1455-1466. |
| 21 | Soares B, Cunha F, Silva I, et al. Sodium hexanoate and dodecanoate salt-based eutectic solvents: density, viscosity, and Kamlet-Taft parameters[J]. Journal of Chemical & Engineering Data, 2021, 66(7): 2793-2802. |
| 22 | Taft R W, Kamlet M J. The solvatochromic comparison method(2): The α-scale of solvent hydrogen-bond donor (HBD) acidities[J]. Journal of the American Chemical Society, 1976, 98(10): 2886-2894. |
| 23 | Weiß N, Schmidt C H, Thielemann G, et al. The physical significance of the Kamlet-Taft π* parameter of ionic liquids[J]. Physical Chemistry Chemical Physics, 2021, 23(2): 1616-1626. |
| 24 | Duereh A, Guo H X, Honma T, et al. Solvent polarity of cyclic ketone (cyclopentanone, cyclohexanone): alcohol (methanol, ethanol) renewable mixed-solvent systems for applications in pharmaceutical and chemical processing[J]. Industrial & Engineering Chemistry Research, 2018, 57(22): 7331-7344. |
| 25 | Rosés M, Ortega J, Bosch E. Variation of E T 30 polarity and the Kamlet-Taft solvatochromic parameters with composition in alcohol-alcohol mixtures[J]. Journal of Solution Chemistry, 1995, 24(1): 51-63. |
| 26 | Kamlet M J, Taft R W. The solvatochromic comparison method(Ⅰ): The β-scale of solvent hydrogen-bond acceptor (HBA) basicities[J]. Journal of the American Chemical Society, 1976, 98(2): 377-383. |
| 27 | Marcus Y. The properties of organic liquids that are relevant to their use as solvating solvents[J]. Chemical Society Reviews, 1993, 22(6): 409-416. |
| 28 | 邹建卫, 俞庆森, 商志才. 醇类溶剂溶剂化显色极性的理论分析[J]. 化学学报, 2000, 58(10): 1247-1253. |
| Zou J W, Yu Q S, Shang Z C. Theoretical analysis of solvatochromic polarity scales on alcoholic solvents[J]. Acta Chimica Sinica, 2000, 58(10): 1247-1253. | |
| 29 | 刘汉文, 胡彩玲. 醇类溶剂的溶剂化显色参数 E T N 的相关分析[J]. 湘潭大学自然科学学报, 2005, 27(3): 67-70. |
| Liu H W, Hu C L. Relevant analysis of solvatochromic parameter on alcoholic solvents[J]. Natural Science Journal of Xiangtan University, 2005, 27(3): 67-70. | |
| 30 | Laurence C, Mansour S, Vuluga D, et al. Theoretical, semiempirical, and experimental solvatochromic comparison methods for the construction of the α1 scale of hydrogen-bond donation of solvents[J]. The Journal of Organic Chemistry, 2022, 87(9): 6273-6287. |
| 31 | 冯流, 韩朔睽, 王连生, 等. 苯系物Lewis酸碱性定量及其应用[J]. 环境化学, 1995, 14(5): 417-424. |
| Feng L, Han S K, Wang L S, et al. A new quantiative parameter of the Lewis acidity and basicity of benzene and its derivatives[J]. Environmental Chemistry, 1995, 14(5): 417-424. | |
| 32 | Brehmer T, Duong B, Boeker P, et al. Simulation of gas chromatographic separations and estimation of distribution-centric retention parameters using linear solvation energy relationships[J]. Journal of Chromatography A, 2024, 1717: 464665. |
| 33 | 韩长日. 溶剂极性的ET 经验参数及其应用[J]. 化学通报, 1985, 48(7): 40-43. |
| Han C R. ET empirical parameter of solvent polarity and its application[J]. Chemistry, 1985, 48(7): 40-43. | |
| 34 | Liu M S, Wang X, Jiang Y C, et al. Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: current state-of-the art of catalyst development and reaction analysis[J]. Catalysis Reviews, 2019, 61(2): 214-269. |
