化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5320-5336.DOI: 10.11949/0438-1157.20200332
收稿日期:2020-03-30
修回日期:2020-06-10
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
王志远
作者简介:王志远(1983—),男,博士,讲师,基金资助:
Zhiyuan WANG( ),Xudong DING,Boyan WANG,Zhihong XING
),Xudong DING,Boyan WANG,Zhihong XING
Received:2020-03-30
Revised:2020-06-10
Online:2020-11-05
Published:2020-11-05
Contact:
Zhiyuan WANG
摘要:
以石脑油为裂解原料,考察了硫化物和硫/磷化合物的添加方式对热裂解结焦行为的影响。采用Raman光谱、XRD、SEM和XPS等检测手段表征了HP40试样的氧化层和焦炭的形貌与结构。结果表明,原料连续注入硫化物条件下,磷化物的加入使得焦炭结构改变,显示出优异的抗结焦效果。硫化物和硫/磷化合物预处理导致氧化层中Fe含量升高,抑制结焦效果有限。硫/磷预处理与原料连续注入硫/磷化合物联合方式与原料连续注入硫/磷化合物方式的抗结焦效果接近,但前者在初期的抑制效果更明显。所有添加方式都会引起结焦层中无定形焦炭含量升高,焦炭的石墨化程度降低。热裂解焦炭缩合程度较高,硫化物和硫/磷化合物减少了催化结焦的生成,在一定程度上提高了焦层中氢含量。
中图分类号:
王志远,丁旭东,王博研,邢志宏. 硫化物和硫/磷化合物的添加方式对石脑油热裂解结焦影响的研究[J]. 化工学报, 2020, 71(11): 5320-5336.
Zhiyuan WANG,Xudong DING,Boyan WANG,Zhihong XING. Addition methods of sulfur and sulfur/phosphorus-based compounds on coking behavior during thermal cracking of naphtha[J]. CIESC Journal, 2020, 71(11): 5320-5336.
| 参数 | 数值 |
|---|---|
| 空气预氧化实验 | |
| 温度/K | 1073 |
| 空气流量/(ml/min) | 700 |
| 时间/min | 600 |
| 清焦 | |
| 温度/K | 1073-1123 |
| 空气流量/(ml/min) | 500 |
| 时间/min | 20 |
| 硫化物和硫/磷化合物预处理实验 | |
| 温度/K | 1103 |
| 去离子水流量/(ml/min) | 1 |
| DMDS质量浓度/(μg/gwater) | 500 |
| TPPI质量浓度/(μg/gwater) | 100 |
| 时间/min | 120 |
| 热裂解实验 | |
| 温度/K | 1123 |
| 石脑油流量/(ml/min) | 2 |
| 去离子水流量/(ml/min) | 0.7 |
表1 实验参数
Table 1 Experimental conditions for each experimental step
| 参数 | 数值 |
|---|---|
| 空气预氧化实验 | |
| 温度/K | 1073 |
| 空气流量/(ml/min) | 700 |
| 时间/min | 600 |
| 清焦 | |
| 温度/K | 1073-1123 |
| 空气流量/(ml/min) | 500 |
| 时间/min | 20 |
| 硫化物和硫/磷化合物预处理实验 | |
| 温度/K | 1103 |
| 去离子水流量/(ml/min) | 1 |
| DMDS质量浓度/(μg/gwater) | 500 |
| TPPI质量浓度/(μg/gwater) | 100 |
| 时间/min | 120 |
| 热裂解实验 | |
| 温度/K | 1123 |
| 石脑油流量/(ml/min) | 2 |
| 去离子水流量/(ml/min) | 0.7 |
| 流程 | 方案 |
|---|---|
| 空气预氧化处理 | preoxidation |
| 采用DMDS预处理 | Pre S |
| 采用DMDS& TPPI预处理 | Pre S/P |
原料连续添加 DMDS+ thiophene+benzothiophene+TEP | sulfides/TEP |
