化工学报 ›› 2020, Vol. 71 ›› Issue (8): 3403-3415.DOI: 10.11949/0438-1157.20200131
孙敬方1( ),葛成艳2(
),葛成艳2( ),安冬琦1,仝庆1,高飞1,董林1(
),安冬琦1,仝庆1,高飞1,董林1( )
)
收稿日期:2020-02-15
修回日期:2020-05-15
出版日期:2020-08-05
发布日期:2020-08-05
通讯作者:
董林
作者简介:孙敬方(1987—),女,博士,中级工程师,基金资助:
Jingfang SUN1( ),Chengyan GE2(
),Chengyan GE2( ),Dongqi AN1,Qing TONG1,Fei GAO1,Lin DONG1(
),Dongqi AN1,Qing TONG1,Fei GAO1,Lin DONG1( )
)
Received:2020-02-15
Revised:2020-05-15
Online:2020-08-05
Published:2020-08-05
Contact:
Lin DONG
摘要:
氧空位(oxygen vacancy, Ov)是金属氧化物缺陷的一种,在多相催化、储能材料、能源化工等众多领域都发挥着重要的作用,因而关于其在理论和实验方面的研究都得到了广泛关注。以稀土CeO2这一广泛应用于能源、环境等领域的催化材料为例,简单归纳了一些氧空位检测的常用表征方法,包括拉曼(Raman)、电子顺磁共振(EPR)、正电子湮没能谱(PALS)、固体核磁(ss-NMR)、X射线光电子能谱(XPS)和扫描隧道显微镜(STM)等,同时对各种表征方法的结果分析进行了举例说明。在此基础上,对氧空位表征技术未来的发展方向提出了一些看法。希望可以对稀土铈基催化材料缺陷相关的表征及研究提供支持。
中图分类号:
孙敬方, 葛成艳, 安冬琦, 仝庆, 高飞, 董林. 稀土铈基催化材料氧空位的表征方法综述[J]. 化工学报, 2020, 71(8): 3403-3415.
Jingfang SUN, Chengyan GE, Dongqi AN, Qing TONG, Fei GAO, Lin DONG. Review on characterization methods of oxygen vacancy in rare earth cerium-based catalysts[J]. CIESC Journal, 2020, 71(8): 3403-3415.
| Surface | M1 | ΔEvac/eV | ΔQM1/e | ΔQM2/e | ΔQM3/e | ΔQM4/e | |
|---|---|---|---|---|---|---|---|
| (111) | Ce | +2.76 | +0.32 | +0.32 | 0.00 | +0.06 | +3.01 |
| Zr | +1.63 | +0.02 | +0.32 | +0.28 | +0.06 | +2.02 | |
| Pd | +0.71 | +0.43 | +0.05 | +0.05 | +0.03 | +2.78 | |
| (110) | Ce | +2.10 | +0.32 | +0.32 | -0.01 | -0.01 | +3.91 |
| Zr | +1.24 | +0.08 | +0.31 | +0.26 | +0.06 | +4.78 | |
| Pd | -0.09 | +0.38 | +0.06 | +0.04 | +0.03 | +0.82 | |
| (100) | Ce | +2.26 | +0.35 | +0.37 | +0.02 | +0.01 | — |
| Zr | +1.84 | +0.07 | +0.30 | +0.26 | +0.03 | — | |
| Pd | -0.05 | +0.51 | +0.08 | +0.01 | +0.02 | — |
表1 不同暴露晶面CeO2氧空位生成能[26]
Table 1 Oxygen vacancy formation energy of each surface of ceria[26]
| Surface | M1 | ΔEvac/eV | ΔQM1/e | ΔQM2/e | ΔQM3/e | ΔQM4/e | |
|---|---|---|---|---|---|---|---|
| (111) | Ce | +2.76 | +0.32 | +0.32 | 0.00 | +0.06 | +3.01 |
| Zr | +1.63 | +0.02 | +0.32 | +0.28 | +0.06 | +2.02 | |
| Pd | +0.71 | +0.43 | +0.05 | +0.05 | +0.03 | +2.78 | |
| (110) | Ce | +2.10 | +0.32 | +0.32 | -0.01 | -0.01 | +3.91 |
| Zr | +1.24 | +0.08 | +0.31 | +0.26 | +0.06 | +4.78 | |
| Pd | -0.09 | +0.38 | +0.06 | +0.04 | +0.03 | +0.82 | |
| (100) | Ce | +2.26 | +0.35 | +0.37 | +0.02 | +0.01 | — |
| Zr | +1.84 | +0.07 | +0.30 | +0.26 | +0.03 | — | |
| Pd | -0.05 | +0.51 | +0.08 | +0.01 | +0.02 | — |
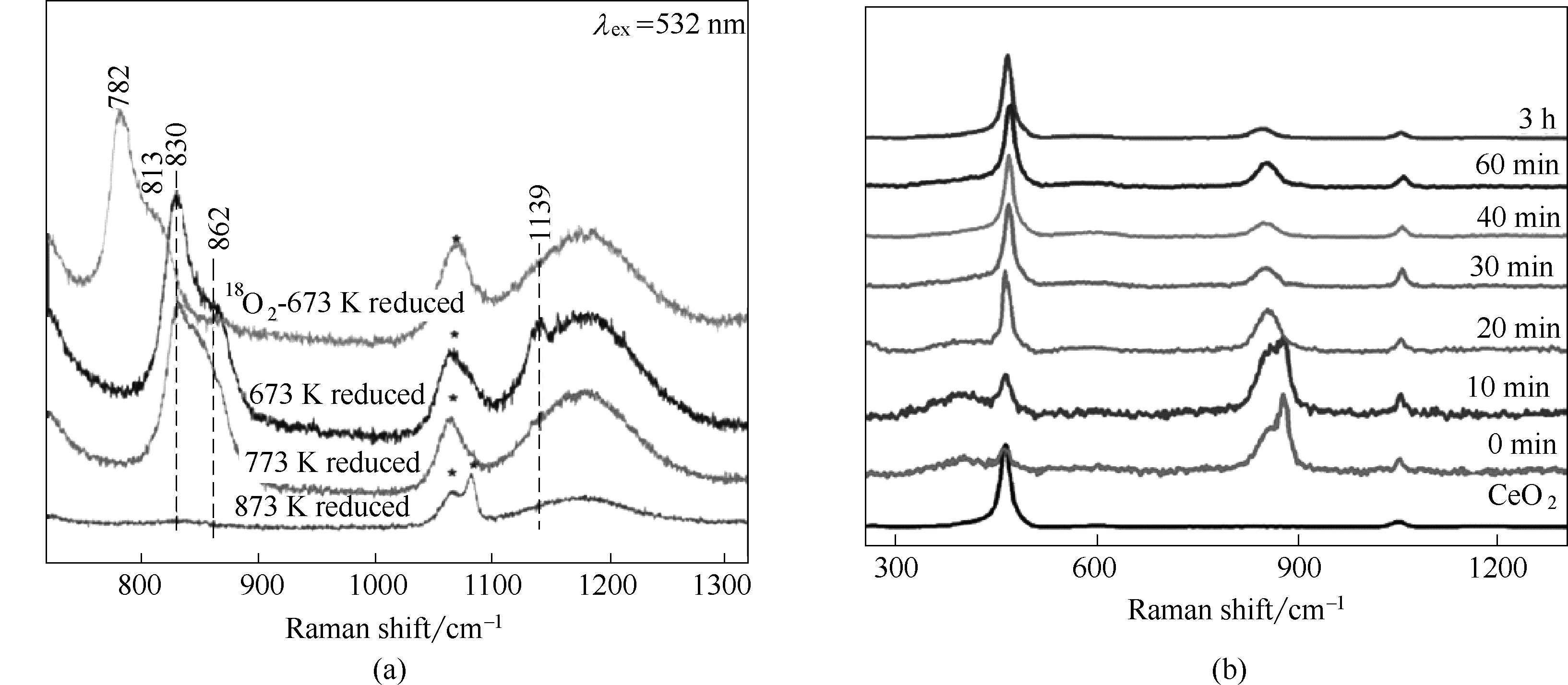
图4 (a)不同温度还原后CeO2纳米棒在室温的O2吸附拉曼结果[39]; (b) CeO2纳米颗粒与H2O2反应拉曼结果[43]
Fig.4 (a) Raman spectra of O2 adsorption at room temperature on different temperature reduced ceria nanorodsm[39]; (b) Raman spectra of CeO2 nanoparticles during the reaction with H2O2[43]
| Signal | EPR parameters | Proposed assignment |
|---|---|---|
