化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2611-2623.DOI: 10.11949/0438-1157.20230254
周小文1,3( ), 杜杰2,3, 张战国1,3(
), 杜杰2,3, 张战国1,3( ), 许光文1,3(
), 许光文1,3( )
)
收稿日期:2023-03-17
修回日期:2023-06-03
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
张战国,许光文
作者简介:周小文(1998—),女,硕士研究生,603901789@qq.com
基金资助:
Xiaowen ZHOU1,3( ), Jie DU2,3, Zhanguo ZHANG1,3(
), Jie DU2,3, Zhanguo ZHANG1,3( ), Guangwen XU1,3(
), Guangwen XU1,3( )
)
Received:2023-03-17
Revised:2023-06-03
Online:2023-06-05
Published:2023-07-27
Contact:
Zhanguo ZHANG, Guangwen XU
摘要:
开发高效廉价铁基载氧体是天然气化学链重整制氢技术走向应用的关键。为探究高效铁基载氧体设计的基本依据,利用自行设计的脉冲反应器和气体产物全量同步在线分析系统,在800℃和无内外扩散影响的条件下研究了不同Fe2O3质量分数的Fe2O3-Al2O3载氧体的甲烷脉冲法还原特性。结果表明:Fe2O3的还原反应依两段机理进行,随载氧体颗粒内Fe2O3含量的多少可停止于Fe3O4,也可完全进行至FeO;气相产物中CO2与CO的摩尔比随CH4脉冲次数的变化规律也与Fe2O3含量密切相关。对用α-Al2O3粉末稀释高Fe2O3质量分数载氧体粉末的方法制备的低Fe2O3质量分数颗粒进行的脉冲还原实验结果,进一步揭示单位时间进入单个载氧体颗粒内的CH4量与其Fe2O3含量的摩尔比决定单个颗粒以及整个床层的Fe2O3还原度。最后对还原度和CH4转化率以及CO2选择性数据进行关联分析,发现只有将载氧体的还原过程止于生成Fe3O4阶段才能得到较高的CO2选择性,从而达到低成本回收高纯CO2的目的。
中图分类号:
周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623.
Xiaowen ZHOU, Jie DU, Zhanguo ZHANG, Guangwen XU. Study on the methane-pulsing reduction characteristics of Fe2O3-Al2O3 oxygen carrier[J]. CIESC Journal, 2023, 74(6): 2611-2623.
| Fe2O3质量分数/% | 比表面积/(m2/g) |
|---|---|
| 0 | 131.9 |
| 10 | 118.2 |
| 20 | 104.7 |
| 40 | 67.8 |
| 60 | 47.7 |
| 80 | 17.4 |
表1 不同Fe2O3质量分数Fe2O3-Al2O3载氧体的BET比表面积
Table 1 BET surface area of Fe2O3-Al2O3 oxygen carriers with different Fe2O3 contents
| Fe2O3质量分数/% | 比表面积/(m2/g) |
|---|---|
| 0 | 131.9 |
| 10 | 118.2 |
| 20 | 104.7 |
| 40 | 67.8 |
| 60 | 47.7 |
| 80 | 17.4 |
| Fe2O3质量分数/% | 晶粒尺寸/nm |
|---|---|
| 10 | <5 |
| 20 | 20 |
| 40 | 21 |
| 60 | 21 |
| 80 | 21 |
表2 不同质量分数Fe2O3在Fe2O3-Al2O3载氧体中的晶粒尺寸
Table 2 Crystallite size of Fe2O3 in different Fe2O3-Al2O3 oxygen carriers
| Fe2O3质量分数/% | 晶粒尺寸/nm |
|---|---|
| 10 | <5 |
| 20 | 20 |
| 40 | 21 |
| 60 | 21 |
| 80 | 21 |

图10 40%(质量)Fe2O3-Al2O3和80%(质量)Fe2O3-Al2O3经不同次数脉冲反应后的XRD谱图
