化工学报 ›› 2021, Vol. 72 ›› Issue (10): 5402-5411.DOI: 10.11949/0438-1157.20210376
收稿日期:2021-03-15
修回日期:2021-06-24
出版日期:2021-10-05
发布日期:2021-10-05
通讯作者:
李文坡
作者简介:张璐璐(1996—),女,硕士研究生,基金资助:
Lulu ZHANG( ),Bochuan TAN,Wenpo LI(
),Bochuan TAN,Wenpo LI( )
)
Received:2021-03-15
Revised:2021-06-24
Online:2021-10-05
Published:2021-10-05
Contact:
Wenpo LI
摘要:
采用简单的一步电沉积方法制备了Cu2+掺杂的MnO2材料。通过XRD、SEM、TEM等方法对材料进行了表征,结果表明其具有蓬松多孔的纳米结构,作为电池正极材料时有利于Zn2+的存储,具有较高的电池容量。与未掺杂样品相比Cu2+掺杂的MnO2材料有更好的电化学性能,在电流密度为200 mA/g时,比容量达到235 mAh/g,增加了47.8%,阻抗也由997.3 Ω降到了564.3 Ω。
中图分类号:
张璐璐,谭伯川,李文坡. Cu2+掺杂MnO2作为水系锌离子电池正极材料的合成与电化学性能[J]. 化工学报, 2021, 72(10): 5402-5411.
Lulu ZHANG,Bochuan TAN,Wenpo LI. Synthesis and electrochemical properties of Cu2+-doped MnO2 as cathode materials for aqueous zinc ion batteries[J]. CIESC Journal, 2021, 72(10): 5402-5411.

图6 MnO2和Cu0.12MnO2 材料的 CV曲线(a);200 mA/g下GCD曲线(b);不同电流密度下GCD曲线(c);EIS曲线对比(d)
Fig.6 CV curves (a),GCD curves at 200 mA/g (b), GCD curves at different current densities (c) and EIS curves (d) of MnO2 and Cu0.12MnO2

图7 Cu0.12MnO2不同扫速下的CV图 (a), 电化学动力学分析图(b),GITT(c)及扩散系数分析图(d);MnO2的GITT(e)及扩散系数分析图(f)
Fig.7 CV diagram at different sweep speeds (a) , electrochemical kinetic analysis diagram (b) and GITT (c) and diffusion coefficient analysis (d) of Cu0.12MnO2; GITT (e) and diffusion coefficient analysis (f) of MnO2
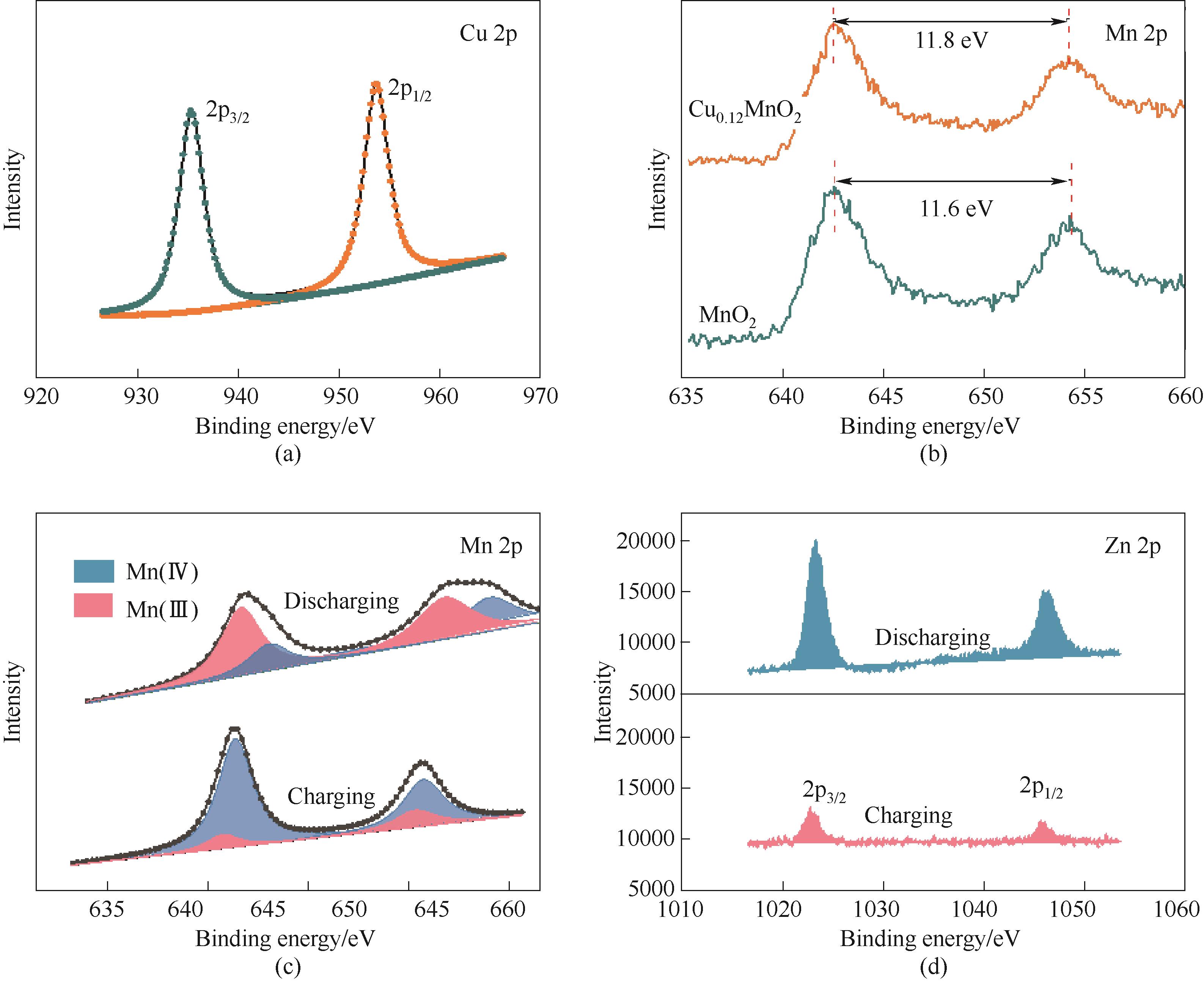
图8 Cu0.12MnO2中Cu的XPS谱图 (a);掺杂前后Mn的XPS谱图(b); 充电和放电状态下Mn(c)和 Zn(d)的XPS谱图
Fig.8 XPS of Cu in Cu0.12MnO2 (a); XPS of Mn before and after doping (b); XPS of Mn (c) and Zn (d) under charging and discharging conditions respectively
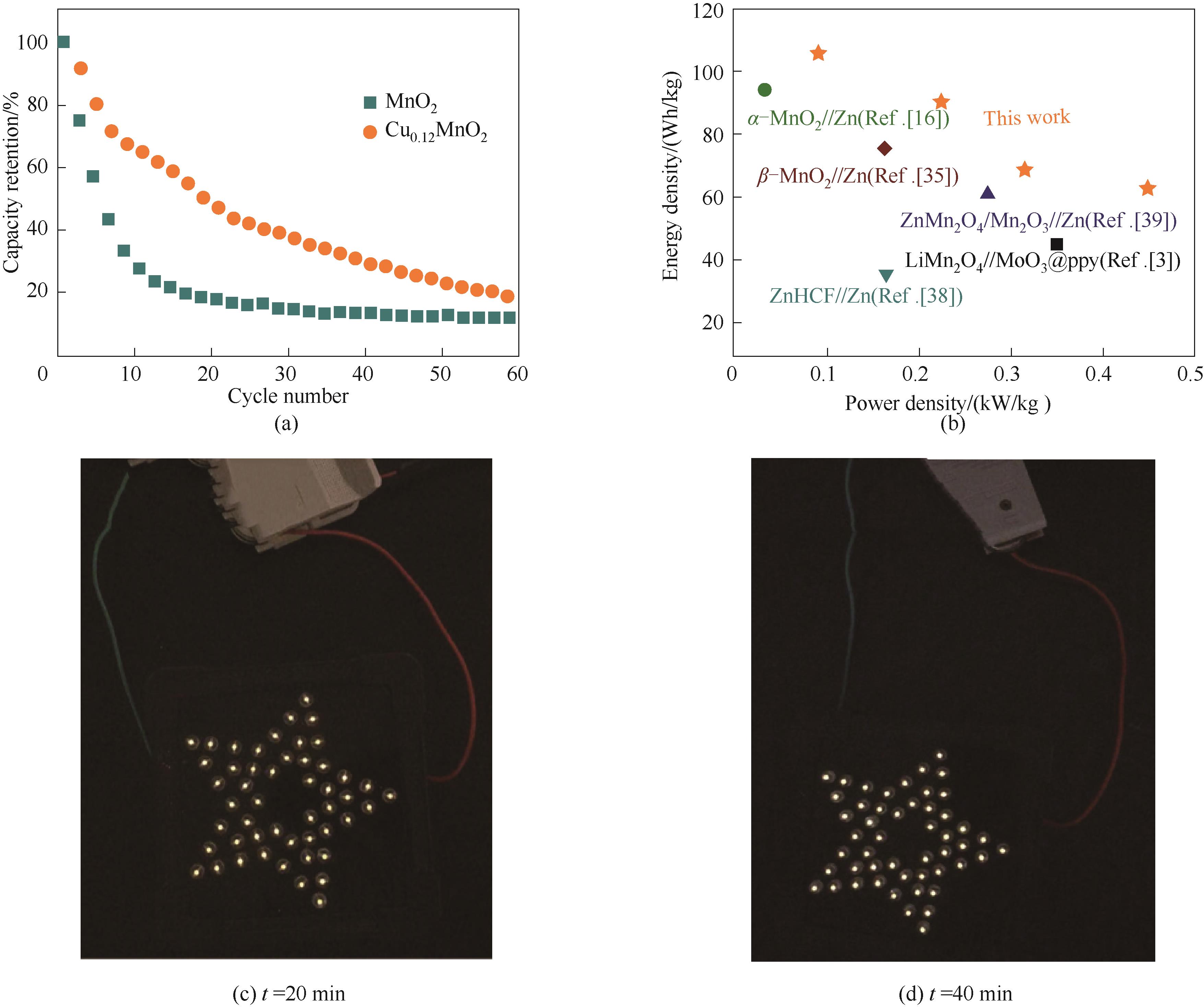
图9 MnO2和Cu0.12MnO2的循环对比图(a);Ragone图(b);MnO2 (c)和Cu0.12MnO2 (d)点灯应用图
Fig.9 Cyclic comparison of MnO2 and Cu0.12MnO2 (a) ; Ragone plot of the Zn//Cu2+ doped MnO2 aqueous battery(b); MnO2 (c)and Cu0.12MnO2 (d) lighting application diagram
| 1 | Chen S, Song Y, Zhou X J, et al. Co(OH)F nanorods@KxMnO2 nanosheet core-shell structured arrays for pseudocapacitor application[J]. RSC Advances, 2019, 9(62): 36208-36212. |
