化工学报 ›› 2020, Vol. 71 ›› Issue (1): 409-416.DOI: 10.11949/0438-1157.20191271
收稿日期:2019-09-24
修回日期:2019-10-24
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
张鹏飞
作者简介:孟敏珊(1996—),女,硕士研究生,基金资助:
Minshan MENG( ),Jiahua ZHAO,Pengfei ZHANG(
),Jiahua ZHAO,Pengfei ZHANG( )
)
Received:2019-09-24
Revised:2019-10-24
Online:2020-01-05
Published:2020-01-05
Contact:
Pengfei ZHANG
摘要:
有序介孔碳是一种具有宽孔径、规则孔道结构、高比表面积和大孔容的纳米结构材料,具有很高的导电性及化学稳定性,是一种非常优良的载体材料。过渡金属碳化物因其结构相似性,具有一系列类似贵金属的性质,可作为贵金属替代材料,用于多相催化过程。但过渡金属碳化物多数粒径大、比表面积低,不利于催化活性,因此,采用熔盐法合成了多种有序介孔碳负载的过渡金属碳化物,并通过SEM、TEM、XRD、BET等方法对样品进行了一系列表征。结果表明,该方法能有效制备碳负载的TiC、Mo2C等金属碳化物,且具有较小的颗粒尺寸和较高的比表面积,将有较好的催化应用前景。
中图分类号:
孟敏珊, 赵佳华, 张鹏飞. 熔盐法合成有序介孔碳负载的金属碳化物[J]. 化工学报, 2020, 71(1): 409-416.
Minshan MENG, Jiahua ZHAO, Pengfei ZHANG. Synthesis of carbides supported on ordered mesoporous carbon by molten salt method[J]. CIESC Journal, 2020, 71(1): 409-416.
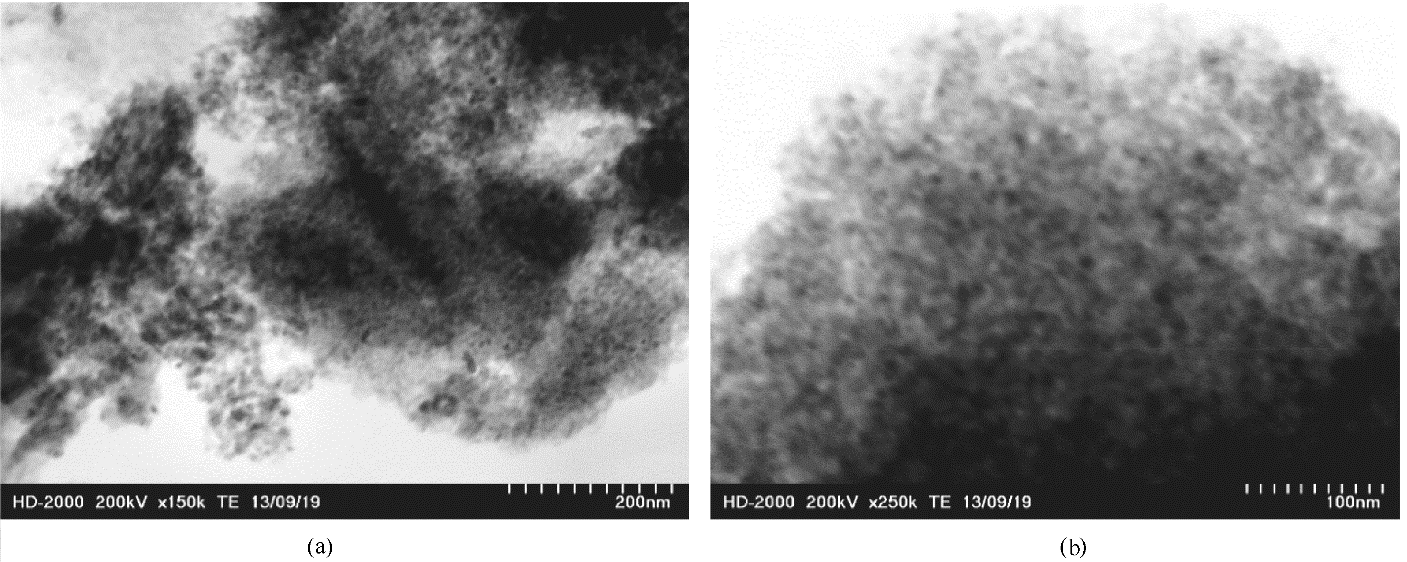
图3 有序介孔碳负载碳化钛的透射电子显微镜图及粒径分布图
Fig.3 Transmission electron microscopy images of ordered mesoporous carbon-supported titanium carbide and image of particle size distribution

图6 活化后的有序介孔碳载体(OM carbon)的BET曲线(a)和孔径分布(b)
Fig.6 BET curve (a) and pore size distribution (b) of activated ordered mesoporous carbon carrier (OM carbon)
| 材料名称 | 比表面积/(m2/g) | 孔容/(cm3/g) | 孔径/nm |
|---|---|---|---|
| TiC@C | 211 | 0.14 | 7.8 |
| Mo2C@C | 438 | 0.58 | 7.6 |
| Fe3C@C | 157 | 0.13 | 7.1 |
| OM carbon | 1810 | 1.16 | 6.8 |
表1 合成样品的孔道参数
Table 1 Channel structure parameters for as-prepared samples
| 材料名称 | 比表面积/(m2/g) | 孔容/(cm3/g) | 孔径/nm |
|---|---|---|---|
| TiC@C | 211 | 0.14 | 7.8 |
| Mo2C@C | 438 | 0.58 | 7.6 |
| Fe3C@C | 157 | 0.13 | 7.1 |
| OM carbon | 1810 | 1.16 | 6.8 |
| 1 | 凌晓凤, 顾娟, 李健生, 等. 一步法合成载铁有序介孔碳材料的形成机理[J]. 化工进展, 2012, 31(1): 156-162. |
| Ling X F, Gu J, Li J S, et al. Mechanism of the synthesis of ordered Fe-containing mesoporous carbon composite materials in one-pot[J]. Chemical Industry and Engineering Progress, 2012, 31(1): 156-162. | |
| 2 | 赵亚丽, 何臻, 俞强, 等. 简易模板法制备有序介孔碳及其表征[J]. 化工进展, 2014, 33(9): 2392-2397. |
