化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4674-4687.DOI: 10.11949/0438-1157.20200735
收稿日期:2020-06-10
修回日期:2020-08-12
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
孟祥海
作者简介:张睿(1979—),男,博士,副教授,基金资助:
Rui ZHANG( ),Shuyuan DONG,Luo WU,Zhichang LIU,Chunming XU,Haiyan LIU,Xianghai MENG(
),Shuyuan DONG,Luo WU,Zhichang LIU,Chunming XU,Haiyan LIU,Xianghai MENG( )
)
Received:2020-06-10
Revised:2020-08-12
Online:2020-10-05
Published:2020-10-05
Contact:
Xianghai MENG
摘要:
研究了多种离子液体对小分子烃类的溶解性能,发现含有Cu(Ⅰ)的离子液体对烃类具有较高的溶解度和烯烃/烷烃溶解选择性,优选出了Et3NHCl-2.1CuCl离子液体。考察了温度和压力对小分子烃类溶解性能的影响规律,发现低温和高压有利烃类的溶解,烯烃/烷烃溶解选择性随温度和压力的升高而减小;在30℃和0.2 MPa的条件下,烯烃/烷烃溶解选择性均在8.3以上。烃类在离子液体中的初始溶解速率较大,但随时间的延长快速降低,相同条件下烯烃的溶解速率高于烷烃的溶解速率。烯烃/烷烃分离选择性随混合气中烯烃含量的减小而增大。升温可以解吸出离子液体中的烃类,烷烃比烯烃容易解吸,优化条件下的解吸率可达92%以上。离子液体对小分子烷烃和烯烃的吸收分离具有良好的重复使用性能。利用Gaussian 09软件对离子液体的阴离子与烯烃、烷烃的作用进行了计算分析,解释了烯烃和烷烃在不同离子液体中溶解性能差异的原因。
中图分类号:
张睿, 董淑媛, 伍洛, 刘植昌, 徐春明, 刘海燕, 孟祥海. 小分子烷烃与烯烃在离子液体中的溶解性能[J]. 化工学报, 2020, 71(10): 4674-4687.
Rui ZHANG, Shuyuan DONG, Luo WU, Zhichang LIU, Chunming XU, Haiyan LIU, Xianghai MENG. Solubility of light alkanes and alkenes in ionic liquids[J]. CIESC Journal, 2020, 71(10): 4674-4687.
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/乙烷选择性 |
|---|---|---|---|
| Et3NHCl-0.6AlCl3 | 0.0280 | 0.0200 | 1.40 |
| Et3NHCl-0.6FeCl2 | 0.0235 | 0.0190 | 1.23 |
| Et3NHCl-0.6FeCl3 | 0.0231 | 0.0192 | 1.20 |
| Et3NHCl-0.6CuCl | 0.0396 | 0.0168 | 2.36 |
| [BMIM]Cl-0.6AlCl3 | 0.0241 | 0.0186 | 1.30 |
| [BMIM]Cl-0.6CuCl | 0.0291 | 0.0132 | 2.21 |
| Et3NHCl-1.0AlCl3 | 0.0284 | 0.0203 | 1.40 |
| Et3NHCl-1.0FeCl2 | 0.0290 | 0.0205 | 1.42 |
| Et3NHCl-1.0FeCl3 | 0.0281 | 0.0208 | 1.35 |
| Et3NHCl-1.0CuCl | 0.0404 | 0.0165 | 2.45 |
| [BMIM]Cl-1.0CuCl | 0.0300 | 0.0132 | 2.28 |
| [BMIM][BF4] | 0.0177 | 0.0142 | 1.25 |
表1 离子液体种类对乙烯与乙烷的溶解度和溶解选择性的影响
Table 1 Effect of ionic liquid types on solubility and solubility selectivity of ethene and ethane
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/乙烷选择性 |
|---|---|---|---|
| Et3NHCl-0.6AlCl3 | 0.0280 | 0.0200 | 1.40 |
| Et3NHCl-0.6FeCl2 | 0.0235 | 0.0190 | 1.23 |
| Et3NHCl-0.6FeCl3 | 0.0231 | 0.0192 | 1.20 |
| Et3NHCl-0.6CuCl | 0.0396 | 0.0168 | 2.36 |
| [BMIM]Cl-0.6AlCl3 | 0.0241 | 0.0186 | 1.30 |
| [BMIM]Cl-0.6CuCl | 0.0291 | 0.0132 | 2.21 |
| Et3NHCl-1.0AlCl3 | 0.0284 | 0.0203 | 1.40 |
| Et3NHCl-1.0FeCl2 | 0.0290 | 0.0205 | 1.42 |
| Et3NHCl-1.0FeCl3 | 0.0281 | 0.0208 | 1.35 |
| Et3NHCl-1.0CuCl | 0.0404 | 0.0165 | 2.45 |
| [BMIM]Cl-1.0CuCl | 0.0300 | 0.0132 | 2.28 |
| [BMIM][BF4] | 0.0177 | 0.0142 | 1.25 |
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/乙烷选择性 |
|---|---|---|---|
| Et3NHCl-1.0CuCl | 0.0050 | 0.0033 | 1.52 |
| Me3NHCl-1.0CuCl | 0.0048 | 0.0031 | 1.56 |