原料连续添加 DMDS+thiophene+benzothiophene+TPPI | sulfides/TPPI |
采用DMDS预处理,随后原料连续添加 DMDS | Pre S + S |
| 采用DMDS& TPPI预处理,随后原料连续添加DMDS+thiophene+benzothiophene+TPPI | Pre S/P + sulfides/TPPI |
表2 实验方案
Table 2 Experimental plans for each experimental step
| 流程 | 方案 |
|---|---|
| 空气预氧化处理 | preoxidation |
| 采用DMDS预处理 | Pre S |
| 采用DMDS& TPPI预处理 | Pre S/P |
原料连续添加 DMDS+ thiophene+benzothiophene+TEP | sulfides/TEP |
原料连续添加 DMDS+thiophene+benzothiophene+TPPI | sulfides/TPPI |
采用DMDS预处理,随后原料连续添加 DMDS | Pre S + S |
| 采用DMDS& TPPI预处理,随后原料连续添加DMDS+thiophene+benzothiophene+TPPI | Pre S/P + sulfides/TPPI |
| 加速电压/ kV | 分析区域 | Cr原子浓度/% | Mn原子浓度/% | Fe原子浓度/% | Ni原子浓度/% | O原子浓度/% | Si原子浓度/% |
|---|---|---|---|---|---|---|---|
| 10(h≤0.53 μm) | Ⅰ | 13.66 | 2.25 | 1.83 | — | 82.25 | — |
| Ⅱ | 13.76 | 1.85 | 1.50 | — | 82.88 | — | |
| Ⅲ | 16.86 | 1.71 | — | — | 81.42 | — | |
| 15(h≤1.04 μm) | I | 25.36 | 3.47 | 6.97 | 4.42 | 58.35 | 1.40 |
| Ⅱ | 27.58 | 3.56 | 4.48 | 2.05 | 61.44 | 0.88 | |
| Ⅲ | 31.46 | 3.07 | 2.89 | 1.53 | 59.54 | 1.50 |
表3 不同预处理条件下HP40合金表面氧化层元素分布
Table 3 Element concentrations of the oxide films on HP40 alloys
| 加速电压/ kV | 分析区域 | Cr原子浓度/% | Mn原子浓度/% | Fe原子浓度/% | Ni原子浓度/% | O原子浓度/% | Si原子浓度/% |
|---|---|---|---|---|---|---|---|
| 10(h≤0.53 μm) | Ⅰ | 13.66 | 2.25 | 1.83 | — | 82.25 | — |
| Ⅱ | 13.76 | 1.85 | 1.50 | — | 82.88 | — | |
| Ⅲ | 16.86 | 1.71 | — | — | 81.42 | — | |
| 15(h≤1.04 μm) | I | 25.36 | 3.47 | 6.97 | 4.42 | 58.35 | 1.40 |
| Ⅱ | 27.58 | 3.56 | 4.48 | 2.05 | 61.44 | 0.88 | |
| Ⅲ | 31.46 | 3.07 | 2.89 | 1.53 | 59.54 | 1.50 |
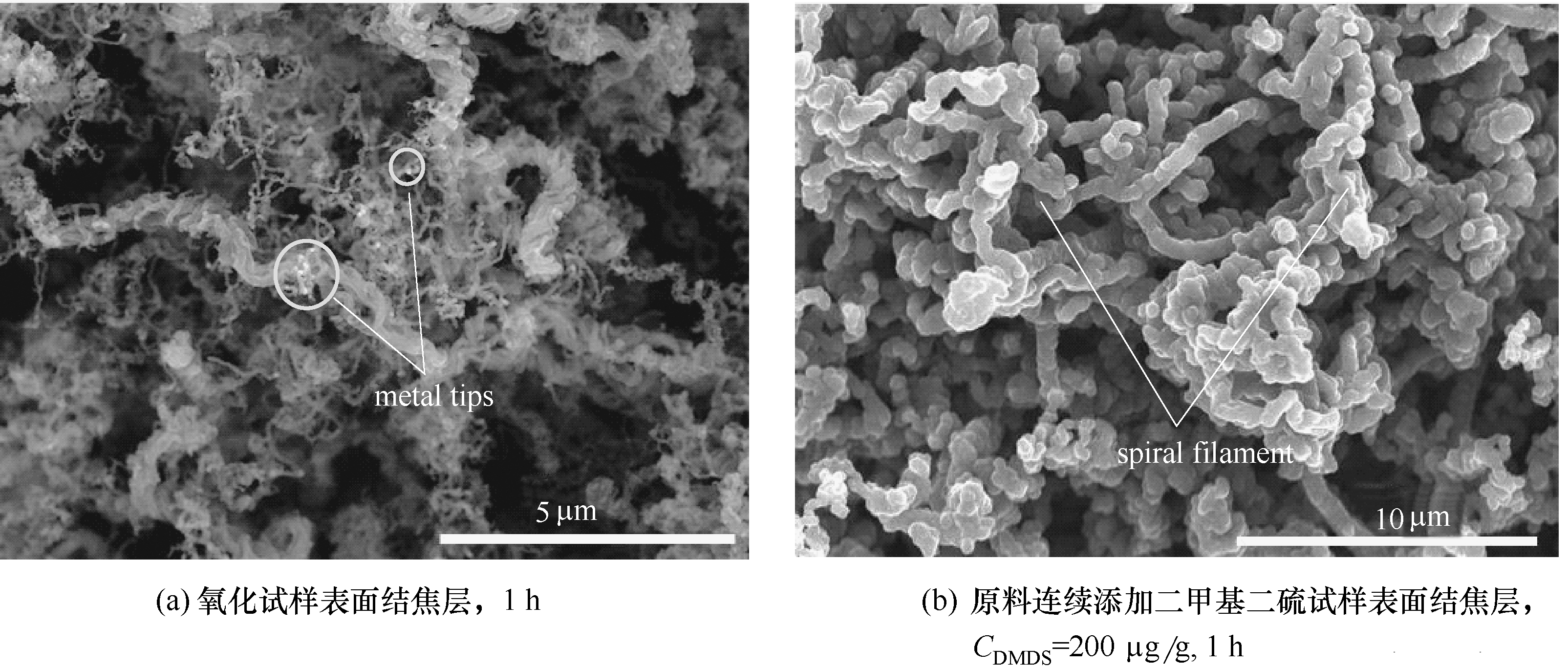
图5 裂解时间1 h条件下,未添加/添加二甲基二硫后HP40合金氧化层表面碳纤维的SEM图
Fig.5 SEM images of carbon fibers on HP40 alloys in the absence/presence of DMDS after cracking time of 1 h
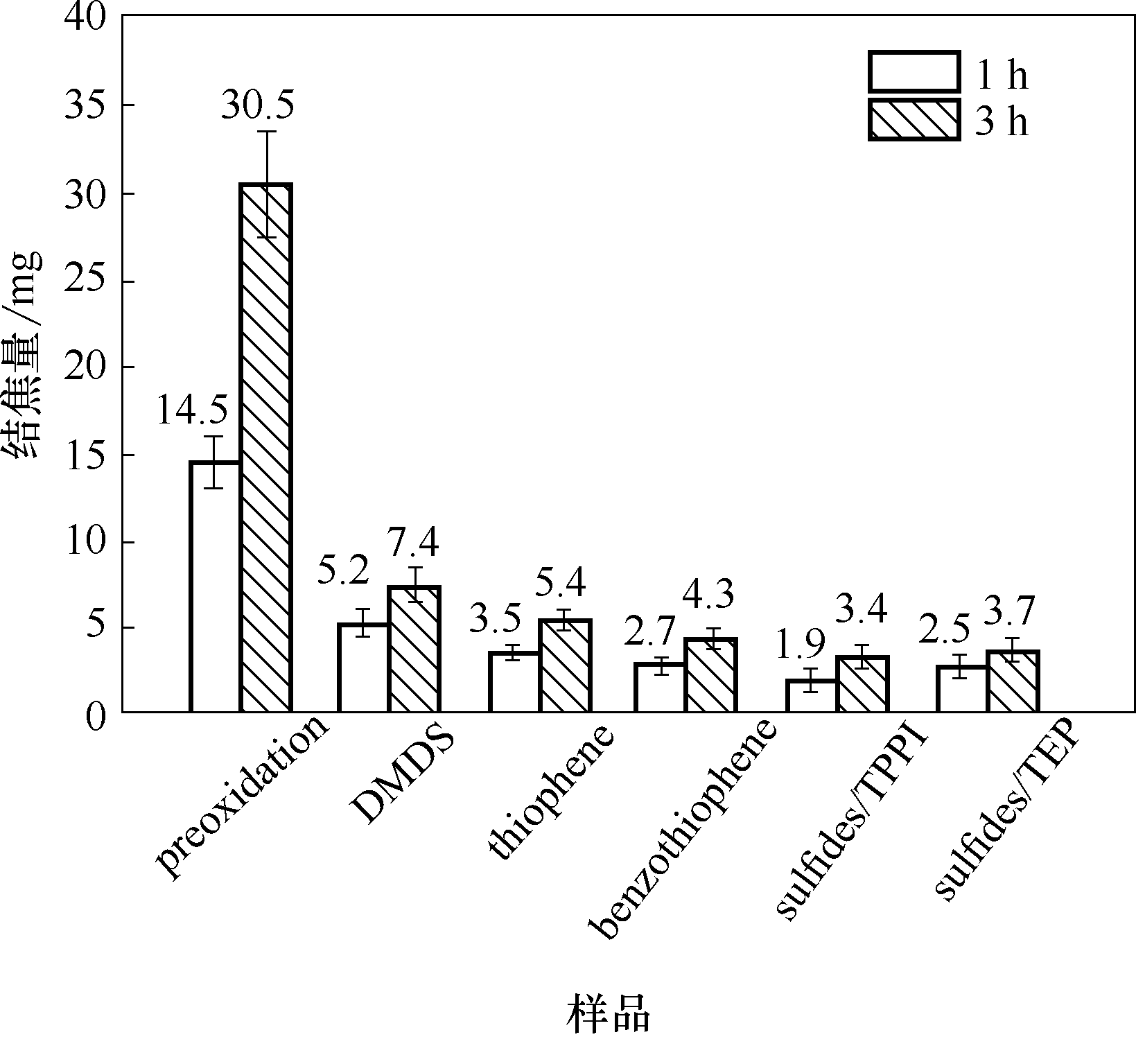
图7 裂解时间1 h和3 h条件下,添加硫化物后HP40合金表面焦炭沉积量情况
Fig.7 The amounts of coke formed on HP40 alloys with the addition of sulfides after cracking time of 1 h and 3 h

图8 裂解时间1 h和3 h、实验方案为“Pre S/P”条件下,HP40合金表面焦炭的SEM图和丝状焦的EDS分析