| Zhao Y L, He Z, Yu Q, et al. Preparation and characterization of ordered mesoporous carbon by simple template method[J]. Chemical Industry and Engineering Progress, 2014, 33(9): 2392-2397. | |
| 3 | Li Z, Jaroniec M. Colloidal imprinting: a novel approach to the synthesis of mesoporous carbons[J]. Journal of the American Chemical Society, 2001, 123(37): 9208-9209. |
| 4 | Ariyadejwanich P, Tanthapanichakoon W, Nakagawa K, et al. Preparation and characterization of mesoporous activated carbon from waste tires[J]. Carbon, 2003, 41(1): 157-164. |
| 5 | Ohkubo T, Miyawaki J, Kaneko K, et al. Adsorption properties of templated mesoporous carbon (CMK-1) for nitrogen and supercritical methane: experiment and GCMC simulation[J]. The Journal of Physical Chemistry B, 2002, 106(25): 6523-6528. |
| 6 | Qin H, Jian R, Bai J, et al. Influence of molecular weight on structure and catalytic characteristics of ordered mesoporous carbon derived from lignin[J]. ACS Omega, 2018, 3(1): 1350-1356. |
| 7 | Li K, Jie S, Yuan L, et al. The synthesis of N, S-codoped ordered mesoporous carbon as an efficient metal-free catalyst for selective oxidation of arylalkanes[J]. Catalysis Communications, 2018, 112: 39-42. |
| 8 | Oyama S T. The Chemistry of Transition Metal Carbides and Nitrides[M]. Dordrecht: Springer, 1996. |
| 9 | 石宝宝, 贾志军, 王毅, 等. 新型二维过渡金属碳化物研究进展[J]. 化工新型材料, 2017, 45(12): 16-20. |
| Shi B B, Jia Z J, Wang Y, et al. Research progress of new two-dimensional transition metal carbides[J]. New Chemical Materials, 2017, 45(12): 16-20. | |
| 10 | 甘赠国, 黄志宇, 庞纪峰, 等. 过渡金属碳化物的催化研究进展[J]. 精细石油化工进展, 2007, 8(6): 37-41. |
| Gan Z G, Huang Z Y, Pang J F, et al. Research development on catalysis of transition metal carbides[J]. Advances in Fine Petrochemicals, 2007, 8(6): 37-41. | |
| 11 | 章永凡, 李俊篯, 丁开宁, 等. 过渡金属碳化物(111)面电子结构的理论研究[J]. 物理化学学报, 2003, 19(1): 40-45. |
| Zhang Y F, Li J Q, Ding K N, et al. Theoretical studies on the geometries and electronic structures of the (111) surfaces of transition-metai carbides[J]. Acta Phys.-Chim. Sin., 2003, 19(1): 40-45. | |
| 12 | Zhang T, Guo X, Zhao Z. Glucose-assisted preparation of a nickel-molybdenum carbide bimetallic catalyst for chemoselective hydrogenation of nitroaromatics and hydrodeoxygenation of m‑cresol[J]. ACS Applied Nano Materials, 2018, 1(7): 3579-3589. |