Fig.10 XRD patterns of 40%(mass) Fe2O3-Al2O3 and 80%(mass) Fe2O3-Al2O3 oxygen carriers recovered after different number of pulse injection
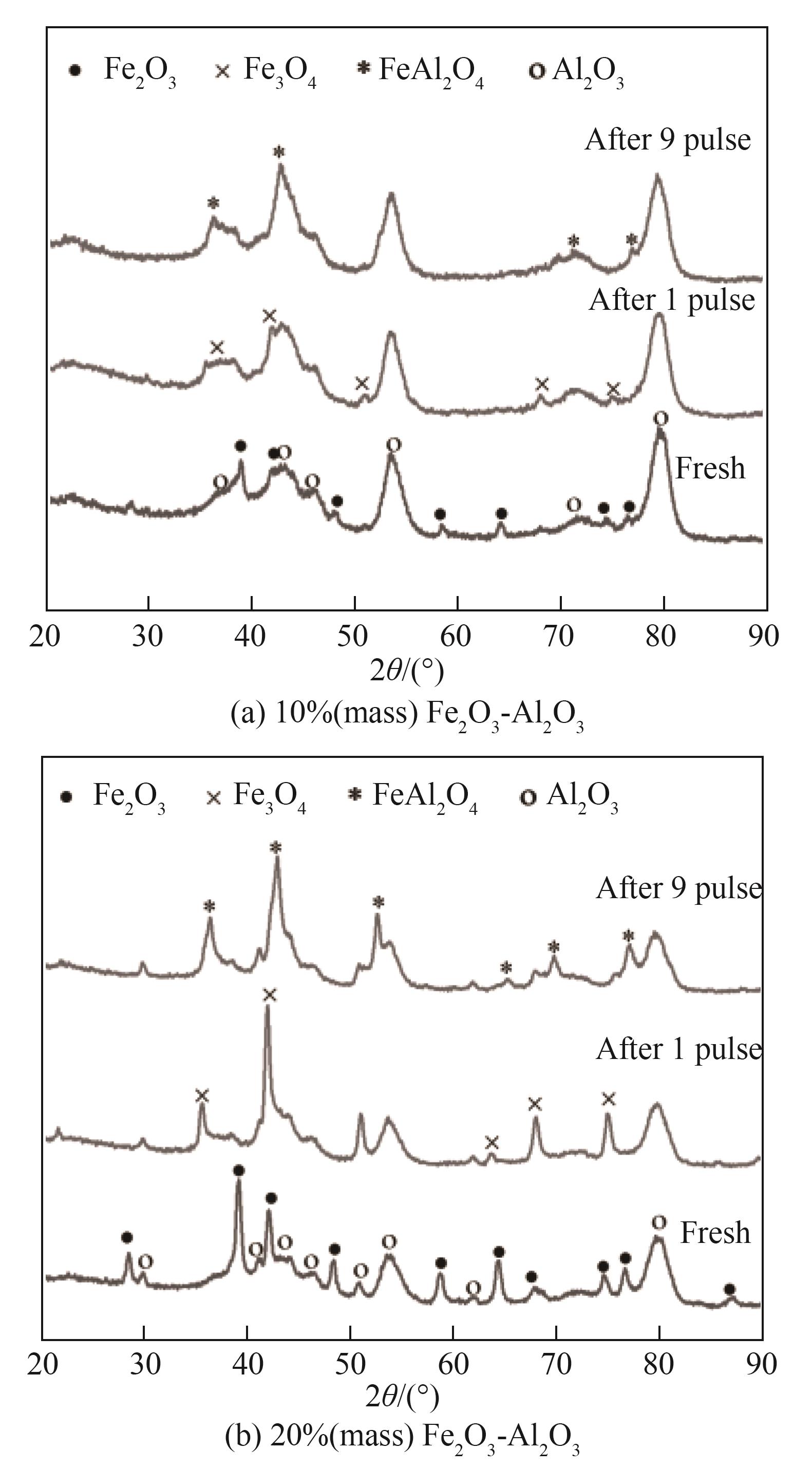
图11 10%(质量)Fe2O3-Al2O3和20%(质量)Fe2O3-Al2O3经第1次和第9次脉冲反应后的XRD谱图
Fig.11 XRD patterns of 10%(mass) Fe2O3-Al2O3 and 20%(mass) Fe2O3-Al2O3 oxygen carriers recovered after the 1st and 9th pulse injections
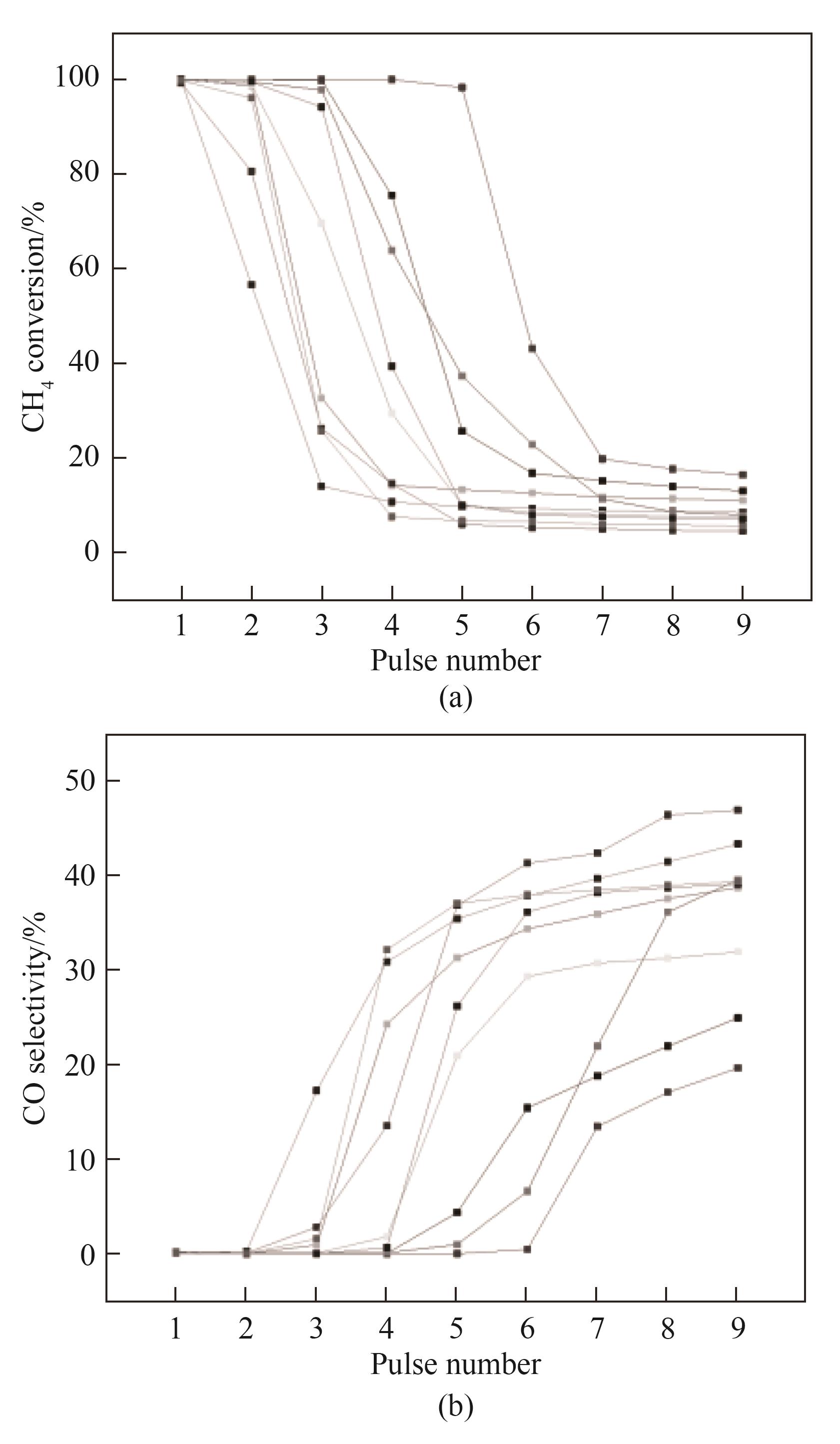
图14 高Fe2O3质量分数Fe2O3-Al2O3载氧体的两段还原特性(图中各组数据分别对应Fe2O3≥40%(质量)的不同载氧体的还原实验)3 Fe3O4 + CH4̿ 9 FeO + CO + 2 H2O(13)4 Fe3O4 + CH4̿ 12 FeO + CO2 + 2 H2O(14)
Fig.14 Two-step reduction behavior of the Fe2O3-Al2O3 oxygen carriers with high Fe2O3 contents

图15 甲烷累积脉冲量与载氧体床层氧化铁含量的摩尔比和甲烷转化率的关联(图中数据源自所有Fe2O3≥40%(质量)的不同载氧体的还原实验)
Fig.15 Correlation of the molar ratio of cumulatively pulsed CH4 to Fe2O3 contained in bed of Fe2O3-Al2O3 with CH4 conversion
| 1 | Richter H J, Knoche K F. Reversibility of combustion processes[M]//ACS Symposium Series. Washington, D.C.: American Chemical Society, 1983: 71-85. |
| 2 | Rydén M, Lyngfelt A. Using steam reforming to produce hydrogen with carbon dioxide capture by chemical-looping combustion[J]. International Journal of Hydrogen Energy, 2006, 31(10): 1271-1283. |
| 3 | 常宏岗. 天然气制氢技术及经济性分析[J]. 石油与天然气化工, 2021, 50(4): 53-57. |
| Chang H G. Technical and economic analysis of hydrogen production from natural gas[J]. Chemical Engineering of Oil & Gas, 2021, 50(4): 53-57. | |
| 4 | Dash S K, Chakraborty S, Elangovan D. A brief review of hydrogen production methods and their challenges[J]. Energies, 2023, 16(3): 1141. |
| 5 | Kathe M V, Empfield A, Na J, et al. Hydrogen production from natural gas using an iron-based chemical looping technology: thermodynamic simulations and process system analysis[J]. Applied Energy, 2016, 165: 183-201. |
| 6 | de Vos Y, Vamvakeros A, Matras D, et al. Sustainable iron-based oxygen carriers for hydrogen production—real-time operando investigation[J]. International Journal of Greenhouse Gas Control, 2019, 88: 393-402. |
