化工学报 ›› 2022, Vol. 73 ›› Issue (7): 2790-2805.DOI: 10.11949/0438-1157.20220325
收稿日期:2022-03-02
修回日期:2022-04-08
出版日期:2022-07-05
发布日期:2022-08-01
通讯作者:
吕波,李春
作者简介:张昕哲(1997—),男,硕士研究生,基金资助:
Xinzhe ZHANG1( ),Wentao SUN2,Bo LYU1(
),Wentao SUN2,Bo LYU1( ),Chun LI1,2(
),Chun LI1,2( )
)
Received:2022-03-02
Revised:2022-04-08
Online:2022-07-05
Published:2022-08-01
Contact:
Bo LYU,Chun LI
摘要:
在植物天然产物合成过程中,氧化反应是其中的关键反应,氧化酶是催化氧化反应不可或缺的生物催化剂,也是利用微生物合成植物天然产物过程中不可或缺的关键酶。介绍了萜类、生物碱、黄酮等植物天然产物骨架的氧化修饰,按照辅基的差异对合成植物天然产物过程中的氧化酶进行分类介绍,阐释了不同辅基参与氧化反应的机理。此外,还介绍了植物天然产物氧化过程在微生物合成过程中的难点,以及提高氧化酶催化效率的方法。最后,对未来合成生物学中氧化酶在微生物合成植物天然产物领域的前景进行了展望。
中图分类号:
张昕哲, 孙文涛, 吕波, 李春. 植物天然产物氧化与微生物制造[J]. 化工学报, 2022, 73(7): 2790-2805.
Xinzhe ZHANG, Wentao SUN, Bo LYU, Chun LI. Oxidative modification of plant natural products and microbial manufacturing[J]. CIESC Journal, 2022, 73(7): 2790-2805.
| 种类 | 辅因子 | 电子供体 | 反应类型 | 举例 | 文献 |
|---|---|---|---|---|---|
| A | FAD | NAD(P)H | 羟基化反应 | 对羟基苯酸3-羟化酶 | [ |
| 磺化氧化反应 | MICAL | [ | |||
| B | FAD | NAD(P)H | 拜尔-维立格氧化反应 | 环己酮单加氧酶 | [ |
| 杂原子氧化反应 | 二甲基苯胺单加氧酶 | [ | |||
| N-羟基化反应 | L-鸟氨酸单加氧酶 | [ | |||
| 氧化脱羧反应 | 吲哚-3-丙酮酸单加氧酶 | [ | |||
| C | FMN | FMNH2 | 光化学反应 | 烷醛单加氧酶 | [ |
| 拜尔-维立格氧化反应 | 二酮环烷单加氧酶 | [ | |||
| 环氧化反应 | 二酮环烷单加氧酶 | [ | |||
| 脱硫反应, 磺化氧化反应 | 烷烃磺酸盐单加氧酶 | [ | |||
| 羟基化反应 | 长链烷烃单氧酶 | [ | |||
| D | FAD/FMN | FADH2/FMNH2 | 羟基化反应 | 对羟基苯乙酸3-羟化酶 | [ |
| N-羟基化反应 | KijD3 糖 N-氧化酶 | [ | |||
| E | FAD | FADH2 | 环氧化反应 | 苯乙烯单加氧酶 | [ |
| F | FAD | FADH2 | 卤代反应 | 色氨酸7-卤化酶 | [ |
| G | FAD | 底物 | 氧化脱羧反应 | 色氨酸2-单加氧酶 | [ |
| H | FMN | 底物 | 氧化脱羧反应 | 乳酸2-单加氧酶 | [ |
| 氧化脱硝反应 | 硝酸单加氧酶 | [ |
表1 黄素依赖的单加氧酶主要类别[11]
Table 1 Main classes of flavin-dependent monooxygenases[11]
| 种类 | 辅因子 | 电子供体 | 反应类型 | 举例 | 文献 |
|---|---|---|---|---|---|
| A | FAD | NAD(P)H | 羟基化反应 | 对羟基苯酸3-羟化酶 | [ |
| 磺化氧化反应 | MICAL | [ | |||
| B | FAD | NAD(P)H | 拜尔-维立格氧化反应 | 环己酮单加氧酶 | [ |
| 杂原子氧化反应 | 二甲基苯胺单加氧酶 | [ | |||
| N-羟基化反应 | L-鸟氨酸单加氧酶 | [ | |||
| 氧化脱羧反应 | 吲哚-3-丙酮酸单加氧酶 | [ | |||
| C | FMN | FMNH2 | 光化学反应 | 烷醛单加氧酶 | [ |
| 拜尔-维立格氧化反应 | 二酮环烷单加氧酶 | [ | |||
| 环氧化反应 | 二酮环烷单加氧酶 | [ | |||
| 脱硫反应, 磺化氧化反应 | 烷烃磺酸盐单加氧酶 | [ | |||
| 羟基化反应 | 长链烷烃单氧酶 | [ | |||
| D | FAD/FMN | FADH2/FMNH2 | 羟基化反应 | 对羟基苯乙酸3-羟化酶 | [ |
| N-羟基化反应 | KijD3 糖 N-氧化酶 | [ | |||
| E | FAD | FADH2 | 环氧化反应 | 苯乙烯单加氧酶 | [ |
| F | FAD | FADH2 | 卤代反应 | 色氨酸7-卤化酶 | [ |
| G | FAD | 底物 | 氧化脱羧反应 | 色氨酸2-单加氧酶 | [ |
| H | FMN | 底物 | 氧化脱羧反应 | 乳酸2-单加氧酶 | [ |
| 氧化脱硝反应 | 硝酸单加氧酶 | [ |
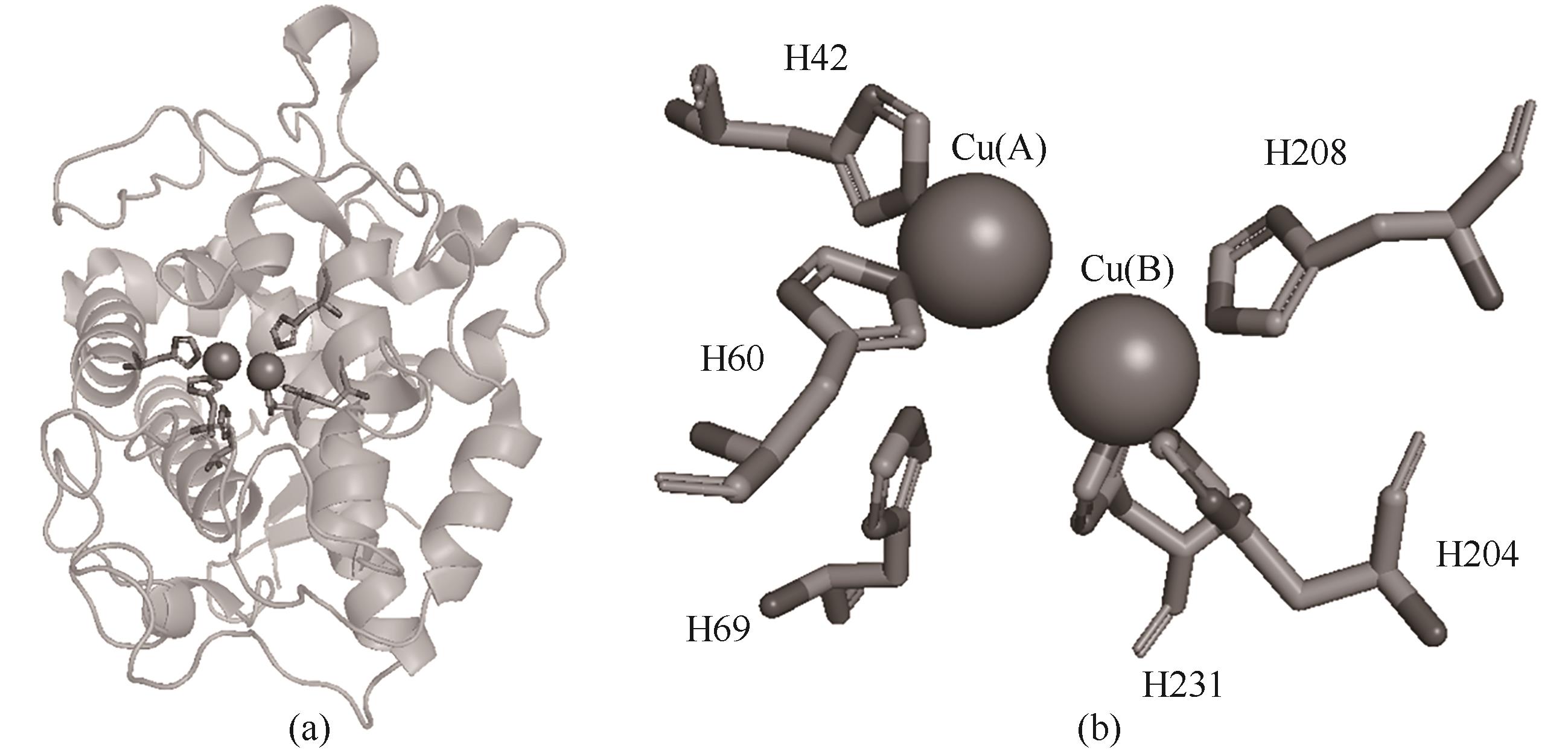
图5 来自巨型芽孢杆菌的酪氨酸酶及其活性位点组氨酸与铜离子的配位(PDB:3NM8)
Fig.5 Tyrosinase from Bacillus megaterium and coordination of its active site histidine with copper ion(PDB:3NM8)
| 氧化酶 | 来源 | 种类 | 反应底物 | 反应产物 | 文献 |
|---|---|---|---|---|---|
| CYP71AV9 | Cynara cardunculus | P450 | 吉玛烯A | 吉玛烯酸 | [ |
| CYP71BL5 | Cynara cardunculus | P450 | 吉玛烯酸 | 6-羟基吉玛烯酸 | [ |
| CYP76AJ1 | Persicaria hydropiper | P450 | 补身醇 | 水蓼二醛 | [ |
| CYP701A26 | Zea mays | P450 | ent-贝壳杉烯 | ent-贝壳杉烯酸 | [ |
| CYP716A179 | Glycyrrhiza uralensis | P450 | 羽扇豆醇 | 桦木酸 | [ |
| β-香树脂醇 | 齐墩果酸 | [ | |||
| α-香树脂醇 | 熊果酸 | [ | |||
| CYP716A175 | Malus pumila | P450 | 日耳曼醇 | 模绕酸 | [ |
| CYP716A141 | Platycodon grandiflorus | P450 | β-香树脂醇 | 马尼拉二醇 | [ |
| CYP716E26 | Solanum lycopersicum | P450 | β-香树脂醇 | 曼陀罗萜二醇 | [ |
| CYP716C49 | Crataegus pinnatifida | P450 | 齐墩果酸 | 山楂酸 | [ |
| 熊果酸 | 科罗索酸 | [ | |||
| 桦木酸 | 麦珠子酸 | [ | |||
| CYP714E19 | Centella asiatica | P450 | 齐墩果酸 | 丝石竹酸 | [ |
| CYP72A68 | Medicago truncatula | P450 | 齐墩果酸 | 常春藤素 | [ |
| CYP72A67 | Medicago truncatula | P450 | 常春藤素 | 贝萼皂苷元 | [ |
| DBOX | Papaver somniferum | 黄素依赖型氧化酶 | 二氢血根碱 | 血根碱 | [ |
| TPOX | Papaver somniferum | 黄素依赖型氧化酶 | 四氢罂粟碱 | 罂粟碱 | [ |
| MAO-N | 黄素依赖型氧化酶 | (±)-毒芹碱 | (R)-毒芹碱 | [ | |
| (±)-胡秃子碱 | (R)-胡秃子碱 | [ | |||
| (±)-细茜花碱 | (R)-细茜花碱 | [ | |||