| 13 | Lin Z, Wan W, Yao S, et al. Cobalt-modified molybdenum carbide as a selective catalyst for hydrodeoxygenation of furfural[J]. Applied Catalysis B Environmental, 2018, 233: 160-166. |
| 14 | 靳广洲, 朱建华, 俱虎良, 等. 碳化钼催化剂的制备及噻吩加氢脱硫性能[J]. 化工学报, 2006, 57(4): 799-804. |
| Jin G Z, Zhu J H, Ju H L, et al. Preparation of molybdenum carbide catalyst and its hydrodesulfurization performance for thiophene[J]. Journal of Chemical Industry and Engineering (China), 2006, 57(4): 799-804. | |
| 15 | Ordan yan S S, Nesmelov D D, Ovsienko A I. Phase formation during reactive sintering of the B4C–SiC–Si (Al) composite (review)[J]. Refractories and Industrial Ceramics, 2018, 58(6): 1-7. |
| 16 | Gaziev G A, Krylov O V, Roginskii S Z, et al. Dehydrogenation of cyclohexane on certain carbides borides and silicides[J]. Dokl. Akad. Nauk SSSR, 1961, 140(4): 863-867. |
| 17 | Levy R L, Boudart M. Platinum-like behavior of tungsten carbide in surface catalysis[J]. Science, 1973, 181(4099): 547-549. |
| 18 | 章永凡, 林伟, 王文峰, 等. 3d过渡金属碳化物相稳定性和化学键的第一性原理研究[J]. 化学学报, 2004, 62(11): 1041-1048. |
| Zhang Y F, Lin W, Wang W F, et al. A first principle study on the phase stability and chemical bonding of the 3d transition metal carbides[J]. Acta Chimica Sinica, 2004, 62(11): 1041-1048. | |
| 19 | Aegerter P A, Quigley W W C, Simpson G J, et al. Thiophene hydrodesulfurization over alumina-supported molybdenum carbide and nitride catalysts: adsorption sites, catalytic activities, and nature of the active surface[J]. Journal of Catalysis, 1996, 164(1): 109-121. |
| 20 | Li S, Lee J S, Hyeon T, et al. Catalytic hydrodenitrogenation of indole over molybdenum nitride and carbides with different structures[J]. Applied Catalysis A General, 1999, 184(1): 1-9. |
| 21 | Nagai M, Kurakami T, Omi S. Activity of carbided molybdena–alumina for CO2 hydrogenation[J]. Catalysis Today, 1998, 45(1): 235-239. |
| 22 | Xiao T C, Hanif A, York A P E, et al. Study on the mechanism of partial oxidation of methane to synthesis gas over molybdenum carbide catalyst[J]. Physical Chemistry Chemical Physics, 2002, 4(18): 4549-4554. |