| 7 | 曹军文, 张文强, 李一枫, 等. 中国制氢技术的发展现状[J]. 化学进展, 2021, 33(12): 2215-2244. |
| Cao J W, Zhang W Q, Li Y F, et al. Current status of hydrogen production in China[J]. Progress in Chemistry, 2021, 33(12): 2215-2244. | |
| 8 | Chiesa P, Lozza G, Malandrino A, et al. Three-reactors chemical looping process for hydrogen production[J]. International Journal of Hydrogen Energy, 2008, 33(9): 2233-2245. |
| 9 | Zhang F, Zhu L, Wang Y, et al. Exergy analysis on the process for three reactors chemical looping hydrogen generation[J]. International Journal of Hydrogen Energy, 2020, 45(46): 24322-24332. |
| 10 | Protasova L, Snijkers F. Recent developments in oxygen carrier materials for hydrogen production via chemical looping processes[J]. Fuel, 2016, 181: 75-93. |
| 11 | Yu Z L, Yang Y Y, Yang S, et al. Iron-based oxygen carriers in chemical looping conversions: a review[J]. Carbon Resources Conversion, 2019, 2(1): 23-34. |
| 12 | Chen S Y, Shi Q L, Xue Z P, et al. Experimental investigation of chemical-looping hydrogen generation using Al2O3 or TiO2-supported iron oxides in a batch fluidized bed[J]. International Journal of Hydrogen Energy, 2011, 36(15): 8915-8926. |
| 13 | 陈庚. 气基还原氧化铁动力学机理研究[D]. 大连: 大连理工大学, 2011. |
| Chen G. The kinetics of the gas-based reduction of iron oxide[D]. Dalian: Dalian University of Technology, 2011. | |
| 14 | 郭培民, 赵沛, 王磊, 等. 氧化铁气基还原过程的气体氧化动力学[J]. 钢铁, 2017, 52(9): 22-26. |
| Guo P M, Zhao P, Wang L, et al. Oxidizing kinetics of reducing gas during iron oxide reduction process[J]. Iron & Steel, 2017, 52(9): 22-26. | |
| 15 | Cho W C, Kim C G, Jeong S U, et al. Activation and reactivity of iron oxides as oxygen carriers for hydrogen production by chemical looping[J]. Industrial & Engineering Chemistry Research, 2015, 54(12): 3091-3100. |