| OC1 type | gz = 2.031—2.030, gx = 2.017, gy = 2.011 | Ce4+-O2- species formed on isolated vacancies of three-dimensional particles |
| g‖= 2.034—2.032, g⊥= 2.011—2.010 | ||
| OC2 | gz = 2.042—2.039, gx = 2.009—2.008 | Ce4+-O2- species formed on isolated vacancies of three-dimensional particles |
| gy = 2.010—2.009 | ||
| OCZ | gz = 2.026—2.025, gx = 2.018—2.017 | Ce4+-O2- species formed on two-dimensional ceria-type patches |
| gy = 2.011 | ||
| OZ | gz = 2.037, 2.032, gy = 2.009 | Zr4+-O2- species |
| gx = 2.002 |
表2 预先吸附O2后的样品在773 K下的EPR结果[46]
Table 2 Characteristic of the EPR signals obtained upon oxygen adsorption on the samples outgessed at 773 K[46]
| Signal | EPR parameters | Proposed assignment |
|---|---|---|
| OC1 type | gz = 2.031—2.030, gx = 2.017, gy = 2.011 | Ce4+-O2- species formed on isolated vacancies of three-dimensional particles |
| g‖= 2.034—2.032, g⊥= 2.011—2.010 | ||
| OC2 | gz = 2.042—2.039, gx = 2.009—2.008 | Ce4+-O2- species formed on isolated vacancies of three-dimensional particles |
| gy = 2.010—2.009 | ||
| OCZ | gz = 2.026—2.025, gx = 2.018—2.017 | Ce4+-O2- species formed on two-dimensional ceria-type patches |
| gy = 2.011 | ||
| OZ | gz = 2.037, 2.032, gy = 2.009 | Zr4+-O2- species |
| gx = 2.002 |
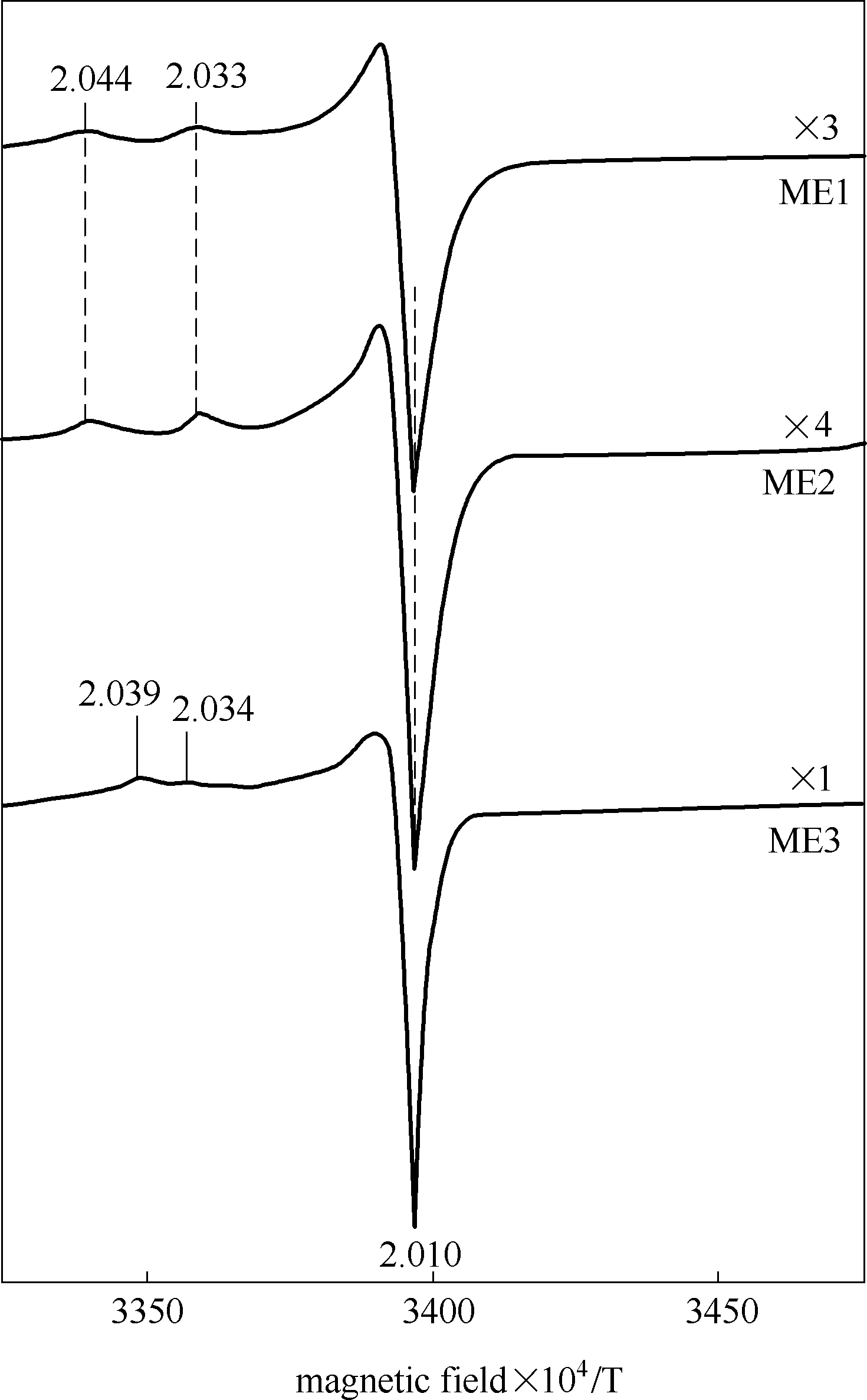
图5 773 K下先抽真空再O2吸附后的样品在77 K下的EPR结果[47]
Fig.5 EPR spectra at 77 K following oxygen adsorption at room temperature on the sample out gassed at 773 K[47]
| Sample | τ1/ps | τ2/ps | τ3/ps | I1/% | I2/% | I3/% | I2/I1 |
|---|---|---|---|---|---|---|---|
| c-CeO2 | 187.0 | 350.2 | 1.50 | 35.99 | 63.16 | 0.85 | 1.75 |
| 1%-Ag/ c-CeO2 | 203.3 | 366.1 | 2.10 | 42.82 | 56.48 | 0.70 | 1.32 |
| 3%-Ag/ c-CeO2 | 198.9 | 360.7 | 1.74 | 40.6 | 58.5 | 0.9 | 1.44 |
| r-CeO2 | 262.0 | 397.0 | 1.90 | 31.2 | 67.9 | 0.9 | 2.18 |
| 1%-Ag/ r-CeO2 | 230.2 | 384.7 | 1.71 | 21.72 | 77.06 | 1.22 | 3.55 |
| 3%-Ag/ r-CeO2 | 250.2 | 409.3 | 2.36 | 41.5 | 57.5 | 1.0 | 1.39 |
表3 不同样品的PALS峰拟合结果和Raman结果[52]
Table 3 Peak-fitting results of PALS spectra and Raman spectra of various samples[52]
| Sample | τ1/ps | τ2/ps | τ3/ps | I1/% | I2/% | I3/% | I2/I1 |
|---|---|---|---|---|---|---|---|
| c-CeO2 | 187.0 | 350.2 | 1.50 | 35.99 | 63.16 | 0.85 | 1.75 |
| 1%-Ag/ c-CeO2 | 203.3 | 366.1 | 2.10 | 42.82 | 56.48 | 0.70 | 1.32 |
| 3%-Ag/ c-CeO2 | 198.9 | 360.7 | 1.74 | 40.6 | 58.5 | 0.9 | 1.44 |
| r-CeO2 | 262.0 | 397.0 | 1.90 | 31.2 | 67.9 | 0.9 | 2.18 |
| 1%-Ag/ r-CeO2 | 230.2 | 384.7 | 1.71 | 21.72 | 77.06 | 1.22 | 3.55 |
| 3%-Ag/ r-CeO2 | 250.2 | 409.3 | 2.36 | 41.5 | 57.5 | 1.0 | 1.39 |

图8 CeO2和CeO2/Co3O4的Ce 3d高分辨XPS结果(a),CeO2和CeO2/Co3O4的O 1s(b)和 Co 2p (c) XPS结果,CeO2和CeO2/Co3O4的Co L-edge XANES结果(d) [63]