| [1] | Ziyang LI, Nan ZHENG, Jiabin FANG, Jinjia WEI. Performance analysis and multi-objective optimization of recompression S-CO2 Brayton cycle [J]. CIESC Journal, 2024, 75(6): 2143-2156. |
| [2] | Chenggong CHANG, Haonan SONG, Feixia LEI, Zichen DI, Fangqin CHENG. Study on the carbon reduction potential of blast furnace injection process using reformed coke oven gas [J]. CIESC Journal, 2024, 75(6): 2344-2352. |
| [3] | Fangtao JIANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN, Jing ZHANG. Efficient synthesis of fluoroethylene carbonate via phase transfer catalysis using [bmim][BF4] [J]. CIESC Journal, 2024, 75(4): 1543-1551. |
| [4] | Ruirui WANG, Ying JIN, Yumei LIU, Mengyue LI, Shengwen ZHU, Ruiyi YAN, Ruixia LIU. Study on design of polymeric ionic liquids and the performance for selective oxidation of cyclohexane [J]. CIESC Journal, 2024, 75(4): 1552-1564. |
| [5] | Jiaqi WANG, Haoqi WEI, Ajing GOU, Jiaxing LIU, Xinlin ZHOU, Kun GE. Study on the formation mechanism of CO2 hydrate under the action of nanoparticles [J]. CIESC Journal, 2024, 75(3): 956-966. |
| [6] | Yongjun XIAO, Zhaochong SHI, Ren WAN, Fan SONG, Changjun PENG, Honglai LIU. Prediction of self-diffusion coefficients of ionic liquids using back-propagation neural networks [J]. CIESC Journal, 2024, 75(2): 429-438. |
| [7] | Rui SUN, Hua TIAN, Zirui WU, Xiaocun SUN, Gequn SHU. Study on the critical properties calculation models of CO2-based binary mixture working fluid [J]. CIESC Journal, 2024, 75(2): 439-449. |
| [8] | Zhi ZHU, Hengjie XU, Wei CHEN, Wenyuan MAO, Qiangguo DENG, Xuejian SUN. Study on critical chocked characteristics of supercritical carbon dioxide spiral groove dry gas seal under thermal-fluid coupling lubrication [J]. CIESC Journal, 2024, 75(2): 604-615. |
| [9] | Zexin ZHANG, Weizhong ZHENG, Yisheng XU, Dongdong HU, Xinyu ZHUO, Yuan ZONG, Weizhen SUN, Ling ZHAO. Research progress of wafer cleaning and selective etching in supercritical carbon dioxide media [J]. CIESC Journal, 2024, 75(1): 110-119. |
| [10] | Ruitao SONG, Pai WANG, Yunpeng WANG, Minxia LI, Chaobin DANG, Zhenguo CHEN, Huan TONG, Jiaqi ZHOU. Numerical simulation of flow boiling heat transfer in pipe arrays of carbon dioxide direct evaporation ice field [J]. CIESC Journal, 2023, 74(S1): 96-103. |
| [11] | Yifei ZHANG, Fangchen LIU, Shuangxing ZHANG, Wenjing DU. Performance analysis of printed circuit heat exchanger for supercritical carbon dioxide [J]. CIESC Journal, 2023, 74(S1): 183-190. |
| [12] | Qi WANG, Bin ZHANG, Xiaoxin ZHANG, Hujian WU, Haitao ZHAN, Tao WANG. Synthesis of isoxepac and 2-ethylanthraquinone catalyzed by chloroaluminate-triethylamine ionic liquid/P2O5 [J]. CIESC Journal, 2023, 74(S1): 245-249. |
| [13] | Ruimin CHE, Wenqiu ZHENG, Xiaoyu WANG, Xin LI, Feng XU. Research progress on homogeneous processing of cellulose in ionic liquids [J]. CIESC Journal, 2023, 74(9): 3615-3627. |
| [14] | Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids [J]. CIESC Journal, 2023, 74(9): 3681-3696. |
| [15] | Meisi CHEN, Weida CHEN, Xinyao LI, Shangyu LI, Youting WU, Feng ZHANG, Zhibing ZHANG. Advances in silicon-based ionic liquid microparticle enhanced gas capture and conversion [J]. CIESC Journal, 2023, 74(9): 3628-3639. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||