化工学报 ›› 2023, Vol. 74 ›› Issue (2): 843-860.DOI: 10.11949/0438-1157.20221063
张娜1( ), 潘鹤林1, 牛波1, 张亚运1(
), 潘鹤林1, 牛波1, 张亚运1( ), 龙东辉1,2
), 龙东辉1,2
收稿日期:2022-07-29
修回日期:2022-11-29
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
张亚运
作者简介:张娜(1997—),女,硕士研究生,zhna135472@163.com
基金资助:
Na ZHANG1( ), Helin PAN1, Bo NIU1, Yayun ZHANG1(
), Helin PAN1, Bo NIU1, Yayun ZHANG1( ), Donghui LONG1,2
), Donghui LONG1,2
Received:2022-07-29
Revised:2022-11-29
Online:2023-02-05
Published:2023-03-21
Contact:
Yayun ZHANG
摘要:
选取2,2'-亚甲基二酚作为酚醛树脂的模型化合物,采用密度泛函理论(DFT),从原子活性和化学键级的视角预测反应趋势,提出反应路径,结合动力学和热力学分析确定各路径优先顺序和产物生成机理。结果表明:由于酚羟基与亚甲基的高活性,酚醛树脂的裂解首先发生脱水缩合反应;亚甲基桥断裂是主要的初始裂解反应,主要生成苯酚及邻甲酚;高活性的酚羟基易解离成 • OH,后续反应中亚甲基被 • OH氧化,经脱羰基和羧基反应生成苯酚、CO和CO2。对于硼改性酚醛树脂的裂解过程,结果显示:硼酸酯结构中的B—O键活性很低,不易断裂,热稳定性增强。亚甲基桥更稳定且脱羰基和羧基的趋势明显减弱,且动力学分析发现其能垒均有增加,表明硼的引入能有效提高其热稳定性和残炭率。
中图分类号:
张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860.
Na ZHANG, Helin PAN, Bo NIU, Yayun ZHANG, Donghui LONG. Density functional theory study on thermal cracking reaction mechanism of phenolic resin[J]. CIESC Journal, 2023, 74(2): 843-860.
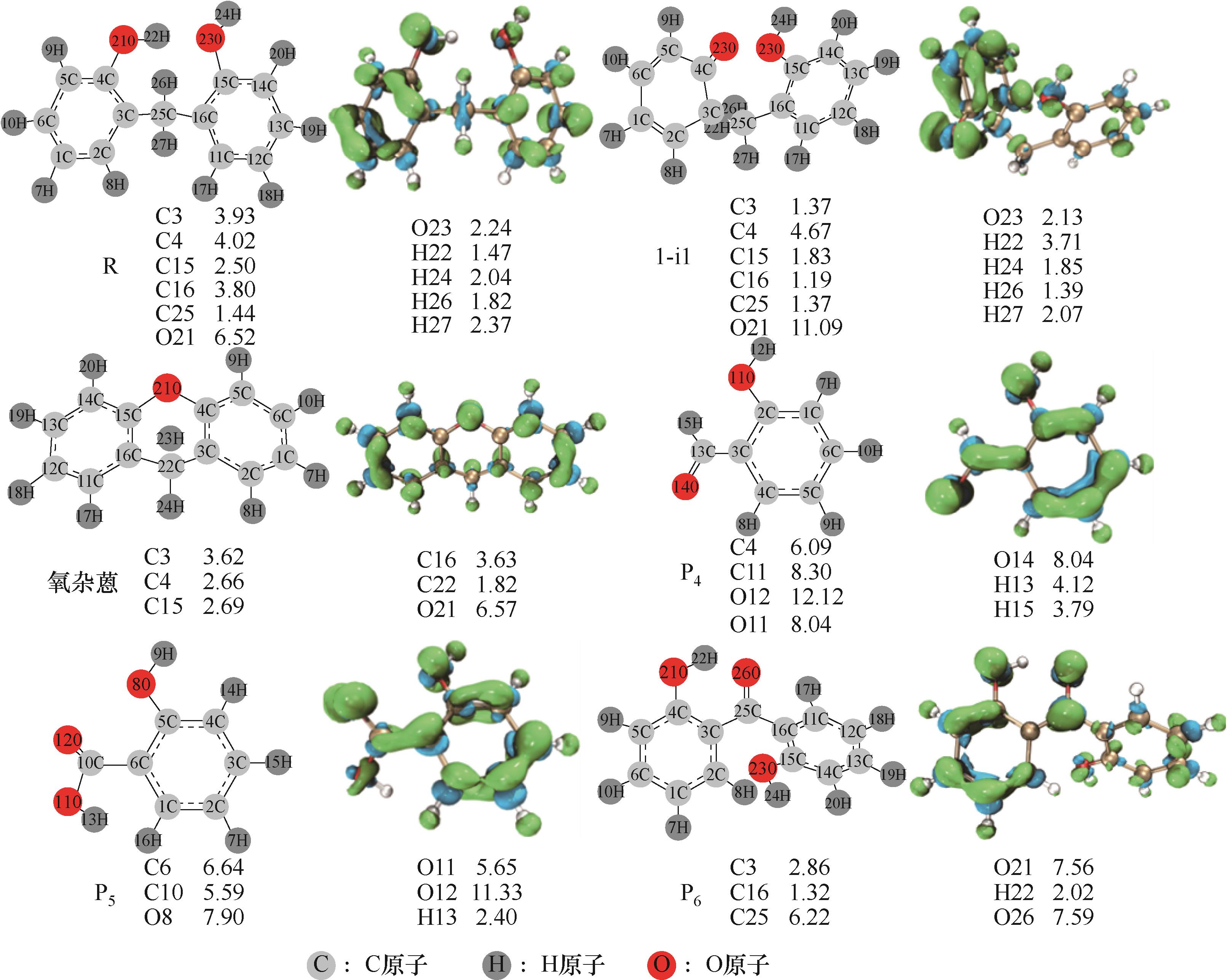
图1 优化后的分子结构以及Fukui函数投影到电子密度等值面图(PR)(左侧是优化后的分子结构,右侧对应的等值面图中绿色区域代表贫电子区,蓝色区域代表富电子区。图下方标示的数值为各反应位点的Fukui函数值,单位为e×100)
Fig.1 Optimized molecular structures and projection of Fukui function to electron density isosurfaces (PR) (on the left is the optimized molecular structures,the green areas in the corresponding isosurfaces on the right represents the electron-poor areas, and the blue areas represents the electron-rich areas, the values marked at the bottom of the figure are the Fukui function values of each reaction site, and the unit is e×100)