| 23 | Sehested J, Jacobsen C J H, Rokni S, et al. Activity and stability of molybdenum carbide as a catalyst for CO2 reforming[J]. Journal of Catalysis, 2001, 201(2): 206-212. |
| 24 | Ranhotra G S, Bell A T, Reimer J A. Catalysis over molybdenum carbides and nitrides(Ⅱ): Studies of CO hydrogenation and C2H6 hydrogenolysis[J]. Journal of Catalysis, 1987, 108(1): 40-49. |
| 25 | Hemming F, Wehrer P, Katrib A, et al. Reactivity of hexanes (2MP, MCP and CH) on W, W2C and WC powders(Ⅱ): Approach to the reaction mechanisms using concepts of organometallic chemistry[J]. Journal of Molecular Catalysis A Chemical, 1997, 124(1): 39–56. |
| 26 | Yuan S D, Hamid S B D, Li Y X, et al. Preparation of Mo2C/HZSM-5 and its catalytic performance for the conversion of n-butane into aromatics[J]. Journal of Molecular Catalysis A Chemical, 2002, 184(1): 257-266. |
| 27 | Zhang H, Cheng Y T, Vispute T P, et al. Catalytic conversion of biomass-derived feedstocks into olefins and aromatics with ZSM-5: the hydrogen to carbon effective ratio[J]. Energy & Environmental Science, 2011, 4(6): 2297-2307. |
| 28 | Ye J, Zhang S, Lee W E. Molten salt synthesis and characterization of SiC coated carbon black particles for refractory castable applications[J]. Journal of the European Ceramic Society, 2013, 33(10): 2023-2029. |
| 29 | Liu X, Antonietti M, Giordano C. Manipulation of phase and microstructure at nanoscale for SiC in molten salt synthesis[J]. Chemistry of Materials, 2013, 25(10): 2021-2027. |
| 30 | Li X, Dong Z, Westwood A, et al. Low-temperature preparation of single crystal titanium carbide nanofibers in molten salts[J]. Crystal Growth & Design, 2011, 11(7): 3122-3129. |
| 31 | Chen D, Zhao J H, Zhang P F, et al. Mechanochemical synthesis of metal–organic frameworks[J]. Polyhedron, 2019, 162: 59-64. |
| 32 | Zhao J H, Shu Y, Zhang P F. Solid-state CTAB-assisted synthesis of mesoporous Fe3O4 and Au@Fe3O4 by mechanochemistry[J]. Chinese Journal of Catalysis, 2019, 40(7): 1078-1084 |