| 2 | Swain N, Mitra A, Saravanakumar B, et al. Construction of three-dimensional MnO2/Ni network as an efficient electrode material for high performance supercapacitors[J]. Electrochimica Acta, 2020, 342: 136041. |
| 3 | Tang W, Liu L L, Zhu Y S, et al. An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2O4[J]. Energy & Environmental Science, 2012, 5(5): 6909. |
| 4 | Kasiri G, Trócoli R, Bani Hashemi A, et al. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries[J]. Electrochimica Acta, 2016, 222: 74-83. |
| 5 | Pang Q, Sun C L, Yu Y H, et al. H2V3O8 nanowire/graphene electrodes for aqueous rechargeable zinc ion batteries with high rate capability and large capacity[J]. Advanced Energy Materials, 2018, 8(19): 1800144. |
| 6 | Zhu C B, Fu Y P, Yu Y. Designed nanoarchitectures by electrostatic spray deposition for energy storage[J]. Advanced Materials, 2019, 31(1): 1803408. |
| 7 | He P, Chen Q, Yan M Y, et al. Building better zinc-ion batteries: a materials perspective[J]. EnergyChem, 2019, 1(3): 100022. |
| 8 | Cheng X Y, Zhou L J, Lu Y Z, et al. Facile activation of commercial Ni foil as robust cathode for advanced rechargeable Ni-Zn battery[J]. Electrochimica Acta, 2018, 263: 311-317. |
| 9 | Kundu D P, Adams B D, Duffort V, et al. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode[J]. Nature Energy, 2016, 1: 16119. |
| 10 | Fang G Z, Zhu C Y, Chen M H, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery[J]. Advanced Functional Materials, 2019, 29(15): 1808375. |
| 11 | Wang G, Kohn B, Scheler U, et al. A high-voltage, dendrite-free, and durable Zn-graphite battery[J]. Advanced Materials, 2020, 32(4): 1905681. |
| 12 | 韩亚娜. Mn3O4@MWCNT复合材料的制备及其在水系锌离子电池中的性能研究[D]. 天津: 天津理工大学, 2019. |
| Han Y N. Preparation and properties of Mn3O4@MWCNT composites materials in aqueous zinc-ion batteries[D]. Tianjin:Tianjin University of Technology, 2019. | |
| 13 | Yu P, Zeng Y X, Zhang H Z, et al. Flexible Zn-ion batteries: recent progresses and challenges[J]. Small, 2019, 15(7): 1804760. |