Fig.8 SEM images of cokes on HP40 alloys under the condition of “ Pre S/P” after cracking time of 1h and 3 h; EDS of cokes on HP40 alloys

图9 裂解时间1 h和3 h、实验方案为Pre S条件下,HP40合金表面焦炭的SEM图和表面丝状焦的EDS分析
Fig.9 SEM images of cokes on HP40 alloys under the condition of Pre S after cracking time of 1h and 3 h;EDS of cokes on HP40 alloys
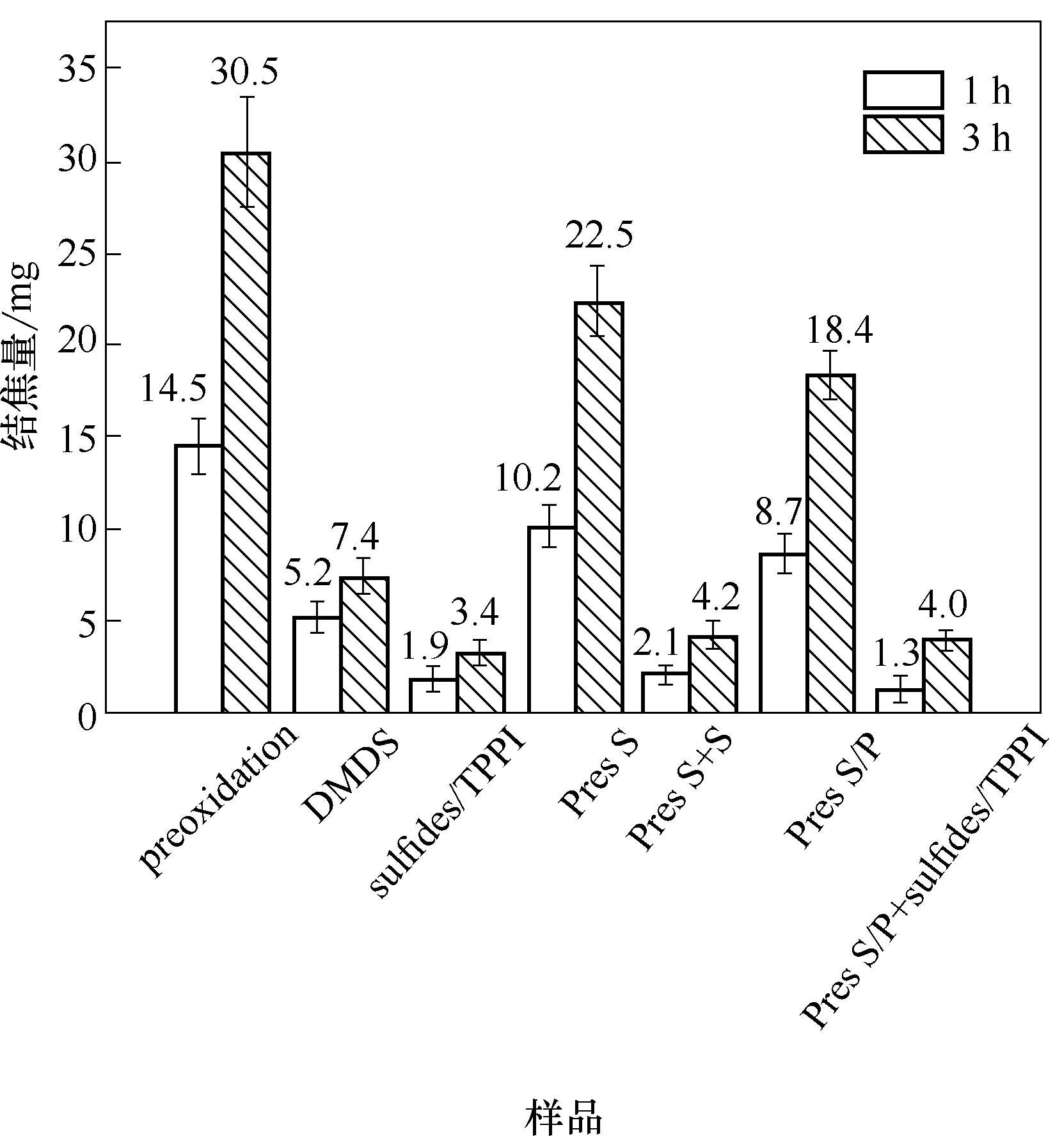
图10 裂解时间1 h和3 h条件下,不同添加方式对HP40合金表面焦炭沉积量的影响
Fig.10 The amounts of coke formed on HP40 alloys with varying the addition methods after cracking time of 1 h and 3 h

图11 裂解时间1 h和3 h、实验方案为Pre S + S条件下HP40合金表面焦炭的SEM图和表面丝状焦的EDS分析
Fig.11 SEM images of cokes on HP40 alloys under the condition of Pre S + S after cracking time of 1 h and 3 h; EDS of coke on HP40 alloys

图12 裂解时间1 h和3 h条件下,硫/磷组分预处理与原料连续注入硫/磷化合物联合操作方式下HP40合金表面焦炭的SEM图
Fig.12 SEM photos of cokes on HP40 alloys with the pretreatment of DMDS/TPPI followed by continuous addition of mixed sulfides/TPPI after cracking time of 1 h and 3 h
| 拟合峰 | Raman位移/cm-1 | 振动模式 | 拟合方式 |
|---|---|---|---|
| G | 1580 | 理想石墨晶格 (E2g-symmetry) | Lorentzian |
| D1 | 1350 | 无序石墨晶格 (graphene layer edge, A1g-symmetry) | Lorentzian |
| D2 | 1620 | 无序石墨晶格(surface graphene layers, E2g-symmetry) | Lorentzian |
| D3 | 1500 | 无定形碳 | Gaussian |
| D4 | 1200 | 无序石墨晶格(A1g-symmetry), polyenes, ionic impurities | Lorentzian |
表4 热裂解结焦一阶拉曼光谱的分峰拟合参数[20-21]
Table 4 The parameters of curve fits for the first-order Raman spectra of cokes on HP40 alloys
| 拟合峰 | Raman位移/cm-1 | 振动模式 | 拟合方式 |
|---|---|---|---|
| G | 1580 | 理想石墨晶格 (E2g-symmetry) | Lorentzian |
| D1 | 1350 | 无序石墨晶格 (graphene layer edge, A1g-symmetry) | Lorentzian |
| D2 | 1620 | 无序石墨晶格(surface graphene layers, E2g-symmetry) | Lorentzian |
| D3 | 1500 | 无定形碳 | Gaussian |
| D4 | 1200 | 无序石墨晶格(A1g-symmetry), polyenes, ionic impurities | Lorentzian |
| 样品 | ΓD1 /cm-1 | ΓD2 /cm-1 | ΓD3 /cm-1 | ΓD4/cm-1 | ΓG/cm-1 | ID1/IG | ID3/IG | IG/IAll |
|---|---|---|---|---|---|---|---|---|
| preoxidation | 167.02 | 23.32 | 171.24 | 112.63 | 58.04 | 2.54 | 0.47 | 0.27 |