| Et2NH2Cl-1.0CuCl | 0.0044 | 0.0032 | 1.38 |
| Me2NH2Cl-1.0CuCl | 0.0034 | 0.0025 | 1.36 |
| EtNH3Cl-1.0CuCl | 0.0025 | 0.0022 | 1.14 |
表2 离子液体的阳离子供体对乙烯与乙烷的溶解度和溶解选择性的影响
Table 2 Effect of cation donor of ionic liquids on solubility and solubility selectivity of ethene and ethane
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/乙烷选择性 |
|---|---|---|---|
| Et3NHCl-1.0CuCl | 0.0050 | 0.0033 | 1.52 |
| Me3NHCl-1.0CuCl | 0.0048 | 0.0031 | 1.56 |
| Et2NH2Cl-1.0CuCl | 0.0044 | 0.0032 | 1.38 |
| Me2NH2Cl-1.0CuCl | 0.0034 | 0.0025 | 1.36 |
| EtNH3Cl-1.0CuCl | 0.0025 | 0.0022 | 1.14 |
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/ 乙烷选择性 |
|---|---|---|---|
| Et3NHCl-1.0CuCl | 0.0404 | 0.0165 | 2.45 |
| Et3NHCl-1.3CuCl | 0.0465 | 0.0159 | 2.92 |
| Et3NHCl-1.5CuCl | 0.0503 | 0.0154 | 3.26 |
| Et3NHCl-1.7CuCl | 0.0624 | 0.0109 | 5.70 |
| Et3NHCl-2.0CuCl | 0.0866 | 0.0102 | 8.47 |
| Et3NHCl-2.1CuCl | 0.0872 | 0.0099 | 8.78 |
| Et3NHCl-2.2CuCl | 0.0872 | 0.0099 | 8.81 |
| Et3NHCl-2.4CuCl | 0.0873 | 0.0100 | 8.71 |
表3 CuCl/Et3NHCl摩尔比对乙烯与乙烷的溶解度和溶解选择性的影响
Table 3 Effect of CuCl/Et3NHCl molar ratio on solubility and solubility selectivity of ethene and ethane
| 离子液体 | 乙烯溶解度/ (mol/mol) | 乙烷溶解度/ (mol/mol) | 乙烯/ 乙烷选择性 |
|---|---|---|---|
| Et3NHCl-1.0CuCl | 0.0404 | 0.0165 | 2.45 |
| Et3NHCl-1.3CuCl | 0.0465 | 0.0159 | 2.92 |
| Et3NHCl-1.5CuCl | 0.0503 | 0.0154 | 3.26 |
| Et3NHCl-1.7CuCl | 0.0624 | 0.0109 | 5.70 |
| Et3NHCl-2.0CuCl | 0.0866 | 0.0102 | 8.47 |
| Et3NHCl-2.1CuCl | 0.0872 | 0.0099 | 8.78 |
| Et3NHCl-2.2CuCl | 0.0872 | 0.0099 | 8.81 |
| Et3NHCl-2.4CuCl | 0.0873 | 0.0100 | 8.71 |
| 温度/℃ | 乙烯/ (mol/mol) | 乙烷/ (mol/mol) | 乙烯/乙烷选择性 | 丙烯/ (mol/mol) | 丙烷/ (mol/mol) | 丙烯/丙烷选择性 | 异丁烯/ (mol/mol) | 异丁烷/ (mol/mol) | 异丁烯/异丁烷选择性 |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 0.0872 | 0.0099 | 8.78 | 0.1366 | 0.0164 | 8.32 | 0.1982 | 0.0234 | 8.48 |
| 40 | 0.0612 | 0.0095 | 6.46 | 0.1123 | 0.0141 | 7.97 | 0.1683 | 0.0209 | 8.07 |
| 50 | 0.0463 | 0.0086 | 5.39 | 0.0802 | 0.0111 | 7.23 | 0.1175 | 0.0151 | 7.78 |
| 60 | 0.0347 | 0.0067 | 5.17 | 0.0522 | 0.0083 | 6.31 | 0.0822 | 0.0107 | 7.65 |
| 70 | 0.0204 | 0.0049 | 4.20 | 0.0302 | 0.0071 | 4.26 | 0.052 | 0.0073 | 7.15 |
表4 温度对小分子烷烃与烯烃溶解度及溶解选择性的影响
Table 4 Effect of temperature on solubility and solubility selectivity of alkanes and alkenes
| 温度/℃ | 乙烯/ (mol/mol) | 乙烷/ (mol/mol) | 乙烯/乙烷选择性 | 丙烯/ (mol/mol) | 丙烷/ (mol/mol) | 丙烯/丙烷选择性 | 异丁烯/ (mol/mol) | 异丁烷/ (mol/mol) | 异丁烯/异丁烷选择性 |
|---|---|---|---|---|---|---|---|---|---|
| 30 | 0.0872 | 0.0099 | 8.78 | 0.1366 | 0.0164 | 8.32 | 0.1982 | 0.0234 | 8.48 |
| 40 | 0.0612 | 0.0095 | 6.46 | 0.1123 | 0.0141 | 7.97 | 0.1683 | 0.0209 | 8.07 |