图3 PR反应路径中涉及断键的化学键的Mayer键级和 Laplacian键级(横坐标中数字“1,2,3,4,5,6”分别表示反应物R的化学键“C3—C25,C16—C25, C4—O21, C15—O23, O21—H22, O23—H24”,“7,8”分别表示水杨醛P4和水杨酸P5中的化学键“C3—C13, C6—C10”,“-1”表示异构化产物1-i1的对应化学键,“-2”表示苯甲酮的对应化学键)
Fig.3 The Mayer bond order and the Laplacian bond order of the chemical bonds involved in the broken bonds in the reaction pathways of PR (the numbers “1, 2, 3, 4, 5, 6” in the abscissa represent the chemical bonds“C3—C25, C16—C25, C4—O21, C15—O23, O21—H22, O23—H24”,“7,8”represent the chemical bonds“C3—C13, C6—C10” in salicylaldehyde P4 and salicylic acid P5 respectively,“-1” represents the corresponding chemical bonds of isomerization product 1-i1,“-2” represents the corresponding chemical bonds of benzophenone)
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(4,21) 1.3837 R(3,25) 1.5265 R(15,23) 1.4096 R(11,17) 1.0859 | A(3,4,21) 123.5698 A(16,15,23) 116.6298 A(3,25,16) 115.1569 A(26,25,27) 106.4195 | D(3,4,21,22) 17.5317 D(16,15,23,24) 177.6034 D(8,2,3,25) -0.5943 D(17,11,16,15) 179.8965 |
| TS1a |  | R(4,21) 1.3313 R(3,25) 1.5717 R(16,25) 1.5149 R(3,22) 1.4548 | A(3,4,21) 107.6645 A(3,25,26) 108.2772 A(15,16,25) 120.5249 A(26,25,27) 107.7724 | D(2,3,4,21) 146.3908 D(2,3,25,16) 57.0082 D(23,15,16,25) 0.7638 D(22,3,25,26) -31.9241 |
| 1-i1 |  | R(4,21) 1.2509 R(3,25) 1.5724 R(15,23) 1.3925 R(11,17) 1.0866 | A(3,4,21) 120.1579 A(16,15,23) 116.1152 A(3,25,16) 116.3922 A(26,25,27) 107.2023 | D(21,4,3,22) 69.4683 D(16,15,23,24) 178.8148 D(8,2,3,25) 42.8377 D(17,11,16,15) 179.6106 |
| 1-i2 |  | R(5,6) 1.433 R(6,13) 1.3991 R(13,14) 1.0799 R(13,15) 1.0825 | A(5,11,12) 109.1272 A(1,6,13) 122.1677 A(5,6,13) 121.3617 A(6,13,14) 121.2142 | D(6,5,11,12) -180.0119 D(7,1,6,13) -0.0014 D(1,6,13,14) -179.9998 D(1,6,13,15) -0.0012 |