| DMDS | 179.87 | 32.68 | 160.33 | 110.39 | 59.51 | 3.05 | 0.50 | 0.23 |
| thiophene | 157.91 | 39.13 | 173.89 | 106.49 | 56.41 | 3.51 | 0.68 | 0.18 |
| benzothiophene | 182.63 | 37.72 | 150.61 | 120.18 | 63.15 | 3.59 | 0.52 | 0.21 |
| sulfides/TPPI | 178.29 | 35.27 | 163.70 | 114.37 | 58.09 | 3.76 | 0.78 | 0.12 |
| Pre S | 150.36 | 38.29 | 170.56 | 126.80 | 57.73 | 2.84 | 0.58 | 0.23 |
| Pre S+ sulfides | 173.09 | 39.38 | 157.12 | 124.15 | 62.63 | 3.61 | 0.56 | 0.18 |
| Pre S/P | 163.53 | 34.37 | 169.85 | 116.62 | 58.74 | 2.70 | 0.47 | 0.25 |
| Pre S/P+sulfides/TPPI | 156.61 | 42.41 | 180.63 | 138.05 | 56.54 | 4.14 | 0.90 | 0.08 |
表5 热裂解结焦拉曼光谱的分峰拟合结果
Table 5 Fitting results of Raman spectra of coke deposits
| 样品 | ΓD1 /cm-1 | ΓD2 /cm-1 | ΓD3 /cm-1 | ΓD4/cm-1 | ΓG/cm-1 | ID1/IG | ID3/IG | IG/IAll |
|---|---|---|---|---|---|---|---|---|
| preoxidation | 167.02 | 23.32 | 171.24 | 112.63 | 58.04 | 2.54 | 0.47 | 0.27 |
| DMDS | 179.87 | 32.68 | 160.33 | 110.39 | 59.51 | 3.05 | 0.50 | 0.23 |
| thiophene | 157.91 | 39.13 | 173.89 | 106.49 | 56.41 | 3.51 | 0.68 | 0.18 |
| benzothiophene | 182.63 | 37.72 | 150.61 | 120.18 | 63.15 | 3.59 | 0.52 | 0.21 |
| sulfides/TPPI | 178.29 | 35.27 | 163.70 | 114.37 | 58.09 | 3.76 | 0.78 | 0.12 |
| Pre S | 150.36 | 38.29 | 170.56 | 126.80 | 57.73 | 2.84 | 0.58 | 0.23 |
| Pre S+ sulfides | 173.09 | 39.38 | 157.12 | 124.15 | 62.63 | 3.61 | 0.56 | 0.18 |
| Pre S/P | 163.53 | 34.37 | 169.85 | 116.62 | 58.74 | 2.70 | 0.47 | 0.25 |
| Pre S/P+sulfides/TPPI | 156.61 | 42.41 | 180.63 | 138.05 | 56.54 | 4.14 | 0.90 | 0.08 |
| 方案 | sp3/sp2比值 |
|---|---|
| preoxidation | 0.17 |
| sulfides/TPPI | 0.23 |
| DMDS | 0.29 |
表6 热裂解结焦的sp3/sp2比值
Table 6 sp3/sp2ratio of coke deposits
| 方案 | sp3/sp2比值 |
|---|---|
| preoxidation | 0.17 |
| sulfides/TPPI | 0.23 |
| DMDS | 0.29 |
| 方案 | H/C 比值 |
|---|---|
| preoxidation | 0.69 |
| sulfides/TPPI | 2.56 |
| 连续添加DMDS | 1.25 |
| Pre S | 0.82 |
表7 热裂解结焦的H/C比值(质量比)
Table 7 TheH/C massratios of coke deposits
| 方案 | H/C 比值 |
|---|---|
| preoxidation | 0.69 |