| 50 | 0.0463 | 0.0086 | 5.39 | 0.0802 | 0.0111 | 7.23 | 0.1175 | 0.0151 | 7.78 |
| 60 | 0.0347 | 0.0067 | 5.17 | 0.0522 | 0.0083 | 6.31 | 0.0822 | 0.0107 | 7.65 |
| 70 | 0.0204 | 0.0049 | 4.20 | 0.0302 | 0.0071 | 4.26 | 0.052 | 0.0073 | 7.15 |
| 压力/MPa | 乙烯/ (mol/mol) | 乙烷/ (mol/mol) | 乙烯/乙烷选择性 | 丙烯/ (mol/mol) | 丙烷/ (mol/mol) | 丙烯/丙烷选择性 | 异丁烯/ (mol/mol) | 异丁烷/ (mol/mol) | 异丁烯/ 异丁烷选择性 |
|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.0872 | 0.0099 | 8.78 | 0.1366 | 0.0164 | 8.32 | 0.1982 | 0.0234 | 8.48 |
| 0.245 | 0.0918 | 0.0152 | 6.02 | 0.1469 | 0.0183 | 8.04 | 0.2272 | 0.0275 | 8.25 |
| 0.3 | 0.1082 | 0.0212 | 5.09 | 0.1847 | 0.0234 | 7.89 | 0.2385 | 0.0311 | 7.67 |
| 0.4 | 0.1476 | 0.0367 | 4.02 | 0.2412 | 0.0422 | 5.72 | — | — | — |
| 0.48 | 0.1872 | 0.0468 | 4.00 | 0.2945 | 0.0523 | 5.63 | — | — | — |
| 0.59 | 0.2249 | 0.0584 | 3.85 | 0.3458 | 0.0642 | 5.39 | — | — | — |
| 0.735 | 0.2369 | 0.0639 | 3.71 | 0.3646 | 0.0685 | 5.33 | — | — | — |
| 0.81 | 0.2409 | 0.0678 | 3.55 | 0.3696 | 0.0702 | 5.27 | — | — | — |
表5 压力对小分子烷烃与烯烃溶解度及溶解选择性的影响
Table 5 Effect of pressure on solubility and solubility selectivity of alkanes and alkenes
| 压力/MPa | 乙烯/ (mol/mol) | 乙烷/ (mol/mol) | 乙烯/乙烷选择性 | 丙烯/ (mol/mol) | 丙烷/ (mol/mol) | 丙烯/丙烷选择性 | 异丁烯/ (mol/mol) | 异丁烷/ (mol/mol) | 异丁烯/ 异丁烷选择性 |
|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.0872 | 0.0099 | 8.78 | 0.1366 | 0.0164 | 8.32 | 0.1982 | 0.0234 | 8.48 |
| 0.245 | 0.0918 | 0.0152 | 6.02 | 0.1469 | 0.0183 | 8.04 | 0.2272 | 0.0275 | 8.25 |
| 0.3 | 0.1082 | 0.0212 | 5.09 | 0.1847 | 0.0234 | 7.89 | 0.2385 | 0.0311 | 7.67 |
| 0.4 | 0.1476 | 0.0367 | 4.02 | 0.2412 | 0.0422 | 5.72 | — | — | — |
| 0.48 | 0.1872 | 0.0468 | 4.00 | 0.2945 | 0.0523 | 5.63 | — | — | — |
| 0.59 | 0.2249 | 0.0584 | 3.85 | 0.3458 | 0.0642 | 5.39 | — | — | — |
| 0.735 | 0.2369 | 0.0639 | 3.71 | 0.3646 | 0.0685 | 5.33 | — | — | — |
| 0.81 | 0.2409 | 0.0678 | 3.55 | 0.3696 | 0.0702 | 5.27 | — | — | — |
| 吸收剂 | 压力/MPa | 温度/℃ | 烯烃 | 烷烃 | 选择性 | 文献 |
|---|---|---|---|---|---|---|
| [Emim][Tf2N]- 1.8 mol/L Ag[Tf2N] | 0.1 | 30 | ~0.34① | ~0.007① | ~71 | [ |
| [Emim][Tf2N]- 1.8 mol/L Ag[Tf2N] | 0.4 | 30 | ~0.42① | ~0.035① | ~20 | [ |
| [Emim][TfO]- 1.2 mol/L Ag[TfO] | 0.1 | 30 | ~0.18① | ~0.004① | ~44 | [ |
| [Emim][TfO]- 1.2 mol/L Ag[TfO] | 0.4 | 30 | ~0.28① | ~0.019① | ~20 | [ |
| [Bmim][BF4]- 1 mol/L Ag[BF4] | 0.1 | 25 | ~1.30② | ~0.028② | 46.6 | [ |
| [Bmim][BF4]- 1 mol/L Ag[BF4] | 0.2 | 25 | ~1.52② | ~0.054② | 27.9 | [ |
| [Bmim][BF4]- 0.5 mol/L Ag[BF4] | 0.2 | 25 | ~0.85② | ~0.056② | 15.2 | [ |
| [Bmpy][BF4]- 1 mol/L Ag[BF4] | 0.1 | 25 | ~1.72② | ~0.028② | 61.3 | [ |
| [Bmpy][BF4]- 1 mol/L Ag[BF4] | 0.2 | 25 | ~1.98② | ~0.057② | 34.9 | [ |