| FsDAAO | Aspergillus niger | 黄素依赖型氧化酶 | 四氢异喹啉 | (S)-四氢异喹啉 | [ |
| HypC | Aspergillus nidulans | 蒽酮氧化酶 | 降散盘衣酸蒽酮 | 降散盘衣酸 | [ |
| TpcL | Aspergillus fumigatus | 蒽酮氧化酶 | 大黄素蒽酮 | 大黄素 | [ |
| Trametes pubescens漆酶 | Trametes pubescens | 漆酶 | 葡萄糖 | 葡萄糖醛酸 | [ |
| 桃柘酚 | 二聚桃柘酚 | [ | |||
| CYP106A1 | Bacillus megaterium | P450 | 强的松醇 | 15β-羟基强的松 | [ |
| 地塞米松 | 11-羰基地塞米松 | [ |
表2 天然产物氧化酶催化举例
Table 2 Examples of natural product oxidase catalysis
| 氧化酶 | 来源 | 种类 | 反应底物 | 反应产物 | 文献 |
|---|---|---|---|---|---|
| CYP71AV9 | Cynara cardunculus | P450 | 吉玛烯A | 吉玛烯酸 | [ |
| CYP71BL5 | Cynara cardunculus | P450 | 吉玛烯酸 | 6-羟基吉玛烯酸 | [ |
| CYP76AJ1 | Persicaria hydropiper | P450 | 补身醇 | 水蓼二醛 | [ |
| CYP701A26 | Zea mays | P450 | ent-贝壳杉烯 | ent-贝壳杉烯酸 | [ |
| CYP716A179 | Glycyrrhiza uralensis | P450 | 羽扇豆醇 | 桦木酸 | [ |
| β-香树脂醇 | 齐墩果酸 | [ | |||
| α-香树脂醇 | 熊果酸 | [ | |||
| CYP716A175 | Malus pumila | P450 | 日耳曼醇 | 模绕酸 | [ |
| CYP716A141 | Platycodon grandiflorus | P450 | β-香树脂醇 | 马尼拉二醇 | [ |
| CYP716E26 | Solanum lycopersicum | P450 | β-香树脂醇 | 曼陀罗萜二醇 | [ |
| CYP716C49 | Crataegus pinnatifida | P450 | 齐墩果酸 | 山楂酸 | [ |
| 熊果酸 | 科罗索酸 | [ | |||
| 桦木酸 | 麦珠子酸 | [ | |||
| CYP714E19 | Centella asiatica | P450 | 齐墩果酸 | 丝石竹酸 | [ |
| CYP72A68 | Medicago truncatula | P450 | 齐墩果酸 | 常春藤素 | [ |
| CYP72A67 | Medicago truncatula | P450 | 常春藤素 | 贝萼皂苷元 | [ |
| DBOX | Papaver somniferum | 黄素依赖型氧化酶 | 二氢血根碱 | 血根碱 | [ |
| TPOX | Papaver somniferum | 黄素依赖型氧化酶 | 四氢罂粟碱 | 罂粟碱 | [ |
| MAO-N | 黄素依赖型氧化酶 | (±)-毒芹碱 | (R)-毒芹碱 | [ | |
| (±)-胡秃子碱 | (R)-胡秃子碱 | [ | |||
| (±)-细茜花碱 | (R)-细茜花碱 | [ | |||
| FsDAAO | Aspergillus niger | 黄素依赖型氧化酶 | 四氢异喹啉 | (S)-四氢异喹啉 | [ |
| HypC | Aspergillus nidulans | 蒽酮氧化酶 | 降散盘衣酸蒽酮 | 降散盘衣酸 | [ |
| TpcL | Aspergillus fumigatus | 蒽酮氧化酶 | 大黄素蒽酮 | 大黄素 | [ |
| Trametes pubescens漆酶 | Trametes pubescens | 漆酶 | 葡萄糖 | 葡萄糖醛酸 | [ |
| 桃柘酚 | 二聚桃柘酚 | [ | |||
| CYP106A1 | Bacillus megaterium | P450 | 强的松醇 | 15β-羟基强的松 | [ |
| 地塞米松 | 11-羰基地塞米松 | [ |
| 1 | Newman D J, Cragg G M. Natural products as sources of new drugs over the last 25 years[J]. Journal of Natural Products, 2007, 70(3): 461-477. |
| 2 | Rishton G M. Natural products as a robust source of new drugs and drug leads: past successes and present day issues[J]. The American Journal of Cardiology, 2008, 101(10): S43-S49. |
| 3 | Miettinen K, Pollier J, Buyst D, et al. The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis [J]. Nature Communications, 2017, 8: 14153. |
| 4 | Seki H, Sawai S, Ohyama K, et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin[J]. The Plant Cell, 2011, 23(11): 4112-4123. |
| 5 | 范炳芝, 王一鑫, 廉霄甜, 等. 三萜类化合物抗病毒的构效关系及其作用机制研究进展[J]. 化工学报, 2020, 71(9): 4071-4101. |
| Fan B Z, Wang Y X, Lian X T, et al. Structure-activity relationships and mechanisms of triterpenoids against virus[J]. CIESC Journal, 2020, 71(9): 4071-4101. | |
| 6 | Marsafari M, Samizadeh H, Rabiei B, et al. Biotechnological production of flavonoids: an update on plant metabolic engineering, microbial host selection, and genetically encoded biosensors[J]. Biotechnology Journal, 2020, 15(8): 1900432. |
| 7 | Wani M C, Wall M E. Plant antitumor agents(Ⅱ): Structure of two new alkaloids from Camptotheca acuminata [J]. The Journal of Organic Chemistry, 1969, 34(5): 1364-1367. |
| 8 | Meunier B, de Visser S P, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome p450 enzymes[J]. Chemical Reviews, 2004, 104(9): 3947-3980. |
| 9 | van Berkel W J H, Kamerbeek N M, Fraaije M W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts[J]. Journal of Biotechnology, 2006, 124(4): 670-689. |
| 10 | Rolff M, Schottenheim J, Decker H, et al. Copper-O2 reactivity of tyrosinase models towards external monophenolic substrates: molecular mechanism and comparison with the enzyme[J]. Chemical Society Reviews, 2011, 40(7): 4077-4098. |