| sulfides/TPPI | 2.56 |
| 连续添加DMDS | 1.25 |
| Pre S | 0.82 |
| 1 | Sarris S A, Olahova N, Verbeken K, et al. Optimization of the in-situ pretreatment of high temperature Ni-Cr alloys for ethane steam cracking[J]. Industrial & Engineering Chemistry Research, 2017, 56(6): 1424-1438. |
| 2 | Reyniers M F, Froment G F. Influence of metal surface and sulfur addition on coke deposition in the thermal cracking of hydrocarbons[J]. Industrial & Engineering Chemistry Research, 1995, 34(3): 773-785. |
| 3 | Singh A, Paulson S, Farag H, et al. Role of presulfidation and H2S cofeeding on carbon formation on SS304 alloy during the ethane-steam cracking process at 700℃[J]. Industrial & Engineering Chemistry Research, 2018, 57(4): 1146-1158. |
| 4 | Wang J, Reyniers M F, Van Geem K M, et al. Influence of silicon and silicon/sulfur-containing additives on coke formation during steam cracking of hydrocarbons[J]. Industrial & Engineering Chemistry Research, 2008, 47(5): 1468-1482. |
| 5 | Kumar S. Triethyl phosphite additive-based fouling inhibition studies[J]. Industrial & Engineering Chemistry Research, 1999, 38(4): 1364-1368. |
| 6 | Wang J D, Reyniers M F, Martin G B. The influence of phosphorous containing compounds on steam cracking of n-hexane[J]. Journal of Analytical and Applied Pyrolysis, 2006, 77(2): 133-148. |
| 7 | Bao B B, Liu J L, Xu H, et al. Effect of selective oxidation and sulphur/phosphorus-containing compounds on coking behaviour during light naphtha thermal cracking[J]. The Canadian Journal of Chemical Engineering, 2017, 95(8): 1480-1488. |
| 8 | 屈笑雨, 刘京雷, 徐宏, 等. 25Cr35NiNb 合金表面Al-Si-Cr 涂层抑制结焦性能[J]. 化工学报, 2015, 66(3): 1059-1065. |
| Qu X Y, Liu J L, Xu H, et al. Anti-coking characteristics of Al-Si-Cr coating on 25Cr35NiNb alloy[J]. CIESC Journal, 2015, 66(3): 1059-1065. | |
| 9 | Bao B B, Liu J L, Xu H, et al. Inhibitory effect of MnCr2O4 spinel coating on coke formation during light naphtha thermal cracking[J].RSC Advances, 2016, 6(73): 68934-68941. |