| 14 | Ghosh M, Vijayakumar V, Anothumakkool B, et al. Nafion ionomer-based single component electrolytes for aqueous Zn/ MnO2 batteries with long cycle life[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(13): 5040-5049. |
| 15 | Li S W, Liu Y C, Zhao X D, et al. Sandwich-like heterostructures of MoS2/graphene with enlarged interlayer spacing and enhanced hydrophilicity as high-performance cathodes for aqueous zinc-ion batteries[J]. Advanced Materials, 2021, 33(12): 2007480. |
| 16 | Alfaruqi M H, Gim J, Kim S, et al. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode[J]. Journal of Power Sources, 2015, 288: 320-327. |
| 17 | Park J H, Choi W Y, Lee S, et al. Graphene intercalated free-standing carbon paper coated with MnO2 for anode materials of lithium ion batteries[J]. Electrochimica Acta, 2020, 348: 136310. |
| 18 | Alfaruqi M H, Gim J, Kim S, et al. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications[J]. Electrochemistry Communications, 2015, 60: 121-125. |
| 19 | Cheng G, Xie S L, Lan B, et al. Phase controllable synthesis of three-dimensional star-like MnO2 hierarchical architectures as highly efficient and stable oxygen reduction electrocatalysts[J]. Journal of Materials Chemistry A, 2016, 4(42): 16462-16468. |
| 20 | Wang D H, Wang L F, Liang G J, et al. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery[J]. ACS Nano, 2019, 13(9): 10643-10652. |
| 21 | Lee J, Ju J B, Cho W I, et al. Todorokite-type MnO2 as a zinc-ion intercalating material[J]. Electrochimica Acta, 2013, 112: 138-143. |
| 22 | Alfaruqi M H, Mathew V, Gim J, et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system[J]. Chemistry of Materials, 2015, 27(10): 3609-3620. |
| 23 | Liu G X, Huang H W, Bi R, et al. K+ pre-intercalated manganese dioxide with enhanced Zn2+ diffusion for high rate and durable aqueous zinc-ion batteries[J]. Journal of Materials Chemistry A, 2019, 7(36): 20806-20812. |
| 24 | 韩世昌, 邹正光, 吕婷婷, 等. Ag掺杂VO2(B)正极材料的合成及其电化学性能[J]. 化工学报, 2018, 69(4): 1741-1748. |
| Han S C, Zou Z G, Lyu T T, et al. Sythesis and electrochemical performance of Ag-doped VO2(B) as cathode materials[J]. CIESC Journal, 2018, 69(4): 1741-1748. | |