| 11 | Huijbers M M E, Montersino S, Westphal A H, et al. Flavin dependent monooxygenases[J]. Archives of Biochemistry and Biophysics, 2014, 544: 2-17. |
| 12 | Chordia S, Narasimhan S, Lucini Paioni A, et al. In vivo assembly of artificial metalloenzymes and application in whole-cell biocatalysis[J]. Angewandte Chemie International Edition, 2021, 60(11): 5913-5920. |
| 13 | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| Sun W T, Li C. Design and construction of microbial cell factory for biosynthesis of plant natural products[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1202-1214. | |
| 14 | 薛海洁, 王颖, 李春. 植物天然产物的微生物合成与转化[J]. 化工学报, 2019, 70(10): 3825-3835. |
| Xue H J, Wang Y, Li C. Microbial synthesis and transformation of plant-derived natural products[J]. CIESC Journal, 2019, 70(10): 3825-3835. | |
| 15 | Sun W T, Qin L, Xue H J, et al. Novel trends for producing plant triterpenoids in yeast[J]. Critical Reviews in Biotechnology, 2019, 39(5): 618-632. |
| 16 | 朱明, 王彩霞, 李春. 工程化酿酒酵母合成植物三萜类化合物[J]. 化工学报, 2015, 66(9): 3350-3356. |
| Zhu M, Wang C X, Li C. Engineered saccharomyces cerevisiae for biosynthesis of plant triterpenoids[J]. CIESC Journal, 2015, 66(9): 3350-3356. | |
| 17 | Karp F, Mihaliak C A, Harris J L, et al. Monoterpene biosynthesis: specificity of the hydroxylations of (-)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata), and perilla (Perilla frutescens) leaves[J]. Archives of Biochemistry and Biophysics, 1990, 276(1): 219-226. |
| 18 | Covello P S, Teoh K H, Polichuk D R, et al. Functional genomics and the biosynthesis of artemisinin[J]. Phytochemistry, 2007, 68(14): 1864-1871. |
| 19 | Ro D K, Bohlmann J. Diterpene resin acid biosynthesis in loblolly pine (Pinus taeda): functional characterization of abietadiene/levopimaradiene synthase (PtTPS-LAS) cDNA and subcellular targeting of PtTPS-LAS and abietadienol/abietadienal oxidase (PtAO, CYP720B1)[J]. Phytochemistry, 2006, 67(15): 1572-1578. |
| 20 | 周蕾, 刘卓刚. 熊果酸抗肿瘤作用机制的研究进展[J]. 医药导报, 2011, 30(4): 490-494. |
| Zhou L, Liu Z G. Research progress of ursolic acid's antitumor mechanism[J]. Herald of Medicine, 2011, 30(4): 490-494. | |
| 21 | 陈芳玲, 楼雅楠, 孔祥倩, 等. 三萜类化合物抗肿瘤及其作用机制的研究进展[J]. 中医药导报, 2018, 24(17): 45-49. |
| Chen F L, Lou Y N, Kong X Q, et al. Research progress on antitumor effect and mechanisms of three terpenoids[J]. Guiding Journal of Traditional Chinese Medicine and Pharmacy, 2018, 24(17): 45-49. | |
| 22 | 李诺楠, 李春. 糖基转移酶在三萜皂苷合成中的应用[J]. 化工学报, 2019, 70(10): 3869-3879. |
| Li N N, Li C. Applications of glycosyltransferases in synthesis of triterpenoid saponins[J]. CIESC Journal, 2019, 70(10): 3869-3879. | |
| 23 | Nabavi S M, Šamec D, Tomczyk M, et al. Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering[J]. Biotechnology Advances, 2020, 38: 107316. |
| 24 | Xie F, Lang Q Y, Zhou M, et al. The dietary flavonoid luteolin inhibits Aurora B kinase activity and blocks proliferation of cancer cells[J]. European Journal of Pharmaceutical Sciences, 2012, 46(5): 388-396. |
| 25 | 翟广玉, 马海英, 郜蕾. 槲皮素及其衍生物的抗肿瘤活性研究进展[J]. 化学试剂, 2015, 37(2): 97-103. |
| Zhai G Y, Ma H Y, Gao L. Progress of antitumor activity of quercetin and derivatives[J]. Chemical Reagents, 2015, 37(2): 97-103. | |
| 26 | Akhlaghi M, Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury[J]. Journal of Molecular and Cellular Cardiology, 2009, 46(3): 309-317. |
| 27 | 冯甜, 王力彬, 周楠, 等. 根皮素的研究进展[J]. 转化医学杂志, 2017, 6(1): 42-46. |
| Feng T, Wang L B, Zhou N, et al. Advance in studies on phloretin[J]. Translational Medicine Journal, 2017, 6(1): 42-46. | |
| 28 | 马琮鉴, 高健美, 孔浩, 等. 根皮苷药理作用研究进展[J]. 医药导报, 2020, 39(3): 360-364. |