| 1-i3 |  | R(3,4) 1.4523 R(4,5) 1.4523 R(5,6) 1.3748 R(4,11) 1.2514 | A(4,5,6) 120.9385 A(3,4,5) 116.9288 A(6,5,9) 122.1827 A(5,4,11) 121.5356 | D(9,5,6,10) -0.0001 D(9,5,4,11) 0.0001 D(2,3,4,11) 0.0001 D(6,5,4,11) -179.9999 |
| P1 |  | R(5,6) 1.4045 R(6,13) 1.5095 R(13,14) 1.0973 R(13,15) 1.0973 | A(5,11,12) 109.606 A(1,6,13) 121.6233 A(5,6,13) 120.4383 A(6,13,14) 112.0314 | D(6,5,11,12) -0.0037 D(7,1,6,13) 0.0006 D(1,6,13,14) 119.5144 D(1,6,13,15) -119.5242 |
| P3 |  | R(3,4) 1.3967 R(4,5) 1.3963 R(5,6) 1.3934 R(4,12) 1.3671 | A(4,5,6) 119.8367 A(3,4,5) 119.9397 A(6,5,10) 120.2222 A(4,12,13) 109.1344 | D(3,4,12,13) -179.9897 D(10,5,4,12) -0.001 D(3,4,12,13) 179.9582 D(5,4,12,13) -0.0447 |
| TS1c |  | R(3,4) 1.4079 R(2,8) 1.0837 R(13,15) 1.3486 R(17,18) 0.9812 | A(4,11,12) 111.8752 A(6,5,9) 120.3442 A(3,13,14) 115.9687 A(16,17,18) 122.7612 | D(8,2,3,4) -179.5535 D(5,4,11,12) 1.5985 D(2,3,13,14) -158.9475 D(13,16,17,18) 110.7506 |
表1 PR热解反应路径中的反应物、重要的过渡态、中间体和产物的优化几何结构
Table 1 Optimized geometries of reactants, important transition states, intermediates and products in the pyrolysis reaction pathways of PR
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(4,21) 1.3837 R(3,25) 1.5265 R(15,23) 1.4096 R(11,17) 1.0859 | A(3,4,21) 123.5698 A(16,15,23) 116.6298 A(3,25,16) 115.1569 A(26,25,27) 106.4195 | D(3,4,21,22) 17.5317 D(16,15,23,24) 177.6034 D(8,2,3,25) -0.5943 D(17,11,16,15) 179.8965 |
| TS1a |  | R(4,21) 1.3313 R(3,25) 1.5717 R(16,25) 1.5149 R(3,22) 1.4548 | A(3,4,21) 107.6645 A(3,25,26) 108.2772 A(15,16,25) 120.5249 A(26,25,27) 107.7724 | D(2,3,4,21) 146.3908 D(2,3,25,16) 57.0082 D(23,15,16,25) 0.7638 D(22,3,25,26) -31.9241 |
| 1-i1 |  | R(4,21) 1.2509 R(3,25) 1.5724 R(15,23) 1.3925 R(11,17) 1.0866 | A(3,4,21) 120.1579 A(16,15,23) 116.1152 A(3,25,16) 116.3922 A(26,25,27) 107.2023 | D(21,4,3,22) 69.4683 D(16,15,23,24) 178.8148 D(8,2,3,25) 42.8377 D(17,11,16,15) 179.6106 |