| 25 | 侯忠良, 邹正光, 吴一, 等. Cr掺杂VO2(B)正极材料的合成及其电化学性能[J]. 化工学报, 2017, 68(4): 1691-1701. |
| Hou Z L, Zou Z G, Wu Y, et al. Sythesis and electrochemical performance of Cr-doped VO2(B) cathode materials[J]. CIESC Journal, 2017, 68(4): 1691-1701. | |
| 26 | Xiong T, Zhang Y X, Lee W S V, et al. Defect engineering in manganese-based oxides for aqueous rechargeable zinc-ion batteries: a review[J]. Advanced Energy Materials, 2020, 10(34): 2001769. |
| 27 | Sun T J, Nian Q S, Zheng S B, et al. Layered Ca0.28MnO2∙0.5H2O as a high performance cathode for aqueous zinc-ion battery[J]. Small, 2020, 16(17): 2000597. |
| 28 | Zhang M S, Wu W X, Luo J W, et al. A high-energy-density aqueous zinc–manganese battery with a La–Ca co-doped ε-MnO2 cathode[J]. Journal of Materials Chemistry A, 2020, 8(23): 11642-11648. |
| 29 | Huang Z H, Song Y, Feng D Y, et al. High mass loading MnO2 with hierarchical nanostructures for supercapacitors[J]. ACS Nano, 2018, 12(4): 3557-3567. |
| 30 | Jiang Y L, Yuan L, Wang X Y, et al. Jahn-Teller disproportionation induced exfoliation of unit-cell scale ε-MnO2[J]. Angewandte Chemie International Edition, 2020, 59(50): 22659-22666. |
| 31 | Hong W, Shao M P, Zhu T L, et al. To promote ozone catalytic decomposition by fabricating manganese vacancies in ε-MnO2 catalyst via selective dissolution of Mn-Li precursors[J]. Applied Catalysis B:Environmental, 2020, 274: 119088. |
| 32 | Lin M, Chen Z L. A facile one-step synthesized epsilon-MnO2 nanoflowers for effective removal of lead ions from wastewater[J]. Chemosphere, 2020, 250: 126329. |
| 33 | Khan A, Zhang K K, Taraqqi-A-Kamal A, et al. Degradation of antibiotics in aqueous media using manganese nanocatalyst-activated peroxymonosulfate[J]. Journal of Colloid and Interface Science, 2021, 599: 805-818. |
| 34 | Fic K, Lota G, Meller M, et al. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors[J]. Energy & Environmental Science, 2012, 5(2): 5842-5850. |
| 35 | Huang J, Wang Z, Hou M, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery[J]. Nature Communications, 2018, 9(1): 2906. |
