化工学报 ›› 2022, Vol. 73 ›› Issue (7): 2885-2894.DOI: 10.11949/0438-1157.20211747
收稿日期:2021-12-10
修回日期:2022-03-21
出版日期:2022-07-05
发布日期:2022-08-01
通讯作者:
王沛
作者简介:王沛(1986—),男,博士,教授,基金资助:Received:2021-12-10
Revised:2022-03-21
Online:2022-07-05
Published:2022-08-01
Contact:
Pei WANG
摘要:
热化学循环太阳燃料技术过程所涉及的多孔介质中复杂反应及热质传递过程,尚未建立较为完善的数学模型。以多孔氧化铈热化学循环解水过程为研究对象,将颗粒尺度的氧输运与宏观尺度的热质输运相耦合,提出完整的光热驱动条件下多孔介质非热质平衡模型,实验数据对比验证了动力学及热质输运模型的可靠性,分析了两种尺度(颗粒及床层)下,非热平衡效应、入射辐射热流、反应物浓度对动态过程的影响。入射辐射在床层的体积效应下,轴向的温度梯度使得缺陷反应的热力学平衡控制最大氧空位浓度出现在床层前侧,在缺陷反应的动态过程中,氧化过程相较于还原反应更快,提高多孔载氧体反应器的产物H2浓度应主要从还原阶段中反应过程及条件出发。可为该类问题的建模和过程设计提供较为完整的理论基础和参考路径。
中图分类号:
王沛, 魏荣阔. 光热驱动多孔氧化铈热化学循环解水制氢非热质平衡模型[J]. 化工学报, 2022, 73(7): 2885-2894.
Pei WANG, Rongkuo WEI. Thermal-mass nonequilibrium model for water splitting hydrogen production by solar thermochemical cycle of porous cerium oxide[J]. CIESC Journal, 2022, 73(7): 2885-2894.
| 参数 | 数值 |
|---|---|
| 多孔床(氧化铈) | |
| 床层孔隙率φ/孔径dp | 0.8/2 mm |
| 氧化铈密度 | |
| 晶格常数a* | 0.54112 nm |
| 晶格氧扩散系数 | |
| 表面摩尔密度 | |
| 体积摩尔密度 | |
| 颗粒表面积 | 3.14 |
| 比表面积αsf[ | |
| 反应床厚度L | 21.6 mm/50 mm |
| 多孔体积密度 | |
| 动力学参数[ | |
| 反应式(9)正反应活化能 | -7 kJ/mol |
| 反应式(9)逆反应活化能 | -230 kJ/mol |
| 反应式(10)正反应活化能 | -190 kJ/mol |
| 反应式(10)逆反应活化能 | -102 kJ/mol |
| 反应式(9)正反应动力学参数 | 130 |
| 反应式(9)逆反应动力学参数 | |
| 反应式(10)正反应动力学参数 | |
| 反应式(10)逆反应动力学参数 | |
| CeO2体积缺陷平衡反应摩尔焓 | -113.7 J/mol |
| CeO2体积缺陷平衡反应摩尔熵 | -42.1 J/(mol·K) |
| 实验参数 | |
| 还原气浓度 | |
| 氧化气浓度 | |
| 进气压力 | |
| 进气流量 | 0.2 g/s |
| 进气黏度 | |
| 进气密度 | |
| 进气温度 | 100℃ |
| 床层温度 | 恒温1000℃/ 辐射热通量1 MW/m2 |
| H2初始摩尔分数 | 0.1/0.143/0.2/0.3/0.4/0.5/0.6 |
| H2O初始摩尔分数 | 0.1/0.2/0.26/0.3/0.4 |
表1 模型部分变量参数参考值
Table 1 Values of some model variable parameters
| 参数 | 数值 |
|---|---|
| 多孔床(氧化铈) | |
| 床层孔隙率φ/孔径dp | 0.8/2 mm |
| 氧化铈密度 | |
| 晶格常数a* | 0.54112 nm |
| 晶格氧扩散系数 | |
| 表面摩尔密度 | |
| 体积摩尔密度 | |
| 颗粒表面积 | 3.14 |
| 比表面积αsf[ | |
| 反应床厚度L | 21.6 mm/50 mm |
| 多孔体积密度 | |
| 动力学参数[ | |
| 反应式(9)正反应活化能 | -7 kJ/mol |
| 反应式(9)逆反应活化能 | -230 kJ/mol |
| 反应式(10)正反应活化能 | -190 kJ/mol |
| 反应式(10)逆反应活化能 | -102 kJ/mol |
| 反应式(9)正反应动力学参数 | 130 |
| 反应式(9)逆反应动力学参数 | |
| 反应式(10)正反应动力学参数 | |
| 反应式(10)逆反应动力学参数 | |
| CeO2体积缺陷平衡反应摩尔焓 | -113.7 J/mol |
| CeO2体积缺陷平衡反应摩尔熵 | -42.1 J/(mol·K) |
| 实验参数 | |
| 还原气浓度 | |
| 氧化气浓度 | |
| 进气压力 | |
| 进气流量 | 0.2 g/s |
| 进气黏度 | |
| 进气密度 | |
| 进气温度 | 100℃ |
| 床层温度 | 恒温1000℃/ 辐射热通量1 MW/m2 |
| H2初始摩尔分数 | 0.1/0.143/0.2/0.3/0.4/0.5/0.6 |
| H2O初始摩尔分数 | 0.1/0.2/0.26/0.3/0.4 |
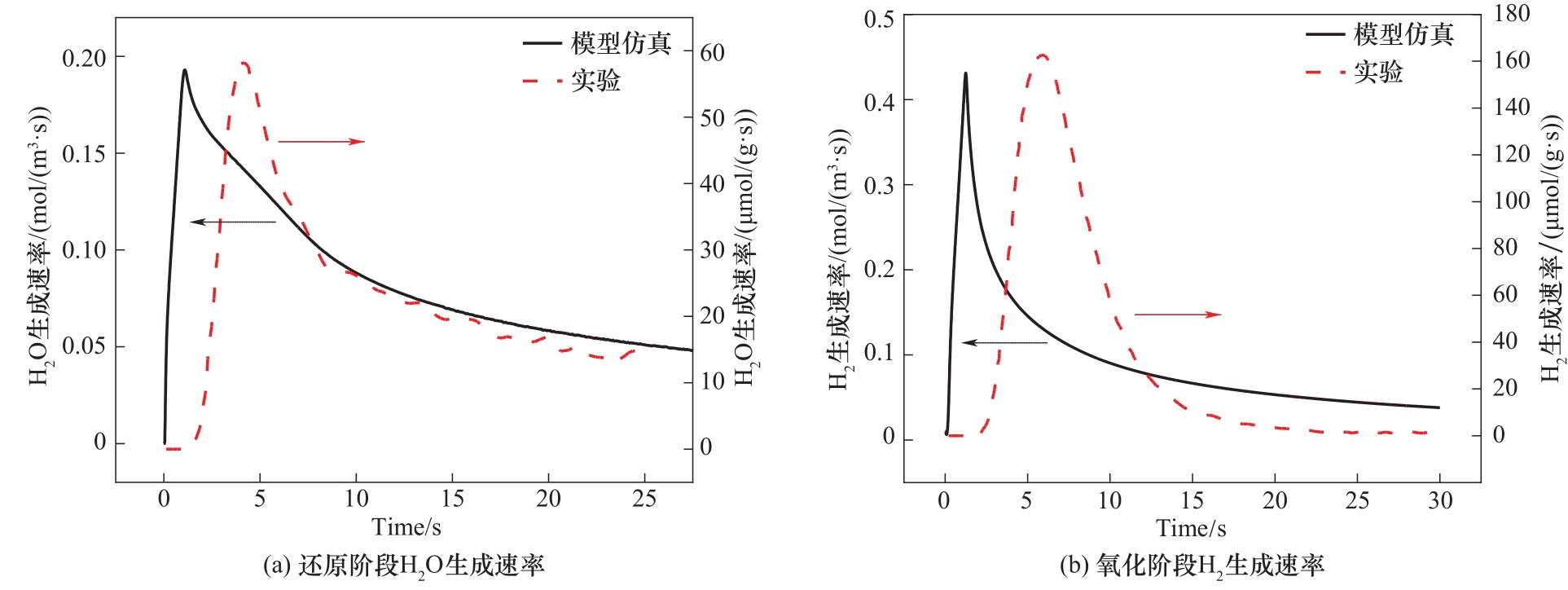
图2 氧化铈颗粒还原和氧化阶段生成物产生速率与参考实验[29]对比