| 16 | Svoboda K, Slowinski G, Rogut J, et al. Thermodynamic possibilities and constraints for pure hydrogen production by iron based chemical looping process at lower temperatures[J]. Energy Conversion and Management, 2007, 48(12): 3063-3073. |
| 17 | Jafarian M, Arjomandi M, Nathan G J. Thermodynamic potential of high temperature chemical looping combustion with molten iron oxide as the oxygen carrier[J]. Chemical Engineering Research and Design, 2017, 120: 69-81. |
| 18 | 李然家, 沈师孔. 晶格氧用于甲烷氧化制合成气的研究——氧化铁的氧化还原性能[J]. 分子催化, 2001, 15(3): 181-186. |
| Li R J, Shen S K. Study on lattice oxygen used in the conversion of methane to synthesis gas—redox performance of Fe2O3 catalyst[J]. Journal of Molecular Catalysis (China), 2001, 15(3): 181-186. | |
| 19 | Cetinkaya S, Eroglu S. A single-step process for direct reduction of iron oxide to sponge iron by undiluted methane[J]. JOM, 2017, 69(6): 993-998. |
| 20 | 刘丰. 化学链燃烧中铁基复合载氧体与CO的反应特性及机理研究[D]. 武汉: 华中科技大学, 2020. |
| Liu F. Research on the reaction characteristics and mechanism of Fe-based composite oxygen carriers with CO during chemical-looping combustion[D]. Wuhan: Huazhong University of Science and Technology, 2020. | |
| 21 | 李振山, 鲍金花, 孙宏明, 等.以煤为燃料的化学链燃烧研究进展[J].中国电机工程学报, 2014, 34(29): 5131-5139. |
| Li Z S, Bao J H, Sun H M, et al. Research and development of coal-fueled chemical looping combustion[J]. Proceedings of the CSEE, 2014, 34(29): 5131-5139. | |
| 22 | 马哲. 纳米/多级孔*BEA及MFI型分子筛的合成及性能研究[D]. 长春: 吉林大学, 2022. |
| Ma Z. Synthesis and properties of nano/multi-porous *BEA and MFI molecular sieves[D]. Changchun: Jilin University, 2022. | |
| 23 | 梁皓,宋喜军,尹泽群, 等. 化学链制氢中Fe2O3/LaFeO3载氧体的性能研究[J]. 燃料化学学报, 2013, 41(12): 1512-1519. |
| Liang H, Song X J, Yin Z Q, et al. Performance of Fe2O3/LaFeO3 as oxygen carrier in chemical-looping hydrogen generation[J]. Journal of Fuel Chemistry and Technology, 2013, 41(12): 1512-1519. | |
| 24 | Dai X P, Li R J, Yu C C, et al. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A= La, Nd, Eu) perovskite-type oxides as oxygen storage[J]. The Journal of Physical Chemistry B, 2006, 110(45): 22525-22531. |