| Ma C J, Gao J M, Kong H, et al. Research progress of pharmacological effects of phlorizin[J]. Herald of Medicine, 2020, 39(3): 360-364. | |
| 29 | Schläger S, Dräger B. Exploiting plant alkaloids[J]. Current Opinion in Biotechnology, 2016, 37: 155-164. |
| 30 | Fraaije M W, Mattevi A. Flavoenzymes: diverse catalysts with recurrent features[J]. Trends in Biochemical Sciences, 2000, 25(3): 126-132. |
| 31 | Leys D, Scrutton N S. Sweating the assets of flavin cofactors: new insight of chemical versatility from knowledge of structure and mechanism[J]. Current Opinion in Structural Biology, 2016, 41: 19-26. |
| 32 | Piano V, Palfey B A, Mattevi A. Flavins as covalent catalysts: new mechanisms emerge[J]. Trends in Biochemical Sciences, 2017, 42(6): 457-469. |
| 33 | de Colibus L, Mattevi A. New frontiers in structural flavoenzymology[J]. Current Opinion in Structural Biology, 2006, 16(6): 722-728. |
| 34 | Chaiyen P, Fraaije M W, Mattevi A. The enigmatic reaction of flavins with oxygen[J]. Trends in Biochemical Sciences, 2012, 37(9): 373-380. |
| 35 | Ortiz-Maldonado M, Ballou D P, Massey V. Use of free energy relationships to probe the individual steps of hydroxylation of p-hydroxybenzoate hydroxylase: studies with a series of 8-substituted flavins[J]. Biochemistry, 1999, 38(25): 8124-8137. |
| 36 | Senn H M, Thiel S, Thiel W. Enzymatic hydroxylation in p-hydroxybenzoate hydroxylase: a case study for QM/MM molecular dynamics[J]. Journal of Chemical Theory and Computation, 2005, 1(3): 494-505. |
| 37 | Palfey B A, Moran G R, Entsch B, et al. Substrate recognition by “password” in p-hydroxybenzoate hydroxylase[J]. Biochemistry, 1999, 38(4): 1153-1158. |
| 38 | Massey V. Activation of molecular oxygen by flavins and flavoproteins[J]. Journal of Biological Chemistry, 1994, 269(36): 22459-22462. |
| 39 | Entsch B, van Berkel W J H. Structure and mechanism of para-hydroxybenzoate hydroxylase[J]. The FASEB Journal, 1995, 9(7): 476-483. |
| 40 | Esposito A, Ventura V, Petoukhov M V, et al. Human MICAL1: activation by the small GTPase Rab8 and small-angle X-ray scattering studies on the oligomerization state of MICAL1 and its complex with Rab8[J]. Protein Science, 2019, 28(1): 150-166. |
| 41 | Romero E, Castellanos J R G, Mattevi A, et al. Characterization and crystal structure of a robust cyclohexanone monooxygenase[J]. Angewandte Chemie International Edition, 2016, 55(51): 15852-15855. |
| 42 | Oae S, Mikami A, Matsuura T, et al. Comparison of sulfide oxygenation mechanism for liver microsomal FAD-containing monooxygenase with that for cytochrome P-450[J]. Biochemical and Biophysical Research Communications, 1985, 131(2): 567-573. |
| 43 | Pohlmann V, Marahiel M A. Delta-amino group hydroxylation of L-ornithine during coelichelin biosynthesis[J]. Organic & Biomolecular Chemistry, 2008, 6(10): 1843-1848. |
| 44 | Zhao Y D. Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants[J]. Molecular Plant, 2012, 5(2): 334-338. |
| 45 | Tinikul R, Pitsawong W, Sucharitakul J, et al. The transfer of reduced flavin mononucleotide from LuxG oxidoreductase to luciferase occurs via free diffusion[J]. Biochemistry, 2013, 52(39): 6834-6843. |
| 46 | Willetts A, Kelly D. Flavin-dependent redox transfers by the two-component diketocamphane monooxygenases of camphor-grown pseudomonas putida NCIMB 10007[J]. Microorganisms, 2016, 4(4): 38. |
| 47 | Hille R, Miller S, Palfey B. Handbook of Flavoproteins[M]. Berlin, Germany: De Gruyter, 2013. |
| 48 | Li L, Liu X Q, Yang W, et al. Crystal structure of long-chain alkane monooxygenase (LadA) in complex with coenzyme FMN: unveiling the long-chain alkane hydroxylase[J]. Journal of Molecular Biology, 2008, 376(2): 453-465. |