Fig.2 Comparison of product generation rates of ceria particles in reduction and oxidation stages with reference experiments[29]

图6 还原步不同qin床层温度Ts及氧空位浓度VO??非稳态变化 (Dp=500 μm)
Fig.6 Bed temperature Ts and oxygen vacancy concentration VO?? unsteady changes with different qin in the reduction step (Dp=500 μm)
| 1 | Roeb M, Müller-Steinhagen H. Concentrating on solar electricity and fuels[J]. Science, 2010, 329(5993): 773-774. |
| 2 | Bayon A, de la Calle A, Ghose K K, et al. Experimental, computational and thermodynamic studies in perovskites metal oxides for thermochemical fuel production: a review[J]. International Journal of Hydrogen Energy, 2020, 45(23): 12653-12679. |
| 3 | Pereira C A, Coelho P M, Fernandes J F, et al. Study of an energy mix for the production of hydrogen[J]. International Journal of Hydrogen Energy, 2017, 42(2): 1375-1382. |
| 4 | Yadav D, Banerjee R. A review of solar thermochemical processes[J]. Renewable and Sustainable Energy Reviews, 2016, 54: 497-532. |
| 5 | Zhang B, Zhang S X, Yao R, et al. Progress and prospects of hydrogen production: opportunities and challenges[J]. Journal of Electronic Science and Technology, 2021, 19(2): 100080. |
| 6 | Voitic G, Hacker V. Recent advancements in chemical looping water splitting for the production of hydrogen[J]. RSC Advances, 2016, 6(100): 98267-98296. |
| 7 | Luo M, Yi Y, Wang S Z, et al. Review of hydrogen production using chemical-looping technology[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 3186-3214. |
| 8 | Protasova L, Snijkers F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes[J]. Fuel, 2016, 181: 75-93. |
| 9 | Agrafiotis C, Roeb M, Sattler C. A review on solar thermal syngas production via redox pair-based water/carbon dioxide splitting thermochemical cycles[J]. Renewable and Sustainable Energy Reviews, 2015, 42: 254-285. |
| 10 | Krenzke P T, Fosheim J R, Davidson J H. Solar fuels via chemical-looping reforming[J]. Solar Energy, 2017, 156: 48-72. |
| 11 | Furler P, Scheffe J R, Steinfeld A. Syngas production by simultaneous splitting of H2O and CO2 via ceria redox reactions in a high-temperature solar reactor[J]. Energy Environ. Sci., 2012, 5(3): 6098-6103. |
| 12 | Furler P, Scheffe J, Gorbar M, et al. Solar thermochemical CO2 splitting utilizing a reticulated porous ceria redox system[J]. Energy & Fuels, 2012, 26(11): 7051-7059. |
| 13 | Haeussler A, Abanades S, Costa Oliveira F A, et al. Solar redox cycling of ceria structures based on fiber boards, foams, and biomimetic cork-derived ecoceramics for two-step thermochemical H2O and CO2 splitting[J]. Energy & Fuels, 2020, 34(7): 9037-9049. |