Fig.8 High-resolution Ce 3d XPS spectra of CeO2 and CeO2/Co3O4(a). High-resolution O 1s (b) and Co 2p (c) XPS spectra of CeO2 and CeO2/Co3O4. Co L-edge XANES of CeO2 and CeO2/Co3O4 (d) [63]
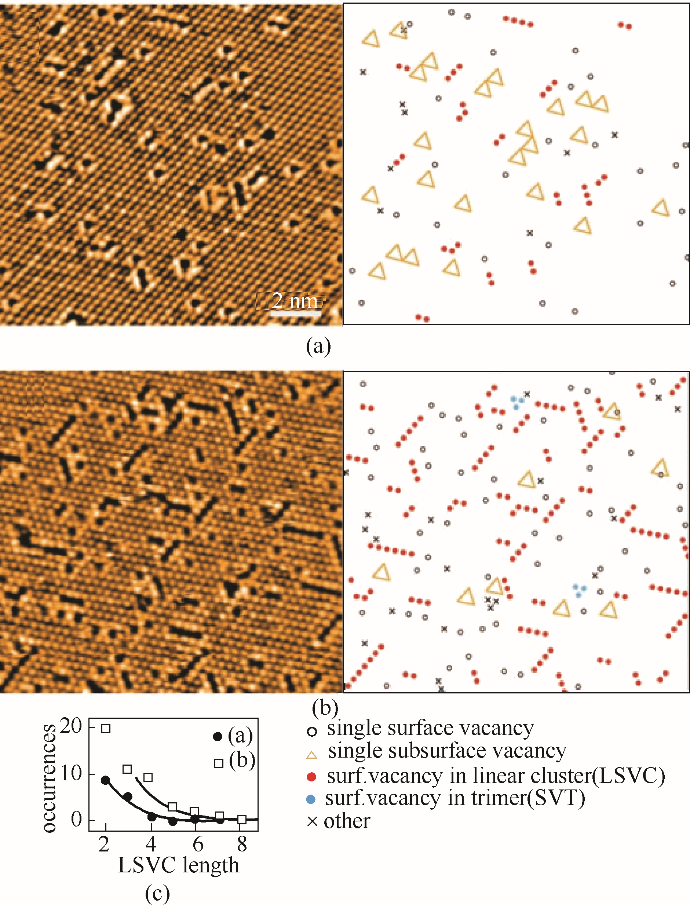
图10 900℃加热1 min(a)和5 min(b)后的CeO2(111)面的STM结果以及对应出现的各种氧空位信息。由图(a)和图(b)中统计出来的表面线性氧空位团簇(LSVC)分布图(c)[71]
Fig.10 STM images of the CeO2(111) surface obtained after 1 min (a) and 5 min (b) of annealing at 900℃, with corresponding representations of the observed defects. (c) Histogram of the LSVC distribution as evaluated from (a) (solid circles) and (b) (open squares)[71]
| 1 | Tompkins F C. Superficial chemistry and solid imperfections[J]. Nature, 1960, 186: 3-6. |
| 2 | Lou Y, Ma J, Cao X, et al. Promoting effects of In2O3 on Co3O4 for CO oxidation: tuning O2 activation and CO adsorption strength simultaneously[J]. ACS Catal., 2014, 4: 4143-4152. |
| 3 | Sayle D C, Maicaneanu S A, Watson G W. Atomistic models for CeO2 (111), (110) and (100) nanoparticles, supported on yttrium-stabilized zirconia[J]. J. Am. Chem. Soc., 2002, 124: 11429-11439. |
| 4 | Over H, Kim Y D, Seitsonen A P, et al. Atomic-scale structure and catalytic reactivity of the RuO2 (110) surface[J]. Science, 2000, 287: 1474-1476. |
| 5 | Schaub R E, Wahlstrom A, Rønnau E, et al. Oxygen-mediated diffusion of oxygen vacancies on the TiO2 (110) surface[J]. Science, 2003, 299: 377-379. |
| 6 | Li D, Yu Q, Li S S, et al. The remarkable enhancement of CO-pretreated CuO Mn2O3/γ-Al2O3 supported catalyst for the reduction of NO with CO: the formation of surface synergetic oxygen vacancy[J]. Chem. Eur. J., 2011, 17: 5668-5679. |
| 7 | Dong L, Zhang L, Sun C, et al. Study of the properties of CuO/VOx/Ti0.5Sn0.5O2 catalysts and their activities in NO+CO reaction[J]. ACS Catal., 2011, 1: 468-480. |
| 8 | Liu X, Zhou K, Wang L, et al. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. J. Am. Chem. Soc., 2009, 131: 3140-3141. |
| 9 | Wu Z, Li M, Overbury S H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes[J]. J. Catal., 2012, 285: 61-73. |
| 10 | Xiong J, Di J, Xia J, et al. Surface defect engineering in 2D nanomaterials for photocatalysis[J]. Adv. Funct. Mater., 2018, 28: 1801983. |
| 11 | Wang G, Yang Y, Han D, et al. Oxygen defective metal oxides for energy conversion and storage[J]. Nano Today, 2017, 13: 23-39. |
| 12 | Fallya F, Perrichona V, Vidal H, et al. Lavalleye modification of the oxygen storage capacity of CeO2-ZrO2 mixed oxides after redox cycling aging[J]. Catal. Today, 2000, 59: 373-386. |
| 13 | KasÏpar J, Fornasiero P, Graziani M. Use of CeO2-based oxides in the three-way catalysis[J]. Catal. Today, 1999, 50: 285-298. |
| 14 | Gamboa-Rosales N K, Ayastuy J L, Boukhaa Z, et al. Ceria-supported Au-CuO and Au-Co3O4 catalysts for CO oxidation: an 18O/16O isotopic exchange study[J]. Appl. Catal. B: Environ., 2015, 168/169: 87-97. |