| 10 | Yan N, Luo J L, Chuang K T. Improved coking resistance of direct ethanol solid oxide fuel cells with a Ni-Sx anode[J]. Journal of Power Sources, 2014, 250(15): 212-219. |
| 11 | Sanchez-Tovar R, Leiva-Garcia R, Garcia-Anton J. Characterization of thermal oxide films formed on a duplex stainless steel by means of confocal-Raman microscopy and electrochemical techniques[J]. Thin Solid Films, 2015, 576(2): 1-10. |
| 12 | Ramya S, Anita T, Shaikh H, et al. Laser Raman microscopic studies of passive films formed on type 316LN stainless steels during pitting in chloride solution[J]. Corrosion Science, 2010, 52(6): 2114-2121. |
| 13 | Oblonsky L J, Devine T M. A Surface enhanced Raman spectroscopic study of the passive films formed in borate buffer on iron, nickel, chromium and stainless steel[J]. Corrosion Science, 1995, 37(11): 17-41. |
| 14 | Olahova N, Sarris S A, Reyniers M F, et al. Coking tendency of 25Cr-35Ni alloys: influence of temperature, sulfur addition, and cyclic aging[J]. Industrial & Engineering Chemistry Research, 2018, 57(9): 3138-3148. |
| 15 | Wang B, Wang S X, Liu B, et al. Oxide film prepared by selective oxidation of stainless steel and anti-coking behavior during n-hexane thermal cracking[J]. Surface & Coating Technology, 2019, 378(25): 12492-12499. |
| 16 | Boehm H P. Carbon from carbon monoxide disproportionation on nickel and iron catalysts: morphological studies and possible growth mechanisms[J]. Carbon, 1973, 11(6): 583-595. |
| 17 | Bennett M J, Chaffey G H, Myatt B L, et al. Inhibition by sulfur poisoning of the heterogeous decomposition of acetone[C]// Lyle F A, Baker R T K. Coke Formation on Metal Surfaces. Washington D C, America: America Chemical Society, 1982: 223-238. |
| 18 | Salaha M B, Sabot R, Refait P, et al. Passivation behaviour of stainless steel (UNS N-08028) in industrial or simplified phosphoric acid solutions at different temperatures[J]. Corrosion Science, 2015, 99: 320-332. |