| 49 | Sucharitakul J, Chaiyen P, Entsch B, et al. Kinetic mechanisms of the oxygenase from a two-component enzyme, p-hydroxyphenylacetate 3-hydroxylase from acinetobacter baumannii[J]. Journal of Biological Chemistry, 2006, 281(25): 17044-17053. |
| 50 | Bruender N A, Thoden J B, Holden H M. X-Ray structure of kijd3, a key enzyme involved in the biosynthesis of D-kijanose[J]. Biochemistry, 2010, 49(17): 3517-3524. |
| 51 | Kantz A, Chin F, Nallamothu N, et al. Mechanism of flavin transfer and oxygen activation by the two-component flavoenzyme styrene monooxygenase[J]. Archives of Biochemistry and Biophysics, 2005, 442(1): 102-116. |
| 52 | Yeh E, Blasiak L C, Koglin A, et al. Chlorination by a long-lived intermediate in the mechanism of flavin-dependent halogenases[J]. Biochemistry, 2007, 46(5): 1284-1292. |
| 53 | Fitzpatrick P F. Oxidation of amines by flavoproteins[J]. Archives of Biochemistry and Biophysics, 2010, 493(1): 13-25. |
| 54 | Maeda-Yorita K, Aki K, Sagai H, et al. L-lactate oxidase and L-lactate monooxygenase: mechanistic variations on a common structural theme[J]. Biochimie, 1995, 77(7/8): 631-642. |
| 55 | Gadda G, Francis K. Nitronate monooxygenase, a model for anionic flavin semiquinone intermediates in oxidative catalysis[J]. Archives of Biochemistry and Biophysics, 2010, 493(1): 53-61. |
| 56 | Iacovino L G, Magnani F, Binda C. The structure of monoamine oxidases: past, present, and future[J]. Journal of Neural Transmission, 2018, 125(11): 1567-1579. |
| 57 | Batista V F, Galman J L, Pinto D C G A, et al. Monoamine oxidase: tunable activity for amine resolution and functionalization[J]. ACS Catalysis, 2018, 8(12): 11889-11907. |
| 58 | Duan J Q, Li B B, Qin Y C, et al. Recent progress in directed evolution of stereoselective monoamine oxidases[J]. Bioresources and Bioprocessing, 2019, 6: 37. |
| 59 | Haberska K, Vaz-Domínguez C, de Lacey A L, et al. Direct electron transfer reactions between human ceruloplasmin and electrodes[J]. Bioelectrochemistry, 2009, 76(1/2): 34-41. |
| 60 | Floris G, Medda R, Padiglia A, et al. The physiopathological significance of ceruloplasmin: a possible therapeutic approach[J]. Biochemical Pharmacology, 2000, 60(12): 1735-1741. |
| 61 | Fujieda N, Umakoshi K, Ochi Y, et al. Copper-oxygen dynamics in the tyrosinase mechanism[J]. Angewandte Chemie International Edition, 2020, 59(32): 13385-13390. |
| 62 | Marino S M, Fogal S, Bisaglia M, et al. Investigation of streptomyces antibioticus tyrosinase reactivity toward chlorophenols[J]. Archives of Biochemistry and Biophysics, 2011, 505(1): 67-74. |
| 63 | Muñoz-Muñoz J L, Berna J, García-Molina M D M, et al. Hydroxylation of p-substituted phenols by tyrosinase: further insight into the mechanism of tyrosinase activity[J]. Biochemical and Biophysical Research Communications, 2012, 424(2): 228-233. |
| 64 | Washington C, Maxwell J, Stevenson J, et al. Mechanistic studies of the tyrosinase-catalyzed oxidative cyclocondensation of 2-aminophenol to 2-aminophenoxazin-3-one[J]. Archives of Biochemistry and Biophysics, 2015, 577/578: 24-34. |
| 65 | van Deurzen M P J, van Rantwijk F, Sheldon R A. Selective oxidations catalyzed by peroxidases[J]. Tetrahedron, 1997, 53(39): 13183-13220. |
| 66 | Hager L P, Lakner F J, Basavapathruni A. Chiral synthons via chloroperoxidase catalysis[J]. Journal of Molecular Catalysis B: Enzymatic, 1998, 5(1/2/3/4): 95-101. |
| 67 | Colonna S, Gaggero N, Richelmi C, et al. Recent biotechnological developments in the use of peroxidases[J]. Trends in Biotechnology, 1999, 17(4): 163-168. |
| 68 | Guengerich F P. Mechanisms of cytochrome P450-catalyzed oxidations[J]. ACS Catalysis, 2018, 8(12): 10964-10976. |
| 69 | Guengerich F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. |