| 15 | Liu Z P, Jenkins S J, King D A. Origin and activity of oxidized gold in water-gas-shift catalysis[J]. Phys. Rev. Lett., 2005, 94: 196102. |
| 16 | Iwasaki B, Katsura T. The thermodynamic properties of the nonstoichiometric ceric oxide at temperatures from 900 to 1300 °C [J]. B. Chem. Soc. Jpn., 1971, 44: 1297-1301. |
| 17 | Blumenthal R, Hofmaier R. The temperature and compositional dependence of the electrical conductivity of nonstoichiometric CeO2-x[J]. J. Electrochem. Soc., 1974, 121: 126-131. |
| 18 | VanHandel G, Blumenthal R. The temperature and oxygen pressure dependence of the ionic transference number of nonstoichiometric CeO2-x[J]. J. Electrochem. Soc., 1974, 121: 1198-1202. |
| 19 | Tuller H, Nowick A. Defect structure and electrical properties of nonstoichiometric CeO2 single crystals[J]. J. Electrochem. Soc., 1979, 126: 209-217. |
| 20 | Nagata T, Miyajima K, Hardy R A, et al. Reactivity of oxygen deficient cerium oxide clusters with small gaseous molecules[J]. J. Phys. Chem. A, 2015, 119: 5545-5552. |
| 21 | Blumenthal R, Lee P, Panlener R. Studies of the defect structure of nonstoichiometric cerium dioxide[J]. J. Electrochem. Soc., 1971, 118: 123-129. |
| 22 | Wang X Q, Rodriguez J A, Hanson J C, et al. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: complex interaction between metallic copper and oxygen vacancies of ceria[J]. J. Phys. Chem. B, 2006, 110: 428-434. |
| 23 | Li Y, Maxey E R, Richardson J W, et al. Oxygen non-stoichiometry and thermal-chemical expansion of Ce0.8Y0.2O1.9-δ electrolytes by neutron diffraction[J]. J. Am. Chem. Soc., 2007, 90 (4): 1208-1214. |
| 24 | Harada K, Oishi T, Hamamoto S, et al. Lattice oxygen activity in Pr- and La-doped CeO2 for low-temperature soot oxidation[J]. J. Phys. Chem. C, 2014, 118(1): 559-568. |
| 25 | Yashima M. Invited review: some recent developments in the atomic-scale characterization of structural and transport properties of ceria-based catalysts and ionic conductors[J]. Catal. Today, 2015, 253: 3-19. |
| 26 | Mayernick A D, Janik M J. Methane activation and oxygen vacancy formation over CeO2 and Zr, Pd substituted CeO2 surfaces[J]. J. Phys. Chem. C, 2008, 112: 14955-14964. |
| 27 | Sayle T X T, Parker S C, Catlow C R A. The role of oxygen vacancies on ceria surfaces in the oxidation of carbon monoxide[J]. Surf. Sci., 1994, 316: 329-336. |
| 28 | Liu L J, Cao Y, Sun W J, et al. Morphology and nanosize effects of ceria from different precursors on the activity for NO reduction[J]. Catal. Today, 2011, 175: 48-54. |
| 29 | Li J, Zhang Z Y, Gao W, et al. Pressure regulations on the surface properties of CeO2 nanorods and their catalytic activity for CO oxidation and nitrile hydrolysis reactions[J]. ACS Appl. Mater. & Interfaces, 2016, 8(35): 22988-22996. |