| 19 | Zhou J X, Xu H, Luan X J, et al. Influence of the SiO2/S coating and sulfur/phosphorus-containing coking inhibitor on coke formation during thermal cracking of light naphtha[J]. Fuel Processing Technology, 2012, 104: 198-203. |
| 20 | Reshetenko T V, Avdeeva L B, Ismagilov Z R, et al. Catalytic filamentous carbon: structure and textural properties[J]. Carbon, 2003, 41(8): 1605-1615. |
| 21 | Sadezkv A, Muckernhuber H, Grothe H, et al. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information[J]. Carbon, 2005, 43(8): 1731-1742. |
| 22 | Sheng C D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15): 2316-2324. |
| 23 | Qian Z H, Zhang H F, Dai D J, et al. Study on occurrence of sulfur in different group components of Xinyu clean coking coal[J]. Journal of Fuel Chemistry and Technology, 2014, 42(11): 1286-1294. |
| 24 | Kicinski W, Szala M, Bystrzejewski M. Sulfur-doped porous carbon: synthesis and application[J]. Carbon, 2014, 68: 1-32. |
| 25 | Bu J, Loh G, Gwie C G, DeWiyanti S, et al. Desulfurization of diesel fuels by selective adsorption on activated carbons: competitive adsorption of polycyclic aromatic sulfur heterocycles and polycyclic aromatic Hydrocarbons[J]. Chemical Engineering Journal, 2011, 166(1): 207-217. |
| 26 | Timko M T, Wang J A, Burgess J, et al. Roles of surface chemistry and structural defects of activated carbons in the oxidative desulfurization of benzothiophenes[J]. Fuel, 2016, 163(1): 223-231. |
| 27 | Niakolas D K. Sulfur poisoning of Ni-based anodes for solid oxide fuel cells in H/C-based fuels[J]. Applied Catalysis A: General, 2014, 486(22): 123-142. |
| 28 | Yang Y, Zhang H P, Yan Y. Synthesis of CNTs on stainless steel microfibrous composite by CVD: effect of synthesis condition on carbon nanotube growth and structure[J]. Composites Part B, 2019, 160(1): 369-383. |
| 29 | Yu X, Kang Y, Park H S. Sulfur and phosphorus co-doping of hierarchically porous graphene aerogels for enhancing supercapacitor performance[J]. Carbon, 2016, 101: 49-56. |