| 70 | Coon M J. Cytochrome P450: nature's most versatile biological catalyst[J]. Annual Review of Pharmacology and Toxicology, 2005, 45: 1-25. |
| 71 | 蒋媛媛, 李盛英. 细胞色素P450酶在生物合成及有机合成中的催化功能及其应用[J]. 有机化学, 2018, 38(9): 2307-2323. |
| Jiang Y Y, Li S Y. Catalytic function and application of cytochrome P450 enzymes in biosynthesis and organic synthesis[J]. Chinese Journal of Organic Chemistry, 2018, 38(9): 2307-2323. | |
| 72 | Nelson D R. Cytochrome P450 diversity in the tree of life[J]. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 2018, 1866(1): 141-154. |
| 73 | Matthews S, Belcher J D, Tee K L, et al. Catalytic determinants of alkene production by the cytochrome P450 peroxygenase OleTJE[J]. Journal of Biological Chemistry, 2017, 292(12): 5128-5143. |
| 74 | Roberts G A, Çelik A, Hunter D J B, et al. A self-sufficient cytochrome P450 with a primary structural organization that includes a flavin domain and a[2Fe-2S]redox center[J]. Journal of Biological Chemistry, 2003, 278(49): 48914-48920. |
| 75 | Daiber A, Shoun H, Ullrich V. Nitric oxide reductase (P450nor) from fusarium oxysporum[J]. Journal of Inorganic Biochemistry, 2005, 99(1): 185-193. |
| 76 | Li Z, Jiang Y Y, Guengerich F P, et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications[J]. The Journal of Biological Chemistry, 2020, 295(3): 833-849. |
| 77 | Hannemann F, Bichet A, Ewen K M, et al. Cytochrome P450 systems—biological variations of electron transport chains[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2007, 1770(3): 330-344. |
| 78 | Dydio P, Key H M, Nazarenko A, et al. An artificial metalloenzyme with the kinetics of native enzymes[J]. Science, 2016, 354(6308): 102-106. |
| 79 | Eljounaidi K, Cankar K, Comino C, et al. Cytochrome P450s from Cynara cardunculus L. CYP71AV9 and CYP71BL5, catalyze distinct hydroxylations in the sesquiterpene lactone biosynthetic pathway[J]. Plant Science, 2014, 223: 59-68. |
| 80 | Henquet M G L, Prota N, van der Hooft J J J, et al. Identification of a drimenol synthase and drimenol oxidase from Persicaria hydropiper, involved in the biosynthesis of insect deterrent drimanes[J]. The Plant Journal, 2017, 90(6): 1052-1063. |
| 81 | Mao H J, Shen Q Q, Wang Q. CYP701A26 is characterized as an ent-kaurene oxidase with putative involvement in maize gibberellin biosynthesis[J]. Biotechnology Letters, 2017, 39(11): 1709-1716. |
| 82 | Tamura K, Seki H, Suzuki H, et al. CYP716A179 functions as a triterpene C-28 oxidase in tissue-cultured stolons of Glycyrrhiza uralensis [J]. Plant Cell Reports, 2017, 36(3): 437-445. |
| 83 | Ríos J L, Máñez S. New pharmacological opportunities for betulinic acid[J]. Planta Medica, 2018, 84(1): 8-19. |
| 84 | Wang W W, Chen K, Xia Y J, et al. The hepatoprotection by oleanolic acid preconditioning: focusing on PPARα activation[J]. PPAR Research, 2018, 2018: 3180396. |
| 85 | Tapondjou L A, Lontsi D, Sondengam B L, et al. In vivo anti-nociceptive and anti-inflammatory effect of the two triterpenes, ursolic acid and 23-hydroxyursolic acid, from Cussonia bancoensis [J]. Archives of Pharmacal Research, 2003, 26(2): 143-146. |
| 86 | Yasumoto S, Seki H, Shimizu Y, et al. Functional characterization of CYP716 family P450 enzymes in triterpenoid biosynthesis in tomato[J]. Frontiers in Plant Science, 2017, 8: 21. |
| 87 | Andre C M, Legay S, Deleruelle A, et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids[J]. New Phytologist, 2016, 211(4): 1279-1294. |
| 88 | Tamura K, Teranishi Y, Ueda S, et al. Cytochrome P450 monooxygenase CYP716A141 is a unique β-amyrin C-16β oxidase involved in triterpenoid saponin biosynthesis in platycodon grandiflorus[J]. Plant and Cell Physiology, 2017, 58(5): 874-884. |