| 30 | Liu L Z, Sun J T, Ding J D, et al. Highly active Mn3-xFexO4 spinel with defects for toluene mineralization: insights into regulation of the oxygen vacancy and active metals[J]. Inorg. Chem., 2019, 58(19): 13241-13249. |
| 31 | Sumio K, Ryu F, Masataka O, et al. Oxygen storage capacity of CuMO2 (M = Al, Fe, Mn, Ga) with a delafossite-type structure[J]. Appl. Catal. B: Environ., 2009, 89: 183-188. |
| 32 | Pu Y, Luo Y D, Wei X Q, et al. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: surface Lewis acid/base and oxygen defect[J]. Appl. Catal. B: Environ., 2019, 254: 580-586. |
| 33 | Chen S Q, Li L P, Hu W B, et al. Anchoring high-concentration oxygen vacancies at interfaces of CeO2-x/Cu toward enhanced activity for preferential CO oxidation[J]. ACS Appl. Materi. & Interfaces., 2015, 7(41): 22999-23007. |
| 34 | Anita M D, Nathan A S, Webster A L. Vacancy generation and oxygen uptake in Cu-doped Pr-CeO2 materials using neutron and in situ X-ray diffraction[J]. Inorg. Chem., 2016, 55(24): 12595-12602. |
| 35 | Coduri M, Brunelli M, Scavini M, et al. Rare earth doped ceria: a combined X-ray and neutron pair distribution function study[J]. Zeitschrift für Kristallographie Crystal. Mater., 2012, 227(5): 272. |
| 36 | Liu L J, Cao Y, Sun W J, et al. Morphology and nanosize effects of ceria from different precursors on the activity for NO reduction[J]. Catal. Today, 2011, 175: 48-54. |
| 37 | 孙敬方, 葛成艳, 姚小江, 等. 固相浸渍法制备镍铈催化剂及其在CO氧化反应中的应用[J]. 物理化学学报, 2013, 29(11): 2451-2458. |
| Sun J F, Ge C Y, Yao X J, et al. Preparation of NiO/CeO2 catalysis by solid-state impregnation method and its application in CO oxidation reaction[J]. Acta Phys.-Chim. Sin., 2013, 29(11): 2451-2458. | |
| 38 | Hu Y H, Dong L, Wang J, et al. UV-Raman characterizations of MoO3/ZrO2 catalysts with extremely low MoO3 loadings[J]. Chem. Lett., 2000, (8): 904-905. |
| 39 | Wu Z L, Li M J, Howe J, et al. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption[J]. Langmuir, 2010, 26(21): 16595-16606. |
| 40 | Luo M F, Yan Z L, Jin L Y, et al. Raman spectroscopic study on the structure in the surface and the bulk shell of CexPr1-xO2-δ mixed oxides[J]. J. Phys. Chem. B, 2006, 110: 13068-13071. |
| 41 | Li S P, Lu J Q, Fang P, et al. Effect of oxygen vacancies on electrical properties of Ce0.8Sm0.1Nd0.1O2-δ electrolyte: an in situ Raman spectroscopic study[J]. J. Power Sources, 2009, 193: 93. |
| 42 | Li M J, Feng Z C, Xiong G, et al. Phase transformation in the surface eegion of zirconia detected by UV Raman spectroscopy[J]. J. Phys. Chem. B, 2001, 105(34): 8107-8111. |
| 43 | Wu K, Sun L D, Yan C H. Recent progress in well-controlled synthesis of ceria-based nanocatalysts towards enhanced catalytic performance[J]. Adv. Energy Mater., 2016, 6: 1600501. |