| 1-i2 |  | R(5,6) 1.433 R(6,13) 1.3991 R(13,14) 1.0799 R(13,15) 1.0825 | A(5,11,12) 109.1272 A(1,6,13) 122.1677 A(5,6,13) 121.3617 A(6,13,14) 121.2142 | D(6,5,11,12) -180.0119 D(7,1,6,13) -0.0014 D(1,6,13,14) -179.9998 D(1,6,13,15) -0.0012 |
| 1-i3 |  | R(3,4) 1.4523 R(4,5) 1.4523 R(5,6) 1.3748 R(4,11) 1.2514 | A(4,5,6) 120.9385 A(3,4,5) 116.9288 A(6,5,9) 122.1827 A(5,4,11) 121.5356 | D(9,5,6,10) -0.0001 D(9,5,4,11) 0.0001 D(2,3,4,11) 0.0001 D(6,5,4,11) -179.9999 |
| P1 |  | R(5,6) 1.4045 R(6,13) 1.5095 R(13,14) 1.0973 R(13,15) 1.0973 | A(5,11,12) 109.606 A(1,6,13) 121.6233 A(5,6,13) 120.4383 A(6,13,14) 112.0314 | D(6,5,11,12) -0.0037 D(7,1,6,13) 0.0006 D(1,6,13,14) 119.5144 D(1,6,13,15) -119.5242 |
| P3 |  | R(3,4) 1.3967 R(4,5) 1.3963 R(5,6) 1.3934 R(4,12) 1.3671 | A(4,5,6) 119.8367 A(3,4,5) 119.9397 A(6,5,10) 120.2222 A(4,12,13) 109.1344 | D(3,4,12,13) -179.9897 D(10,5,4,12) -0.001 D(3,4,12,13) 179.9582 D(5,4,12,13) -0.0447 |
| TS1c |  | R(3,4) 1.4079 R(2,8) 1.0837 R(13,15) 1.3486 R(17,18) 0.9812 | A(4,11,12) 111.8752 A(6,5,9) 120.3442 A(3,13,14) 115.9687 A(16,17,18) 122.7612 | D(8,2,3,4) -179.5535 D(5,4,11,12) 1.5985 D(2,3,13,14) -158.9475 D(13,16,17,18) 110.7506 |

图7 优化后的分子结构以及Fukui函数投影到电子密度等值面图(BPR)(左侧是优化后的分子结构,右侧对应的等值面图中绿色区域代表贫电子区,蓝色区域代表富电子区。图中间标示的数值为各反应位点的Fukui函数值,单位为e×100)
Fig.7 Optimized molecular structures and projection of Fukui function to electron density isosurfaces (BPR) (on the left is the optimized molecular structures,the green areas in the corresponding isosurfaces on the right represents the electron-poor areas, and the blue areas represents the electron-rich areas, the values marked in the middle of the figure are the Fukui function values of each reaction site, and the unit is e×100)