| [Bmpy][BF4]- 0.5 mol/L Ag[BF4] | 0.2 | 25 | ~1.00② | ~0.065② | 15.5 | [ |
| [Bmim]Cl-2 mol/L CuCl | 0.1 | 25 | 0.051② | 0.0042② | 12.1 | [ |
| [Bmim]Cl-pyridine-2 mol/L CuCl | 0.1 | 25 | 0.064② | 0.0105② | 6.1 | [ |
| [Bmim][SCN]- 1.5 mol/L CuSCN | 0.1 | 25 | 0.12② | 0.012② | 10 | [ |
| [Emim][SCN]- 1.5 mol/L CuSCN | 0.1 | 25 | 0.09② | 0.011② | 8.18 | [ |
| [Bmim][Br]- 2 mol/L CuBr | 0.1 | 25 | 0.13② | 0.01② | 13 | [ |
| [Bmim][Br]- 2 mol/L CuCl | 0.1 | 25 | 0.12② | 0.01② | 12 | [ |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.0872③ | 0.0099③ | 8.78 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.1366④ | 0.0164④ | 8.32 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.439⑤ | 0.050⑤ | 8.78 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.687② | 0.082② | 8.32 | 本文 |
表6 小分子烷烃与烯烃在不同吸收剂中的溶解性能
Table 6 Solubility of light alkanes and alkenes in different absorbents
| 吸收剂 | 压力/MPa | 温度/℃ | 烯烃 | 烷烃 | 选择性 | 文献 |
|---|---|---|---|---|---|---|
| [Emim][Tf2N]- 1.8 mol/L Ag[Tf2N] | 0.1 | 30 | ~0.34① | ~0.007① | ~71 | [ |
| [Emim][Tf2N]- 1.8 mol/L Ag[Tf2N] | 0.4 | 30 | ~0.42① | ~0.035① | ~20 | [ |
| [Emim][TfO]- 1.2 mol/L Ag[TfO] | 0.1 | 30 | ~0.18① | ~0.004① | ~44 | [ |
| [Emim][TfO]- 1.2 mol/L Ag[TfO] | 0.4 | 30 | ~0.28① | ~0.019① | ~20 | [ |
| [Bmim][BF4]- 1 mol/L Ag[BF4] | 0.1 | 25 | ~1.30② | ~0.028② | 46.6 | [ |
| [Bmim][BF4]- 1 mol/L Ag[BF4] | 0.2 | 25 | ~1.52② | ~0.054② | 27.9 | [ |
| [Bmim][BF4]- 0.5 mol/L Ag[BF4] | 0.2 | 25 | ~0.85② | ~0.056② | 15.2 | [ |
| [Bmpy][BF4]- 1 mol/L Ag[BF4] | 0.1 | 25 | ~1.72② | ~0.028② | 61.3 | [ |
| [Bmpy][BF4]- 1 mol/L Ag[BF4] | 0.2 | 25 | ~1.98② | ~0.057② | 34.9 | [ |
| [Bmpy][BF4]- 0.5 mol/L Ag[BF4] | 0.2 | 25 | ~1.00② | ~0.065② | 15.5 | [ |
| [Bmim]Cl-2 mol/L CuCl | 0.1 | 25 | 0.051② | 0.0042② | 12.1 | [ |
| [Bmim]Cl-pyridine-2 mol/L CuCl | 0.1 | 25 | 0.064② | 0.0105② | 6.1 | [ |
| [Bmim][SCN]- 1.5 mol/L CuSCN | 0.1 | 25 | 0.12② | 0.012② | 10 | [ |
| [Emim][SCN]- 1.5 mol/L CuSCN | 0.1 | 25 | 0.09② | 0.011② | 8.18 | [ |
| [Bmim][Br]- 2 mol/L CuBr | 0.1 | 25 | 0.13② | 0.01② | 13 | [ |
| [Bmim][Br]- 2 mol/L CuCl | 0.1 | 25 | 0.12② | 0.01② | 12 | [ |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.0872③ | 0.0099③ | 8.78 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.1366④ | 0.0164④ | 8.32 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.439⑤ | 0.050⑤ | 8.78 | 本文 |
| Et3NHCl-2.1CuCl | 0.2 | 30 | 0.687② | 0.082② | 8.32 | 本文 |
| 离子液体重复利用次数 | 效率/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 乙烷溶解度 | 乙烯溶解度 | 乙烯/乙烷 选择性 | 丙烷溶解度 | 丙烯溶解度 | 丙烯/丙烷 选择性 | 异丁烷 溶解度 | 异丁烯 溶解度 | 异丁烯/异丁烷选择性 | |
| 1 | 99.81 | 99.64 | 99.83 | 99.27 | 98.52 | 99.25 | 99.01 | 98.33 | 99.31 |