| 44 | Ren C, Yang R C, Li Y Y, et al. Modulating of facetsdependent oxygen vacancies on ceria and its catalytic oxidation performance[J]. Res. Chem. Intermediates, 2019, 45: 3019-3032. |
| 45 | Rakhmatullin R M, Pavlov V V, Semashko V V. EPR study of nanocrystalline CeO2 exhibiting ferromagnetism at room temperature[J]. Phys. Status Solid B, 2016, 253(3): 499-503. |
| 46 | Martínez-Arias A, Fernández-García M, Ana-Belén H, et al. Spectroscopic characterization of heterogeneity and redox effects in zirconium-cerium(1∶1) mixed oxides prepared by microemulsion methods[J]. J. Phys. Chem. B, 2003, 107: 2667-2677. |
| 47 | Maŕıa D, Hernández A, Ana B H, et al. EPR study of the photoassisted formation of radicals on CeO2 nanoparticles employed for toluene photooxidation[J]. Appl. Catal. B: Environ., 2004, 50: 167-175. |
| 48 | Soria J, Conesa J C, Martŕnez-Arias A. Characterization of surface defects in CeO2 modified by incorporation of precious metals from chloride salts precursors: an EPR study using oxygen as probe molecule[J]. Colloids and Surfaces A: Physicochem. Eng. Aspects, 1999, 158: 67-74. |
| 49 | Skaf M, Hany S, Aouad S,et al. Detection of adsorbed O2- species on CeO2 solid impregnated with Ag2+ ions during its thermal treatment under a H2 atmosphere, an EPR study[J]. Phys. Chem. Chem. Phys., 2016, 18: 29381-29386. |
| 50 | Liu X, Zhou K, Wang L, et al. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. J.Am. Chem. Soc., 2009, 131: 3140-3141. |
| 51 | Puska M J, Nieminen R. Theory of positrons in solids and on solid surfaces[J]. Rev. Modern Phys., 1994, 66: 841-897. |
| 52 | Chang S M, Li Q, Hua L, et al. Shape-dependent interplay between oxygen vacancies and Ag-CeO2 interaction in Ag/CeO2 catalysts and their influence on the catalytic activity[J]. J Catal., 2012, 293: 195-204. |
| 53 | Ashbrook S E, Smith M E. Solid State 17O NMR—an introduction to the background principles and applications to inorganic materials[J]. Chem. Soc. Rev., 2006, 35: 718-735. |
| 54 | Peng L, Liu Y, Kim N, et al. Detection of brönsted acid sites in zeolite HY with high-field 17O MAS-NMR techniques[J]. Nat. Mater., 2005, 4: 216-219. |
| 55 | Kim N, Stebbins J F. Vacancy and cation distribution in yttria-doped ceria: an 89Y and 17O MAS NMR study[J]. Chem. Mater., 2007, 19: 5742-5747. |
| 56 | Wang M, Wu X P, Zheng S, et al. Identification of different oxygen species in oxide nanostructures with 17O solid-state NMR spectroscopy[J]. Sci. Adv., 2015, 1(1): e1400133. |
| 57 | Cao Y, Zhao L, Torsten G, et al. Getting insights into the influence of crystal plane effect of shaped ceria on its catalytic performances[J]. J Phys. Chem. C, 2018, 122(35): 20402-20409. |