| 25 | 史广全, 孙永升, 李淑菲, 等. 某鲕状赤铁矿深度还原过程研究[J]. 现代矿业, 2009, 25(8): 29-31. |
| Shi G Q, Sun Y S, Li S F, et al. Study of deep reduction process of an oolitic hematite[J]. Modern Mining, 2009, 25(8): 29-31. | |
| 26 | 王凯莉. 柱状催化剂颗粒随机堆积固定床反应器甲烷化过程模拟及床层结构优化[D]. 乌鲁木齐: 新疆大学, 2018. |
| Wang K L. Randomly packed methanation fixed-bed numerical simulation of chemical reaction with cylindrical catalysts and bed structure optimization[D]. Urumqi: Xinjiang University, 2018. | |
| 27 | 李然家, 余长春, 代小平, 等. 以晶格氧为氧源的甲烷部分氧化制合成气[J].催化学报,2002, 23(4): 381-387. |
| Li R J, Yu C C, Dai X P, et al. Partial oxidation of methane to synthesis gas using lattice oxygen instead of molecular oxygen[J]. Chinese Journal of Catalysis, 2002, 23(4): 381-387. | |
| 28 | Manchili S K, Wendel J, Hryha E, et al. Analysis of iron oxide reduction kinetics in the nanometric scale using hydrogen[J]. Nanomaterials, 2020, 10(7): 1276. |
| 29 | Jozwiak W K, Kaczmarek E, Maniecki T P, et al. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres[J]. Applied Catalysis A: General, 2007, 326(1): 17-27. |
| 30 | 赵全忠, 赵祯霞, 邹昀, 等. Cu-Ni/γ-Al2O3催化剂上二甘醇催化氨化合成吗啉的本征动力学研究[J]. 高校化学工程学报, 2018, 32(6): 1353-1358. |
| Zhao Q Z, Zhao Z X, Zou Y, et al. Intrinsic kinetics of morpholine synthesis via diethylene glycol amination catalyzed by Cu-Ni/γ- Al2O3 [J]. Journal of Chemical Engineering of Chinese Universities, 2018, 32(6): 1353-1358. | |
| 31 | 郭雪岩, 祝俊, 杨帆. 结构化载氧体颗粒化学链燃烧内扩散影响模拟分析[J].能源研究与信息, 2018, 34(3): 151-158, 181. |
| Guo X Y, Zhu J, Yang F. Simulation analysis on the effect of internal diffusion from chemical looping combustion with structured oxygen carrier[J]. Energy Research and Information, 2018, 34(3): 151-158, 181. | |
| 32 | 刘自松, 魏永刚, 李孔斋, 等. Fe2O3/Al2O3氧载体用于甲烷化学链燃烧:负载量与制备方法的影响[J]. 燃料化学学报, 2013, 41(11):1384-1392. |