| 2 | 99.19 | 98.43 | 99.23 | 98.71 | 98.03 | 99.31 | 98.2 | 97.72 | 99.51 |
| 3 | 98.62 | 98.02 | 99.39 | 98.12 | 97.38 | 99.25 | 97.28 | 96.41 | 99.11 |
| 4 | 98.04 | 97.08 | 99.03 | 97.27 | 96.74 | 99.46 | 96.53 | 95.77 | 99.22 |
| 5 | 97.33 | 96.78 | 99.43 | 96.20 | 95.42 | 99.19 | 95.71 | 94.22 | 98.45 |
表7 离子液体对小分子烃类溶解的重复利用效率
Table 7 Reusing efficiency of ionic liquid for dissolution of light hydrocarbons
| 离子液体重复利用次数 | 效率/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 乙烷溶解度 | 乙烯溶解度 | 乙烯/乙烷 选择性 | 丙烷溶解度 | 丙烯溶解度 | 丙烯/丙烷 选择性 | 异丁烷 溶解度 | 异丁烯 溶解度 | 异丁烯/异丁烷选择性 | |
| 1 | 99.81 | 99.64 | 99.83 | 99.27 | 98.52 | 99.25 | 99.01 | 98.33 | 99.31 |
| 2 | 99.19 | 98.43 | 99.23 | 98.71 | 98.03 | 99.31 | 98.2 | 97.72 | 99.51 |
| 3 | 98.62 | 98.02 | 99.39 | 98.12 | 97.38 | 99.25 | 97.28 | 96.41 | 99.11 |
| 4 | 98.04 | 97.08 | 99.03 | 97.27 | 96.74 | 99.46 | 96.53 | 95.77 | 99.22 |
| 5 | 97.33 | 96.78 | 99.43 | 96.20 | 95.42 | 99.19 | 95.71 | 94.22 | 98.45 |
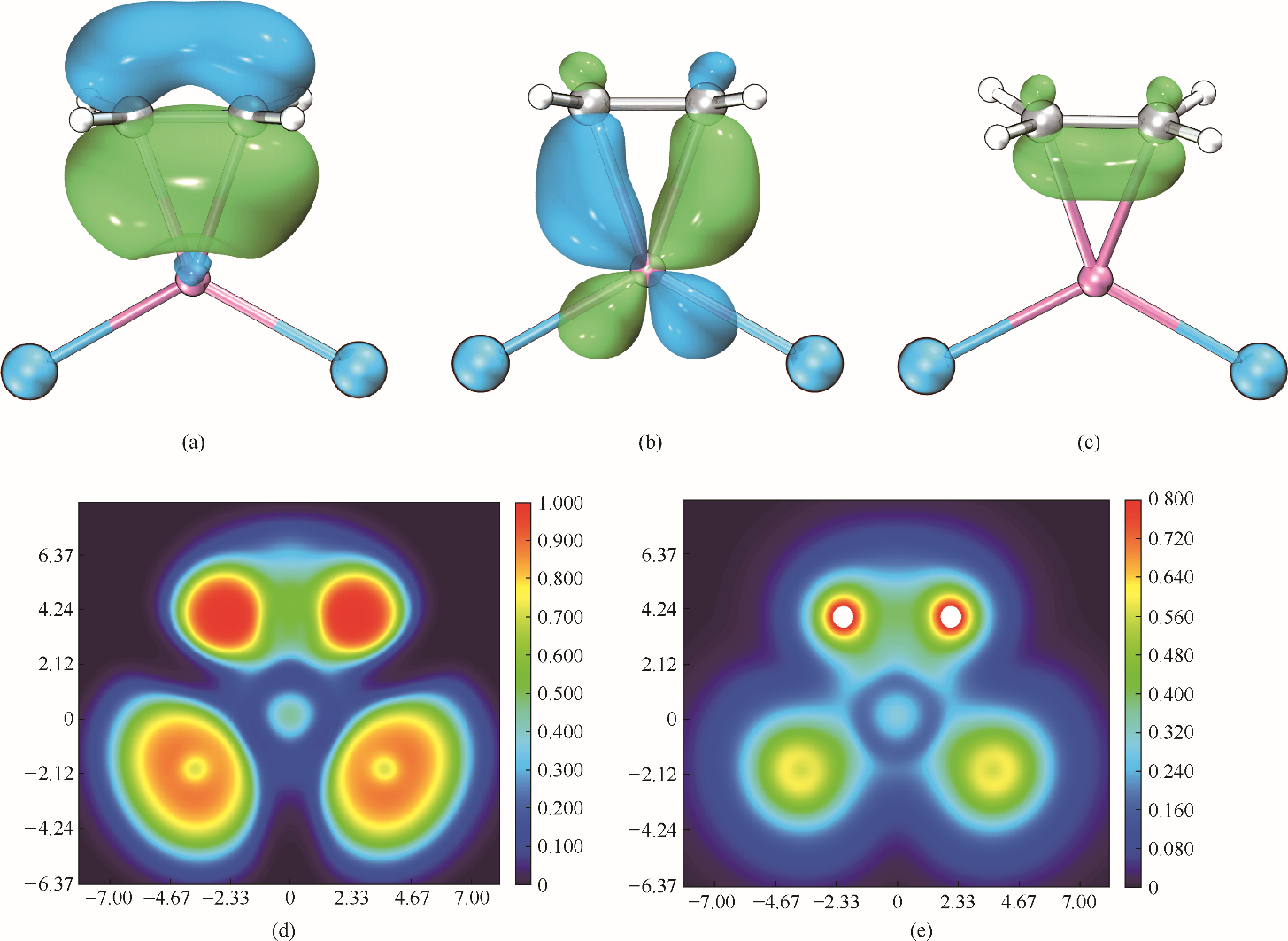
图9 [CuCl2]ˉ-C2H4络合物的定域化轨道[(a)、(b)];π电子密度等值面图(c)(等值面设为0.05);ELF和LOL平面图[(d)、(e)]
Fig.9 LMOs[(a),(b)], π electron density isosurface map (isovalue=0.05)(c), ELF(d) and LOL(e) color filled map of [CuCl2]ˉ-C2H4 complex
| 含Cu阴离子 | 原子电荷 | Cu的亲核指数/eV |
|---|---|---|
| [CuCl2]ˉ | 0.0524 | 1.1281 |
| [Cu2Cl3]ˉ | 0.1304 | 1.2452 |
表8 两种含Cu阴离子中Cu的Hirshfeld原子电荷和亲核指数
Table 8 Hirshfeld atomic charge and nucleophilicity index
| 含Cu阴离子 | 原子电荷 | Cu的亲核指数/eV |
|---|---|---|
| [CuCl2]ˉ | 0.0524 | 1.1281 |
| [Cu2Cl3]ˉ | 0.1304 | 1.2452 |
| 1 | Gao Y F, Neal L, Ding D, et al. Recent advances in intensified ethylene production— a review[J]. ACS Catalysis, 2019, 9: 8592-8621. |
| 2 | 王梦瑶, 周嘉文, 任天华, 等. 催化裂化多产丙烯[J]. 化工进展, 2015, 34(6): 1619-1624. |
| Wang M Y, Zhou J W, Ren T H, et al. Catalytic cracking processes for maximizing propylene production[J]. Chemical Industry and Engineering Progress, 2015, 34(6): 1619-1624. | |
| 3 | Ali M A, Ahmed S, Al-Baghli N, et al. A comprehensive review covering conventional and structured catalysis for methanol to propylene conversion[J]. Catalysis Letters, 2019, 149: 3395-3424. |
| 4 | Shi L, Wang Y, Yan B, et al. Progress in selective oxidative dehydrogenation of light alkanes to olefins promoted by boron nitride catalysts[J]. Chemical Communications, 2018, 54: 10936-10946. |
| 5 | Azhin M, Kaghazchi T, Rahmani M. A review on olefin/paraffin separation using reversible chemical complexation technology[J]. Journal of Industrial and Engineering Chemistry, 2008, 14(5): 622-638. |
| 6 | 杨学萍. 轻质烯烃-烷烃分离新工艺开发进展[J]. 化工进展, 2005, 24(4): 367-371. |
| Yang X P. Advances in light olefin - paraffin separation technology[J]. Chemical Industry and Engineering Progress, 2005, 24(4): 367-371. | |