| 58 | Perras F A, Kobayashi T, Pruski M. Natural bundance 17O DNP two-dimensional and surface-enhanced NMR spectroscopy[J]. J. Am. Chem. Soc., 2015, 137: 8336-8339. |
| 59 | Blanc F, Sperrin L, Jefferson D A, et al. Dynamic nuclear polarization enhanced natural abundance 17O spectroscopy[J]. J. Am. Chem. Soc., 2013, 135: 2975-2978. |
| 60 | Hope M A, Halat D M, Magusin P C, et al. Surface-selective direct 17O DNP NMR of CeO2 nanoparticles[J]. Chem. Commun., 2017, 53: 2142-2145. |
| 61 | Fadley C S, Baird R J, Siekhaus W, et al. Surface analysis and angular distributions in X-ray photoelectron spectroscopy[J]. J. Electron. Spectrosc. Relat. Phenom., 1974, 4: 93-137. |
| 62 | Fadley C S. X-ray photoelectron spectroscopy: progress and perspectives[J]. J. Electron. Spectrosc. Relat. Phenom., 2010, 178/179: 2-32. |
| 63 | Qiu B C, Wang C, Zhang N, et al. CeO2‑induced interfacial Co2+ octahedral sites and oxygen vacancies for water oxidation[J]. ACS Catal., 2019, 9: 6484-6490. |
| 64 | Xu B, Xia L, Zhou F L, et al. Enhancing electrocatalytic N2 reduction to NH3 by CeO2 nanorod with oxygen vacancies[J]. ACS Sustainable Chem. Eng., 2019, 7: 2889-2893. |
| 65 | Nolan M, Parker S C. Watson G W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria[J]. Surf. Sci., 2005, 595: 223-232. |
| 66 | Choi Y M, Abernathy H, Chen H T, et al. Characterization of O2-CeO2 interactions using in situ Raman spectroscopy and first-principle calculations[J]. ChemPhysChem, 2006, 7: 1957-1963. |
| 67 | Qi L, Yu Q, Dai Y, et al. Influence of cerium precursors on the structure and reducibility of mesoporous CuO-CeO2 catalysts for CO oxidation[J]. Appl. Catal. B: Environ., 2012, 119/120: 308-320. |
| 68 | Zhou Y H, Du L Z, Zou Y K, et al. A STM study of Ni-Rh bimetallic particles on reducible CeO2(111)[J]. Surf. Sci., 2019, 681: 47-53. |
| 69 | Nörenberg H, Briggs G A D. The surface structure of CeO2(110) single crystals studied by STM and RHEED[J]. Surf. Sci., 1999, 433/434/435: 127-130. |
| 70 | Nörenberg H, Briggs G A D. Defect formation on CeO2(111) surfaces after annealing studied by STM[J]. Surf. Sci., 1999, 424: L352-L355. |
| 71 | Esch F, Fabris S, Zhou L, et al. Electron localization determines defect formation on ceria substrates[J]. Sci., 2005, 309: 752-755. |
| 72 | Shahed S M F, Hasegawa T, Sainoo Y, et al. STM and XPS study of CeO2(111) reduction by atomic hydrogen[J]. Surf. Sci., 2014: 628: 30-35. |
| 73 | Han Z K, Zhang L, Liu M L. The structure of oxygen vacancies in the near-surface of reduced CeO2 (111) under strain[J]. Frontiers in Chem., 2019, 7: 436. |
| 74 | Han Z K, Yang Y Z, Zhu B. Unraveling the oxygen vacancy structures at the reduced CeO2(111) surface[J]. Phys. Rev. Mater., 2018, 2: 035802. |