| 89 | Yasumoto S, Fukushima E O, Seki H, et al. Novel triterpene oxidizing activity of Arabidopsis thaliana CYP716A subfamily enzymes[J]. FEBS Letters, 2016, 590(4): 533-540. |
| 90 | Dai Z B, Liu Y, Sun Z T, et al. Identification of a novel cytochrome P450 enzyme that catalyzes the C-2α hydroxylation of pentacyclic triterpenoids and its application in yeast cell factories[J]. Metabolic Engineering, 2019, 51: 70-78. |
| 91 | Kim O T, Um Y, Jin M L, et al. A novel multifunctional C-23 oxidase, CYP714E19, is involved in asiaticoside biosynthesis[J]. Plant and Cell Physiology, 2018, 59(6): 1200-1213. |
| 92 | Zhang J S, Dai L H, Yang J G, et al. Oxidation of cucurbitadienol catalyzed by CYP87D18 in the biosynthesis of mogrosides from Siraitia grosvenorii [J]. Plant and Cell Physiology, 2016, 57(5): 1000-1007. |
| 93 | Seki H, Ohyama K, Sawai S, et al. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin[J]. PNAS, 2008, 105(37): 14204-14209. |
| 94 | Tzin V, Snyder J H, Yang D S, et al. Integrated metabolomics identifies CYP72A67 and CYP72A68 oxidases in the biosynthesis of medicago truncatula oleanate sapogenins[J]. Metabolomics, 2019, 15(6): 1-20. |
| 95 | Hagel J M, Beaudoin G A W, Fossati E, et al. Characterization of a flavoprotein oxidase from opium poppy catalyzing the final steps in sanguinarine and papaverine biosynthesis[J]. Journal of Biological Chemistry, 2012, 287(51): 42972-42983. |
| 96 | Ghislieri D, Green A P, Pontini M, et al. Engineering an enantioselective amine oxidase for the synthesis of pharmaceutical building blocks and alkaloid natural products[J]. Journal of the American Chemical Society, 2013, 135(29): 10863-10869. |
| 97 | Ghislieri D, Houghton D, Green A P, et al. Monoamine oxidase (MAO-N) catalyzed deracemization of tetrahydro-β-carbolines: substrate dependent switch in enantioselectivity[J]. ACS Catalysis, 2013, 3(12): 2869-2872. |
| 98 | Xu N N, Ahuja E G, Janning P, et al. Trapped intermediates in crystals of the FMN-dependent oxidase PhzG provide insight into the final steps of phenazine biosynthesis[J]. Acta Crystallographica. Section D, Biological Crystallography, 2013, 69(Pt 8): 1403-1413. |
| 99 | Meng S, Han W, Zhao J, et al. A six-oxidase cascade for tandem C—H bond activation revealed by reconstitution of bicyclomycin biosynthesis[J]. Angewandte Chemie International Edition, 2018, 57(3): 719-723. |
| 100 | Ju S Y, Qian M X, Xu G, et al. Chemoenzymatic approach to (S)-1, 2, 3, 4-tetrahydroisoquinoline carboxylic acids employing D-amino acid oxidase[J]. Advanced Synthesis and Catalysis, 2019, 361(13): 3191-3199. |
| 101 | Couturier M, Bhalara H D, Chawrai S R, et al. Substrate flexibility of the flavin-dependent dihydropyrrole oxidases PigB and HapB involved in antibiotic prodigiosin biosynthesis[J]. ChemBioChem, 2020, 21(4): 523-530. |
| 102 | Pandey R P, Parajuli P, Koffas M A G, et al. Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology[J]. Biotechnology Advances, 2016, 34(5): 634-662. |
| 103 | Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology[J]. Plant Physiology, 2001, 126(2): 485-493. |
| 104 | Choi K Y, Kim T J, Koh S K, et al. A-ring ortho-specific monohydroxylation of daidzein by cytochrome P450s of nocardia farcinica IFM10152[J]. Biotechnology Journal, 2009, 4(11): 1586-1595. |
| 105 | Roh C, Choi K Y, Pandey B P, et al. Hydroxylation of daidzein by CYP107H1 from Bacillus subtilis 168[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 59(4): 248-253. |
| 106 | Pandey B P, Lee N, Choi K Y, et al. Screening of bacterial cytochrome P450s responsible for regiospecific hydroxylation of (iso)flavonoids[J]. Enzyme and Microbial Technology, 2011, 48(4/5): 386-392. |
| 107 | Ehrlich K C, Li P, Scharfenstein L, et al. HypC, the anthrone oxidase involved in aflatoxin biosynthesis[J]. Applied and Environmental Microbiology, 2010, 76(10): 3374-3377. |