| 7 | Lei Z G, Li C Y, Chen B H. Extractive distillation: a review[J]. Separation and Purification Reviews, 2003, 32(2): 121-213. |
| 8 | Matsuyama E, Ikeda A, Komatsuzaki M, et al. High-temperature propylene/propane separation through silica hybrid membranes[J]. Separation and Purification Technology, 2014, 128: 25-30. |
| 9 | Lei Z G, Dai C N, Chen B H. Gas solubility in ionic liquids[J]. Chemical Reviews, 2014, 114(2): 1289-1326. |
| 10 | 赵旭, 邢华斌, 李如龙, 等. 离子液体在气体分离中的应用[J]. 化学进展, 2011, 23(11): 2258-2268. |
| Zhao X, Xing H B, Li R L, et al. Gas separation based on ionic liquids[J]. Progress in Chemistry, 2011, 23(11): 2258-2268. | |
| 11 | 曹领帝, 曾少娟, 张香平, 等. 离子液体吸收分离硫化氢进展[J]. 化工学报, 2015, 66: 1-9. |
| Cao L D, Zeng S J, Zhang X P, et al. Progress on hydrogen sulfide removal using ionic liquids[J]. CIESC Journal, 2015, 66: 1-9. | |
| 12 | 王兰云, 李振东, 位亚南, 等. 基于离子液体吸收法的气体分离研究进展[J]. 煤炭学报, 2018, 43(3): 704-716. |
| Wang L Y, Li Z D, Wei Y N, et al. Gas separation by absorption in pure ionic liquids[J]. Journal of China Coal Society, 2018, 43(3): 704-716. | |
| 13 | Zhang X P, Zhang X C, Dong H F, et al. Carbon capture with ionic liquids: overview and progress[J]. Energy & Environmental Science, 2012, 5: 6668-6681. |
| 14 | Cadena C, Anthony J L, Shah J K, et al. Why is CO2 so soluble in imidazolium-based ionic liquids[J]. Journal of the American Chemical Society, 2004, 126(16): 5300-5308. |
| 15 | 孟祥海, 刘植昌, 张睿, 等. 用于吸收酸性气体的含胺基离子液体及其制备方法与应用: 101993378[P]. 2011-03-30. |
| Meng X H, Liu Z H, Zhang R, et al. Amine-containing ionic liquids used for absorbing acid gases and their preparation and application: 101993378[P]. 2011-03-30. | |
| 16 | Hu P C, Zhang R, Liu Z H, et al. Absorption performance and mechanism of CO2 in aqueous solutions of amine-based ionic liquids[J]. Energy & Fuels, 2015, 29: 6019-6024. |
| 17 | Moura M, Santini C C, Gomes M F C. Gaseous hydrocarbon separations using functionalized ionic liquids[J]. Oil & Gas Science and Technology - Revue d'IFP Energies Nouvelles, 2016, 71(2): 1-11. |
| 18 | Moura L, Darwich W, Santini C C, et al. Imidazolium-based ionic liquids with cyano groups for the selective absorption of ethane and ethylene[J]. Chemical Engineering Journal, 2015, 280: 755-762. |
| 19 | Xing H B, Zhao X, Li R L, et al. Improved efficiency of ethylene/ethane separation using a symmetrical dual nitrile-functionalized ionic liquid[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(11): 1357-1363. |
| 20 | Mokrushin V, Assenbaum D, Paape N, et al. Ionic liquids for propene-propane separation[J]. Chemical Engineering Technology, 2010, 33(1): 63-73. |