| 75 | Wu X P, Gong X Q. Clustering of oxygen vacancies at CeO2(111): critical role of hydroxyls[J]. Phys. Rev. Lett., 2016, 116: 086102. |
| 76 | Lu J L, Gao H J. Shaikhutdinov S,et al. Morphology and defect structure of the CeO2(1 1 1) films grown on Ru(0 0 0 1) as studied by scanning tunneling microscopy[J]. Surf. Sci., 2006, 600: 5004-5010. |
| 77 | Hu S W, Wang Y, Wang W J. Ag nanoparticles on reducible CeO2(111) thin films: effect of thickness and stoichiometry of ceria[J]. J. Phys. Chem. C, 2015, 119(7): 3579-3588. |
| 78 | Torbruegge S, Custance O, Morita S. Manipulation of individual water molecules on CeO2(111)[J]. J. Phys. Condensed Matter, 2012, 24(8): 084010. |
| 79 | Yang F, Choi Y M, Agnoli S, et al. CeO2-CuOx interactions and the controlled assembly of CeO2(111) and CeO2(100) nanoparticles on an oxidized Cu(111) substrate[J]. J. Phys. Chem. C, 2011, 115: 23062-23066. |
| 80 | Aizawa M, Morikawa Y, Namai Y. Oxygen vacancy promoting catalytic dehydration of formic acid on TiO2(110) by in situ scanning tunneling microscopic observation[J]. J. Phys. Chem. B, 2005, 109: 18831-18838. |
| 81 | Koettgen J, Martin M. Coordination numbers in Sm-doped ceria using X‑ray absorption spectroscopy[J]. J. Phys. Chem. C, 2019, 123: 6333-6339. |
| 82 | Varshney M, Sharma A, Chae K H, et al. Electronic structure and dielectric properties of ZrO2-CeO2 mixed oxides[J]. J. Phys. Chem. Solids, 2018, 119: 242-250. |
| 83 | Matthew D, KrchaMichael J J. Examination of oxygen vacancy formation in Mn-doped CeO2(111) using DFT+U and the hybrid functional HSE06[J]. Langmuir, 2013, 29(32): 10120-10131. |
| 84 | Delfina G P, Alfredo J, Beatriz I. Mn-doped CeO2: DFT+U study of a catalyst for oxidation reactions[J]. J. Phys. Chem. C, 2013, 117(35): 18063-18073. |
| 85 | Zhong S Y, Gong X Q. A first-principles molecular dynamics study on the surface lattice oxygen of ceria[J]. Appl. Surf. Sci., 2019, 496(1): 143712. |
| 86 | Han X P, Amrane N, Zhang Z S, et al. Oxygen vacancy ordering and electron localization in CeO2: hybrid functional study[J]. J. Phys. Chem. C, 2016, 120(25): 13325-13331. |
| [1] | 吴馨, 龚建英, 靳龙, 王宇涛, 黄睿宁. 超声波激励下铝板表面液滴群输运特性的研究[J]. 化工学报, 2023, 74(S1): 104-112. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [6] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [7] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [8] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [9] | 陈天华, 刘兆轩, 韩群, 张程宾, 李文明. 喷雾冷却换热强化研究进展及影响因素[J]. 化工学报, 2023, 74(8): 3149-3170. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [12] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [13] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 张希庆, 王琰婷, 徐彦红, 常淑玲, 孙婷婷, 薛定, 张立红. Mg量影响的纳米片负载Pt-In催化异丁烷脱氢性能[J]. 化工学报, 2023, 74(6): 2427-2435. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号