| 36 | Sun W, Wang F, Hou S, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion[J]. Journal of the American Chemical Society, 2017, 139(29): 9775-9778. |
| 37 | Oh W, Park H, Jin B S, et al. Understanding the structural phase transitions in lithium vanadium phosphate cathodes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2020, 8(20): 10331-10336. |
| 38 | Zhang L Y, Chen L, Zhou X F, et al. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system[J]. Advanced Energy Materials, 2015, 5(2): 1400930. |
| 39 | Yang S N, Zhang M S, Wu X W, et al. The excellent electrochemical performances of ZnMn2O4/Mn2O3: the composite cathode material for potential aqueous zinc ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 832: 69-74. |
| [1] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [2] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [3] | 徐东, 田杜, 陈龙, 张禹, 尤庆亮, 胡成龙, 陈韶云, 陈建. 聚苯胺/二氧化锰/聚吡咯复合纳米球的制备及其电化学储能性[J]. 化工学报, 2023, 74(3): 1379-1389. |
| [4] | 徐珂, 史国强, 薛冬峰. 无机杂化钙钛矿团簇材料:介尺度钙钛矿材料发光性质研究[J]. 化工学报, 2022, 73(6): 2748-2756. |
| [5] | 陈婷, 胡泽浩, 秦喆, 陈园虹, 徐彦乔, 林坚, 谢志翔. 有机相微波合成AgInS2量子点及其白光发光二极管应用研究[J]. 化工学报, 2022, 73(11): 5167-5176. |
| [6] | 宋刘斌, 王怡萱, 匡尹杰, 夏宇博, 肖忠良. 钠离子电池中关键材料及技术的发展与前景[J]. 化工学报, 2022, 73(11): 4814-4825. |
| [7] | 彭启, 贾力, 丁艺, 张永欣, 党超, 银了飞. 受限微结构对低表面张力液滴合并弹跳的影响[J]. 化工学报, 2021, 72(4): 1920-1929. |
| [8] | 赵玉海, 罗英武. 可逆失活自由基界面聚合[J]. 化工学报, 2021, 72(2): 653-668. |
| [9] | 张毅舟, 吴籼虹, 王治宇, 邱介山. 镶嵌单层MoS2的生物质基硼氮共掺杂碳纳米片合成与储钠性能[J]. 化工学报, 2021, 72(12): 6371-6379. |
| [10] | 丁鼎, 陆文多, 侯璐, 陆安慧. 纤维状BPO4/SiO2催化剂的制备及其丙烷氧化脱氢性能[J]. 化工学报, 2021, 72(11): 5590-5597. |
| [11] | 王琛璐, 王艳磊, 赵秋, 吕玉苗, 霍锋, 何宏艳. 低维纳米受限离子液体的研究进展[J]. 化工学报, 2021, 72(1): 366-383. |
| [12] | 石向成, 赵志坚, 巩金龙. 遗传算法在催化体系的全局结构优化中的应用[J]. 化工学报, 2021, 72(1): 27-41. |
| [13] | 孙敬方, 葛成艳, 安冬琦, 仝庆, 高飞, 董林. 稀土铈基催化材料氧空位的表征方法综述[J]. 化工学报, 2020, 71(8): 3403-3415. |
| [14] | 王雅倩, 鲁晓, 彭波. 磁场对(C4H9NH3)2(CH3NH3)Pb2I7钙钛矿发光特性调控的研究[J]. 化工学报, 2020, 71(6): 2912-2917. |
| [15] | 胡涛, 张熊, 安亚斌, 李晨, 马衍伟. 锂离子电容器碳正极材料的研究进展[J]. 化工学报, 2020, 71(6): 2530-2546. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号