| 108 | Throckmorton K, Lim F Y, Kontoyiannis D P, et al. Redundant synthesis of a conidial polyketide by two distinct secondary metabolite clusters in Aspergillus fumigatus [J]. Environmental Microbiology, 2016, 18(1): 246-259. |
| 109 | Marzorati M, Danieli B, Haltrich D, et al. Selective laccase-mediated oxidation of sugars derivatives[J]. Green Chemistry, 2005, 7(5): 310. |
| 110 | Baratto L, Candido A, Marzorati M, et al. Laccase-mediated oxidation of natural glycosides[J]. Journal of Molecular Catalysis B: Enzymatic, 2006, 39(1/2/3/4): 3-8. |
| 111 | Ncanana S, Baratto L, Roncaglia L, et al. Laccase-mediated oxidation of totarol[J]. Advanced Synthesis & Catalysis, 2007, 349(8/9): 1507-1513. |
| 112 | Nes W D. Biosynthesis of cholesterol and other sterols[J]. Chemical Reviews, 2011, 111(10): 6423-6451. |
| 113 | Kiss F M, Khatri Y, Zapp J, et al. Identification of new substrates for the CYP106A1-mediated 11-oxidation and investigation of the reaction mechanism[J]. FEBS Letters, 2015, 589(18): 2320-2326. |
| 114 | 姜恬, 冯旭东, 李岩, 等. 底物特异性的生物催化与酶设计改造[J]. 化工进展, 2019, 38(1): 606-614. |
| Jiang T, Feng X D, Li Y, et al. The biocatalysis and enzyme modification of substrate specificity[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 606-614. | |
| 115 | Zhu M, Wang C X, Sun W T, et al. Boosting 11-oxo-β-amyrin and glycyrrhetinic acid synthesis in Saccharomyces cerevisiae via pairing novel oxidation and reduction system from legume plants[J]. Metabolic Engineering, 2018, 45: 43-50. |
| 116 | Kim J E, Son S H, Oh S S, et al. Pairing of orthogonal chaperones with a cytochrome P450 enhances terpene synthesis in Saccharomyces cerevisiae [J]. Biotechnology Journal, 2022, 17(3): 2000452. |
| 117 | Mellor S B, Nielsen A Z, Burow M, et al. Fusion of ferredoxin and cytochrome P450 enables direct light-driven biosynthesis[J]. ACS Chemical Biology, 2016, 11(7): 1862-1869. |
| 118 | Wang X, Pereira J H, Tsutakawa S, et al. Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450[J]. Metabolic Engineering, 2021, 64: 41-51. |
| 119 | Sun W T, Xue H J, Liu H, et al. Controlling chemo- and regioselectivity of a plant P450 in yeast cell toward rare licorice triterpenoid biosynthesis[J]. ACS Catalysis, 2020, 10(7): 4253-4260. |
| [1] | 王雅丽,付友思,陈俊宏,黄佳城,廖浪星,张永辉,方柏山. 酶工程:从人工设计到人工智能[J]. 化工学报, 2021, 72(7): 3590-3600. |
| [2] | 薛海洁, 王颖, 李春. 植物天然产物的微生物合成与转化[J]. 化工学报, 2019, 70(10): 3825-3835. |
| [3] | 于志辉, 黄鹏飞, 汪夏燕. 无定形介孔磷酸锆固定葡萄糖氧化酶的直接电化学[J]. 化工学报, 2016, 67(5): 2161-2168. |
| [4] | 饶超, 董依慧, 庄伟, 邬新兵, 洪启亮, 刘畅, 陆小华. TiO2纳米管阵列孔径调控葡萄糖氧化酶生物传感器性能[J]. 化工学报, 2016, 67(10): 4324-4333. |
| [5] | 朱明, 王彩霞, 李春. 工程化酿酒酵母合成植物三萜类化合物[J]. 化工学报, 2015, 66(9): 3350-3356. |
| [6] | 邬新兵, 蒙萌, 庄伟, 吕玲红, 陆小华. 介孔TiO2固定化葡萄糖氧化酶的直接电化学性能[J]. 化工学报, 2014, 65(5): 1777-1783. |
| [7] | 杨长青, 郑涛, 郁晨晨, 周俊, 何冰芳, 刘晓宁. 触角状环氧链结构对固定化脂肪酶(YCJ01)酶活的影响[J]. 化工学报, 2014, 65(5): 1815-1820. |
| [8] | 陈宏文1,刘 薇1,杜 钰1,陈 国1,方柏山2. 工业微生物还原型辅酶Ⅱ的代谢调控研究进展[J]. 化工进展, 2012, 31(11): 2535-2541. |
| [9] | 彭益强,刘 鹏,邓 峰,刘 宇 . 源于马铃薯的多酚氧化酶活性中心必需基团组成与抑制机理[J]. 化工进展, 2012, 31(02 ): 406-411. |
| [10] | 孙 艳,杨海麟,王 武. 建立高通量筛选耐热胆固醇氧化酶的方法 [J]. CIESC Journal, 2011, 30(3): 612-. |
| [11] | 孙 艳,王长城,张 玲,杨海麟,王 武. 重组大肠杆菌产胆固醇氧化酶的指数流加策略 [J]. CIESC Journal, 2010, 29(1): 130-. |
| [12] | 王秉, 胡智文, 郑海玲, 杨海亮, 黄小芳, 张殿波, 万军民. β-环糊精-Cu(Ⅱ)配合物催化氧化邻苯二酚及其反应动力学 [J]. 化工学报, 2009, 60(6): 1459-1465. |
| [13] | 胡安辉; 郑平; 金仁村. 缺氧胁迫影响硝化污泥活性的机理 [J]. CIESC Journal, 2007, 58(10): 2587-2594. |
| [14] | 肖厚荣, 朱仁发, 徐小龙, 解永树, 刘清亮. 烟草多酚氧化酶固定化新工艺 [J]. 化工学报, 2004, 55(11): 1921-1924. |
| [15] | 刘璘, 施正策, 王权, 金江南, 樊云. 多酚氧化酶-硅藻土耦合系统选择性氧化/吸附邻苯二酚 [J]. 化工学报, 2003, 54(2): 221-225. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号