| 33 | Wang X Q, Lee J S, Tsouris C, et al. Preparation of activated mesoporous carbons for electrosorption of ions from aqueous solutions[J]. Journal of Materials Chemistry, 2010, 20(22): 4602-4608. |
| 34 | Liu Z, Ling X Y, Su X, et al. Carbon-supported Pt and PtRu nanoparticles as catalysts for a direct methanol fuel cell[J]. The Journal of Physical Chemistry B, 2004, 108(24): 8234-8240. |
| 35 | Tian Z Q, Jiang S P, Liang Y M, et al. Synthesis and characterization of platinum catalysts on multiwalled carbon nanotubes by intermittent microwave irradiation for fuel cell applications[J]. The Journal of Physical Chemistry B, 2006, 110(11): 5343-5350. |
| [1] | 党迎喜, 谈朋, 刘晓勤, 孙林兵. 辐射冷却和太阳能加热驱动的CO2变温捕获[J]. 化工学报, 2023, 74(1): 469-478. |
| [2] | 刘学安, 汤丽怡, 覃健, 唐大江, 童张法, 曲慧颖. 热解Ni/Co-ZIF-8制备碳纳米管桥连多孔碳及其在超级电容器中的应用[J]. 化工学报, 2022, 73(7): 3287-3297. |
| [3] | 陈子禾, 赵呈志, 冒文莉, 盛楠, 朱春宇. 定向生物质多孔碳复合相变材料的制备及其热性能研究[J]. 化工学报, 2022, 73(4): 1817-1825. |
| [4] | 任博阳, 车晓刚, 刘思宇, 王满, 韩兴华, 董婷, 杨卷. 熔融盐法制备煤基多孔碳纳米片用于钠离子电池负极[J]. 化工学报, 2022, 73(10): 4745-4753. |
| [5] | 方辉煌, 吴历洁, 陈伟坤, 袁友珠. 生物质基含氧化合物在过渡金属碳化物上加氢脱氧研究进展[J]. 化工学报, 2021, 72(7): 3562-3575. |
| [6] | 那天成, 李祥村, 郭娇, 刘思远, 杨宏杰, 姜贺龙, 姜福林, 贺高红. Fe2C和氮共掺杂的具有有序孔道结构碳膜用于锂硫电池正极[J]. 化工学报, 2021, 72(4): 2283-2292. |
| [7] | 夏争争, 刘加亮, 牛建杰, 胡涵, 赵青山, 吴明铂. 高分散SiO2/石油沥青基多孔碳用于锂离子电池负极[J]. 化工学报, 2020, 71(6): 2752-2759. |
| [8] | 邹雷, 刘国强, 江苗苗, 杨则恒, 张卫新. ZIF-67衍生Co/NC多孔碳材料的改性及其电催化水氧化性能[J]. 化工学报, 2020, 71(6): 2821-2829. |
| [9] | 欧阳金波,陈建,刘峙嵘,周利民,韩方泽,应昕. 生物质源多孔碳制备及其对废水中药物吸附研究进展[J]. 化工学报, 2020, 71(12): 5420-5429. |
| [10] | 张海华,董海泉,李慧,袁璐韫,方哲,程军. 碳化金属-有机骨架强化种间电子传递产甲烷[J]. 化工学报, 2020, 71(12): 5745-5754. |
| [11] | 贺新福, 龙雪颖, 吴红菊, 张凯博, 周均, 李可可, 张亚婷, 邱介山. 氮掺杂石墨烯/多孔碳复合材料的制备及其氧还原催化性能[J]. 化工学报, 2019, 70(6): 2308-2315. |
| [12] | 刘羽, 贺鑫, 周文英, 廖丹葵, 崔学民, 苏耀恩, 童张法. 晶须形中空多孔氮掺杂复合碳电极材料的制备及电化学性能研究[J]. 化工学报, 2018, 69(S1): 115-122. |
| [13] | 陈张豪, 马洪芳, 朱汉飞, 王晓丹, 刘鑫鑫, 许朝贵, 杨娟娟. 秸秆基碳材料在Li2SO4电解液中的电化学性能[J]. 化工学报, 2018, 69(7): 3293-3299. |
| [14] | 王丽, 王兴杰, 李浩, 陈永伟, 李忠. 葡萄糖基多孔碳材料对CO2/CH4的分离性能[J]. 化工学报, 2018, 69(2): 733-740. |
| [15] | 陈敏玲, 王兴杰, 肖静, 夏启斌, 李忠. 淀粉基多孔碳材料的制备及其吸附CO2/CH4性能[J]. 化工学报, 2018, 69(1): 455-463. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号