| 21 | Jacquemin J, Gomes M F C, Husson P, et al. Solubility of carbon dioxide, ethane, methane, oxygen, nitrogen, hydrogen, argon, and carbon monoxide in 1-butyl-3-methylimidazolium tetrafluoroborate between temperatures 283 K and 343 K and at pressures close to atmospheric[J]. Journal of Chemical Thermodynamics, 2006, 38(4): 490-502. |
| 22 | Anthony J L, Maginn E J, Brennecke J F. Solubilities and thermodynamic properties of gases in the ionic liquid 1-n-butyl-3-methylimidazolium hexafluorophosphate[J]. Journal of Physical Chemistry B, 2002, 106(29): 7315-7320. |
| 23 | Lee B C, Outcalt S L. Solubilities of gases in the ionic liquid 1-n-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide[J]. Journal of Chemical and Engineering Data, 2006, 51(3): 892-897. |
| 24 | Fallanza M, González-Miquel M, Ruiz E, et al. Screening of RTILs for propane/propylene separation using COSMO-RS methodology[J]. Chemical Engineering Journal, 2013, 220: 284-293. |
| 25 | Kilaru P K, Condemarin R A, Scovazzo P. Correlations of low-pressure carbon dioxide and hydrocarbon solubilities in imidazolium-, phosphonium-, and ammonium-based room-temperature ionic liquids(1):Using surface tension[J]. Industrial & Engineering Chemistry Research, 2008, 47(3): 900-909. |
| 26 | Palgunadi J, Kim H S, Lee J M, et al. Ionic liquids for acetylene and ethylene separation: material selection and solubility investigation[J]. Chemical Engineering and Processing: Process Intensification, 2010, 49(2): 192-198. |
| 27 | Xing H B, Zhao X, Yang Q W, et al. Molecular dynamics simulation study on the absorption of ethylene and acetylene in ionic liquids[J]. Industrial & Engineering Chemistry Research, 2013, 52(26): 9308-9316. |
| 28 | Huang Y Q, Zhang Y B, Xing H B. Separation of light hydrocarbons with ionic liquids: a review[J]. Chinese Journal of Chemical Engineering, 2019, 27(6): 1374-1382. |
| 29 | Sánchez L M G, Meindersma G W, Haan A B. Potential of silver-based room-temperature ionic liquids for ethylene/ethane separation[J]. Industrial & Engineering Chemistry Research, 2009, 48(23):10650-10656. |
| 30 | Ortiz A, Gorri D, Irabien A, et al. Separation of propylene/propane mixtures using Ag+-RTIL solutions. Evaluation and comparison of the performance of gas-liquid contactors[J]. Journal of Membrane Science, 2010, 360(1/2): 130-141. |
| 31 | Marcos F, Ortiz A, Gorri D, et al. Propylene and propane solubility in imidazolium, pyridinium, and tetralkylammonium based ionic liquids containing a silver salt[J]. Journal of Chemical & Engineering Data, 2013, 58(8): 2147-2153. |
| 32 | Shen X L, Abro R, Alhumaydhi I A, et al. Separation of propylene and propane by functional mixture of imidazolintum chloride ionic liquid - organic solvent - cuprous salt[J]. Separation and Purification Technology, 2017, 175: 177-184. |