| 30 | Cui S H, Li J H, Jayakumar A, et al. Effects of H2S and H2O on carbon deposition over La0.4Sr0.5Ba0.1TiO3/YSZ perovskite anodes in methane fueled SOFCs[J]. Journal of Power Source, 2014, 250(15): 134-142. |
| 31 | 刘玉新, 许志明, 赵锁奇, 等. 哈萨克斯坦及俄罗斯渣油馏分中的硫化物裂解色谱分析[J]. 燃料化学学报, 2008, 36(6): 712-719. |
| Liu Y X, Xu Z M, Zhao S Q, et al. Analysis of sulfur species in the residue fractions of Kazakhstan and Russia crude oils pyrolysis-gas chromatography[J]. Journal of Fuel Chemistry and Technology, 2008, 36(6): 712-719. | |
| 32 | Ren Y, Mahinpey N, Feeitag N. Kinetic model for the combustion of coke derived at different coking temperatures[J]. Energy and Fuels, 2007, 21(1): 82-87. |
| [1] | 郑志航, 马郡男, 闫子涵, 卢春喜. 提升管射流影响区内压力脉动特性研究[J]. 化工学报, 2023, 74(6): 2335-2350. |
| [2] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [3] | 李怀旭, 孙晓岩, 陶少辉, 夏力, 项曙光. 基于分子热力学性质和密度峰聚类的脱硫汽油集总[J]. 化工学报, 2022, 73(12): 5449-5460. |
| [4] | 陈昇, 王梦钶, 鲁波娜, 李秀峰, 刘岑凡, 刘梦溪, 范怡平, 卢春喜. 原料油汽化特性对催化裂化反应结焦过程影响的CFD模拟[J]. 化工学报, 2022, 73(7): 2982-2995. |
| [5] | 郑万鹏, 高小永, 朱桂瑶, 左信. 原油作业过程优化的研究进展[J]. 化工学报, 2021, 72(11): 5481-5501. |
| [6] | 刘晓艺, 李秀萍, 赵荣祥, 张豪. ZrO2/SiO2催化剂的制备及其氧化脱硫性能研究[J]. 化工学报, 2021, 72(11): 5653-5663. |
| [7] | 刘美佳,王刚,张忠东,许顺年,王皓,党法璐,何盛宝. 碳五烷烃裂解制低碳烯烃反应性能的分析[J]. 化工学报, 2021, 72(10): 5172-5182. |
| [8] | 赵岩, 李秀萍, 赵荣祥. 苯酚型低共熔溶剂中硫酸钛作为催化剂高效氧化脱硫[J]. 化工学报, 2021, 72(8): 4391-4400. |
| [9] | 王景效, 贺翔宇, 龚剑洪, 许建良, 刘海峰. 新型催化裂解快速流化床内颗粒浓度分布实验研究[J]. 化工学报, 2021, 72(8): 4104-4110. |
| [10] | 忻睦迪, 邢恩会. 三甲基膦和金属氧化物复合改性ZSM-5分子筛及其裂解性能研究[J]. 化工学报, 2021, 72(5): 2657-2668. |
| [11] | 魏彬, 周鑫, 王耀伟, 郭振莲, 陈小博, 刘熠斌, 杨朝合. 基于改进NSGA-Ⅱ算法的FCC分离系统多目标优化[J]. 化工学报, 2021, 72(5): 2735-2744. |
| [12] | 朱慧红, 茆志伟, 杨涛, 冯翔, 金浩, 彭冲, 杨朝合, 王继锋, 方向晨. 催化剂形貌对沸腾床渣油加氢Ni-Mo/Al2O3 催化剂活性位的影响机制[J]. 化工学报, 2021, 72(4): 2076-2085. |
| [13] | 隋宝宽, 施尧, 林见阳, 刘文洁, 袁胜华, 耿新国, 段学志. 焙烧气氛和孔结构对加氢脱金属催化剂性能的影响[J]. 化工学报, 2021, 72(2): 993-1000. |
| [14] | 吴沛文, 荀苏杭, 蒋伟, 李华明, 朱文帅. 离子液体反应型萃取燃油脱硫研究进展[J]. 化工学报, 2021, 72(1): 276-291. |
| [15] | 张睿, 董淑媛, 伍洛, 刘植昌, 徐春明, 刘海燕, 孟祥海. 小分子烷烃与烯烃在离子液体中的溶解性能[J]. 化工学报, 2020, 71(10): 4674-4687. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号