| 14 | Venstrom L J, Petkovich N, Rudisill S, et al. The effects of morphology on the oxidation of ceria by water and carbon dioxide[J]. Journal of Solar Energy Engineering, 2012, 134(1): 011005. |
| 15 | Shen Y, Zhao K, He F, et al. Synthesis of three-dimensionally ordered macroporous LaFe0.7Co0.3O3 perovskites and their performance for chemical-looping steam reforming of methane[J]. Journal of Fuel Chemistry and Technology, 2016, 44(10): 1168-1176. |
| 16 | Panlener R J, Blumenthal R N, Garnier J E. A thermodynamic study of nonstoichiometric cerium dioxide[J]. Journal of Physics and Chemistry of Solids, 1975, 36(11): 1213-1222. |
| 17 | Mogensen M, Sammes N M, Tompsett G A. Physical, chemical and electrochemical properties of pure and doped ceria[J]. Solid State Ionics, 2000, 129(1/2/3/4): 63-94. |
| 18 | Chueh W C, Haile S M. A thermochemical study of ceria: exploiting an old material for new modes of energy conversion and CO2 mitigation[J]. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences, 2010, 368(1923): 3269-3294. |
| 19 | Zhu X, Wang H, Wei Y G, et al. Hydrogen and syngas production from two-step steam reforming of methane over CeO2-Fe2O3 oxygen carrier[J]. Journal of Rare Earths, 2010, 28(6): 907-913. |
| 20 | Hao Y, Yang C K, Haile S M. Ceria-zirconia solid solutions (Ce1- x Zr x O2- δ, x ≤ 0.2) for solar thermochemical water splitting: a thermodynamic study[J]. Chemistry of Materials, 2014, 26(20): 6073-6082. |
| 21 | Venstrom L J, de Smith R M, Hao Y, et al. Efficient splitting of CO2 in an isothermal redox cycle based on ceria[J]. Energy & Fuels, 2014, 28(4): 2732-2742. |
| 22 | Bulfin B, Lowe A J, Keogh K A, et al. Analytical model of CeO2 oxidation and reduction[J]. The Journal of Physical Chemistry C, 2013, 117(46): 24129-24137. |
| 23 | Sheu E J, Mokheimer E M A, Ghoniem A F. A review of solar methane reforming systems[J]. International Journal of Hydrogen Energy, 2015, 40(38): 12929-12955. |
| 24 | Lyu Y J, Zhu L Y, Agrafiotis C, et al. Solar fuels production: two-step thermochemical cycles with cerium-based oxides[J]. Progress in Energy and Combustion Science, 2019, 75: 100785. |
| 25 | Furler P, Steinfeld A. Heat transfer and fluid flow analysis of a 4 kW solar thermochemical reactor for ceria redox cycling[J]. Chemical Engineering Science, 2015, 137: 373-383. |