| Liu Z S, Wei Y G, Li K Z, et al. Fe2O3/Al2O3 oxygen carriers for chemical looping combustion of methane: influence of Fe2O3 loadings and preparation methods[J]. Journal of Fuel Chemistry and Technology, 2013, 41(11): 1384-1392. | |
| 33 | Kidambi P R, Cleeton J P E, Scott S A, et al. Interaction of iron oxide with alumina in a composite oxygen carrier during the production of hydrogen by chemical looping[J]. Energy & Fuels, 2012, 26(1): 603-617. |
| 34 | 史奇良, 陈时熠, 薛志鹏, 等. 铁基载氧体化学链制氢特性实验研究[J]. 中国电机工程学报, 2011, 31(S1): 168-174. |
| Shi Q L, Chen S Y, Xue Z P, et al. Experimental investigation of chemical looping hydrogen generation using iron oxides as oxygen carrier[J]. Proceedings of the CSEE, 2011, 31(S1): 168-174. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [3] | 刘晓洋, 喻健良, 侯玉洁, 闫兴清, 张振华, 吕先舒. 螺旋微通道对掺氢甲烷爆轰传播的影响[J]. 化工学报, 2023, 74(7): 3139-3148. |
| [4] | 牛超, 沈胜强, 杨艳, 潘泊年, 李熠桥. 甲烷BOG喷射器流动过程计算与性能分析[J]. 化工学报, 2023, 74(7): 2858-2868. |
| [5] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [6] | 李勇, 高佳琦, 杜超, 赵亚丽, 李伯琼, 申倩倩, 贾虎生, 薛晋波. Ni@C@TiO2核壳双重异质结的构筑及光热催化分解水产氢[J]. 化工学报, 2023, 74(6): 2458-2467. |
| [7] | 王泽栋, 石至平, 刘丽艳. 考虑气泡非均匀耗散的矩形反应器声流场数值模拟及结构优化[J]. 化工学报, 2023, 74(5): 1965-1973. |
| [8] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [9] | 张建华, 陈萌萌, 孙雅雯, 彭永臻. 部分短程硝化同步除磷耦合Anammox实现生活污水高效脱氮除磷[J]. 化工学报, 2023, 74(5): 2147-2156. |
| [10] | 张生安, 刘桂莲. 高效太阳能电解水制氢系统及其性能的多目标优化[J]. 化工学报, 2023, 74(3): 1260-1274. |
| [11] | 胡晗, 杨亮, 李春晓, 刘道平. 天然烟浸滤液水合物法储甲烷动力学研究[J]. 化工学报, 2023, 74(3): 1313-1321. |
| [12] | 李雨萧, 王青月, Ho Lim Khak, 李晓辉, Erlita Mastan, 彭博, 王文俊. 自由基聚合反应动力学常数测定技术[J]. 化工学报, 2023, 74(2): 559-570. |
| [13] | 宋悦, 张启成, 彭文朝, 李阳, 张凤宝, 范晓彬. MoS2基单原子催化剂的合成及其在电催化中的应用[J]. 化工学报, 2023, 74(2): 535-545. |
| [14] | 彭晓婉, 郭笑楠, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8浆液法分离CH4/N2的双吸收-吸附塔工艺流程建模与模拟[J]. 化工学报, 2023, 74(2): 784-795. |
| [15] | 付家崴, 陈帅帅, 方凯伦, 蒋新. 微反应器共沉淀反应制备铜锰催化剂[J]. 化工学报, 2023, 74(2): 776-783. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号