| 33 | Ortiz A, Galan L M, Gorri D, et al. Reactive ionic liquid media for the separation of propylene/propane gaseous mixtures[J]. Industrial & Engineering Chemistry Research, 2010, 49(16): 7227-7233. |
| 34 | Yu G R, Zhang L, Alhumaydhi I A, et al. Separation of propylene and propane by alkylimidazolium thiocyanate ionic liquids with Cu+ salt[J]. Separation and Purification Technology, 2015, 156: 356-362. |
| 35 | Yu G R, Deng L Y, Abdeltawab A A, et al. Functional solution composed of Cu(Ⅰ) salt and ionic liquids to separate propylene from propane[J]. Industrial & Engineering Chemistry Research, 2014, 53(34): 13430-13435. |
| 36 | Neese F. The ORCA program system[J]. Wiley Interdisciplinary Reviews: Computational Molecular Science, 2012, 2(1): 73-78. |
| 37 | Lu T, Chen F. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 38 | Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. |
| 39 | Safarik D J, Eldridge R B. Olefin/paraffin separations by reactive absorption: a review[J]. Industrial & Engineering Chemistry Research, 1998, 37(7): 2571-2581. |
| 40 | Pipek J, Mezey P G. A fast intrinsic localization procedure applicable for abinitio and semiempirical linear combination of atomic orbital wave functions[J]. The Journal of Chemical Physics, 1989, 90(9): 4916-4926. |
| 41 | Becke A D, Edgecombe K E. A simple measure of electron localization in atomic and molecular systems[J]. The Journal of Chemical Physics, 1990, 92(9): 5397-5403. |
| 42 | Schmider H L, Becke A D. Chemical content of the kinetic energy density[J]. Journal of Molecular Structure: THEOCHEM, 2000, 527(1/2/3): 51-61. |
| 43 | Davidson E R, Chakravorty S. A test of the Hirshfeld definition of atomic charges and moments[J]. Theoretica Chimica Acta, 1992, 83(5/6): 319-330. |
| 44 | Geerlings P, De Proft F, Langenaeker W. Conceptual density functional theory[J]. Chemical Reviews, 2003, 103(5): 1793-1874. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 吴曦, 区祖迪, 张鑫杰, 徐士鸣, 朱晓静. HFO-1243zf爆燃特性实验研究[J]. 化工学报, 2023, 74(S1): 346-352. |
| [4] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [5] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [6] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [7] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [8] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [9] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [10] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [13] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [14] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [15] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号