| 26 | Patil V R, Kiener F, Grylka A, et al. Experimental testing of a solar air cavity-receiver with reticulated porous ceramic absorbers for thermal processing at above 1000℃[J]. Solar Energy, 2021, 214: 72-85. |
| 27 | Zoller S, Koepf E, Roos P, et al. Heat transfer model of a 50 kW solar receiver-reactor for thermochemical redox cycling using cerium dioxide[J]. Journal of Solar Energy Engineering, 2019, 141(2): 021014. |
| 28 | Wang P, Vafai K, Liu D Y. Analysis of radiative effect under local thermal non-equilibrium conditions in porous media—application to a solar air receiver[J]. Numerical Heat Transfer, Part A: Applications, 2014, 65(10): 931-948. |
| 29 | Zhao Z L, Uddi M, Tsvetkov N, et al. Redox kinetics study of fuel reduced ceria for chemical-looping water splitting[J]. The Journal of Physical Chemistry C, 2016, 120(30): 16271-16289. |
| 30 | Ackermann S, Scheffe J R, Steinfeld A. Diffusion of oxygen in ceria at elevated temperatures and its application to H2O/CO2 splitting thermochemical redox cycles[J]. The Journal of Physical Chemistry C, 2014, 118(10): 5216-5225. |
| 31 | Wang P, Vafai K, Liu D Y, et al. Analysis of collimated irradiation under local thermal non-equilibrium condition in a packed bed[J]. International Journal of Heat and Mass Transfer, 2015, 80: 789-801. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 史昊鹏, 钟达文, 廉学新, 张君峰. 朝下多尺度沟槽翅片结构表面沸腾换热实验研究[J]. 化工学报, 2023, 74(7): 2880-2888. |
| [3] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [4] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [5] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [6] | 贾晓宇, 杨剑, 王博, 林梅, 王秋旺. 金属丝网毛细特性的孔隙尺度数值分析[J]. 化工学报, 2023, 74(5): 1928-1938. |
| [7] | 王晓萱, 胡晓红, 陆雨楠, 王士勇, 凡凤仙. 旋转膜过滤器内部流动特性数值模拟[J]. 化工学报, 2023, 74(4): 1489-1498. |
| [8] | 胡香凝, 尹渊博, 袁辰, 是赟, 刘翠伟, 胡其会, 杨文, 李玉星. 成品油在土壤中运移可视化的实验研究[J]. 化工学报, 2023, 74(4): 1827-1835. |
| [9] | 张银宁, 王进卿, 冯致, 詹明秀, 徐旭, 张光学, 池作和. 升温条件下多孔介质内气泡的生长和聚并行为[J]. 化工学报, 2023, 74(4): 1509-1518. |
| [10] | 杨辉著, 兰精灵, 杨月, 梁嘉林, 吕传文, 朱永刚. 高功率平板热管传热性能的实验研究[J]. 化工学报, 2023, 74(4): 1561-1569. |
| [11] | 张生安, 刘桂莲. 高效太阳能电解水制氢系统及其性能的多目标优化[J]. 化工学报, 2023, 74(3): 1260-1274. |
| [12] | 钱志广, 樊越, 王世学, 岳利可, 王金山, 朱禹. 吹扫条件对PEMFC阻抗弛豫现象和低温启动的影响[J]. 化工学报, 2023, 74(3): 1286-1293. |
| [13] | 宋悦, 张启成, 彭文朝, 李阳, 张凤宝, 范晓彬. MoS2基单原子催化剂的合成及其在电催化中的应用[J]. 化工学报, 2023, 74(2): 535-545. |
| [14] | 徐振和, 李泓江, 高雨, 礼峥, 张含烟, 徐宝彤, 丁茯, 孙亚光. In2O3/Ag:ZnIn2S4“Type Ⅱ”型异质结构材料的制备及可见光催化性能[J]. 化工学报, 2022, 73(8): 3625-3635. |
| [15] | 戚新刚, 路利波, 陈渝楠, 葛志伟, 郭烈锦. 造纸黑液超临界水气化制氢与高附加值化学品回收研究进展[J]. 化工学报, 2022, 73(8): 3338-3354. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