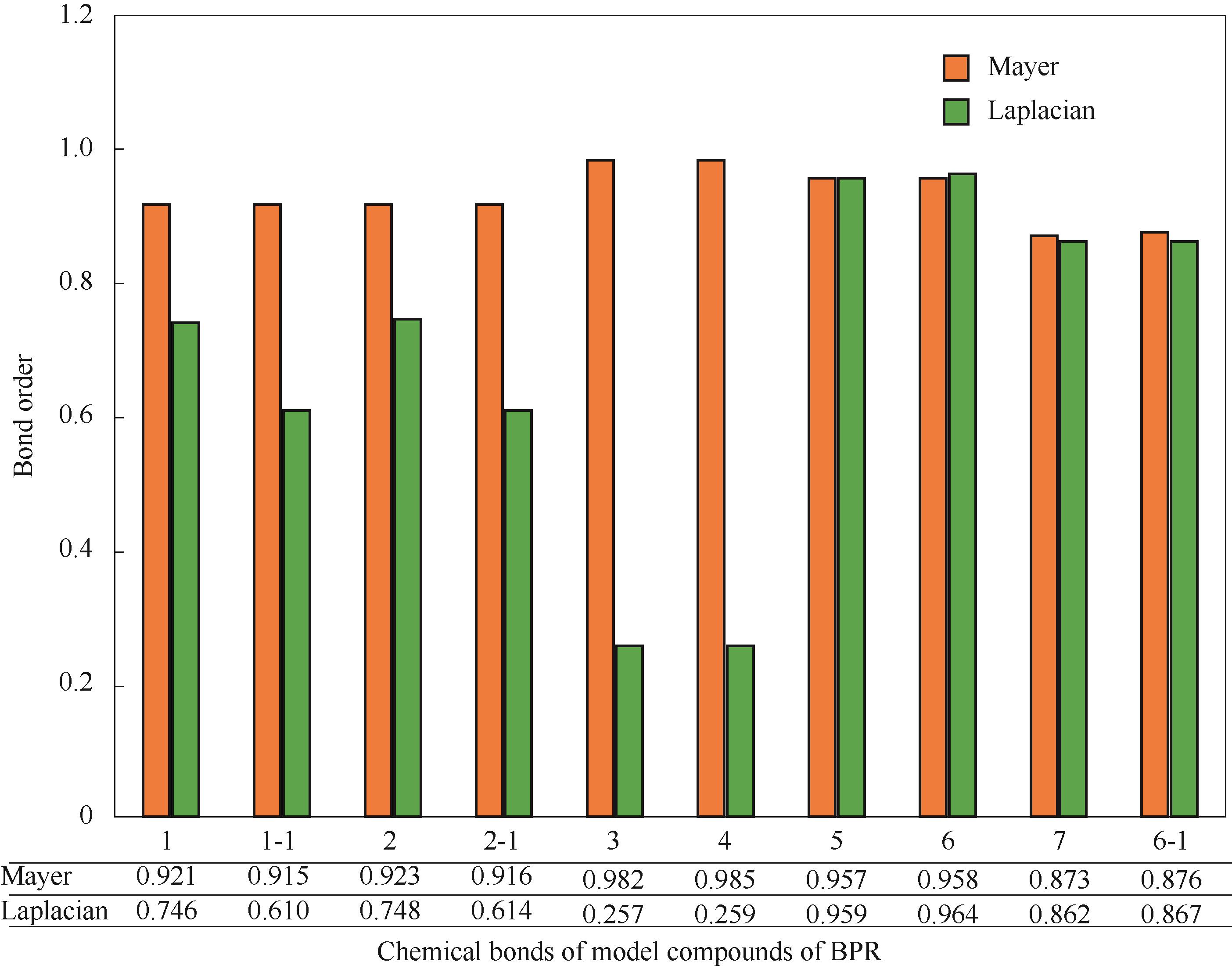
图8 BPR反应路径中涉及断键的化学键的Mayer键级和 Laplacian键级(横坐标中数字“1,3”分别表示反应物R(PR)的化学键“C3—C25, C4—O21”,“2,4”分别表示反应物R(BPR)的化学键“C3—C24, C4—O20”,“5,7”分别表示PR中P4和P5的化学键“C3—C13,C6—C10”,“-1”表示异构化产物1-i1的对应化学键,“6,6-1”分别表示BPR中P4和P5的化学键“C6—C28”)
Fig.8 The Mayer bond order and the Laplacian bond order of the chemical bonds involved in the broken bonds in the reaction pathways of BPR (The numbers “1,3” in the abscissa represent the chemical bonds “C3—C25,C4—O21” of the reactant R (PR),“2,4”represent the chemical bonds “C3—C24,C4—O20” of the reactant R (BPR), and “5,7” represent the chemical bonds “C3—C13,C6—C10” of P4 and P5 in PR respectively, “-1” represents the corresponding chemical bonds of the isomerized product 1-i1,“6,6-1”represent the chemical bonds “C6—C28” of P4 and P5 in BPR respectively)
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(3,4) 1.4129 R(3,24) 1.5263 R(6,10) 1.0856 R(11,16) 1.4053 | A(6,1,7) 120.5751 A(2,3,24) 119.9444 A(3,4,20) 123.7277 A(12,11,17) 119.7931 | D(7,1,6,10) -0.1032 D(8,2,3,4) -179.5135 D(24,3,4,5) -177.5334 D(4,3,24,25) 37.4906 |
| TS1a |  | R(1,7) 1.0847 R(2,8) 1.0872 R(3,21) 1.4603 R(11,16) 1.4039 | A(3,4,20) 108.8091 A(21,3,24) 89.6951 A(4,5,6) 116.4379 A(12,13,14) 118.4517 | D(2,3,4,20) -149.7406 D(4,3,24,26) 90.1564 D(21,4,5,9) -113.5019 D(15,16,24,3) 65.6825 |
| 1-i1 |  | R(2,8) 1.0868 R(3,21) 1.1056 R(3,24) 1.5676 R(6,10) 1.087 | A(3,2,8) 116.7196 A(4,3,21) 105.3531 A(21,3,24) 104.907 A(12,11,16) 121.5719 | D(7,1,2,3) 178.9128 D(8,2,3,4) -174.8452 D(21,3,4,20) -69.4398 D(21,3,24,26) 179.7412 |
| P4 |  | R(6,28) 1.4711 R(13,15) 1.3738 R(19,23) 1.0851 R(22,24) 1.3993 | A(6,1,7) 117.5481 A(2,3,9) 121.2674 A(13,14,17) 125.9009 A(17,18,20) 119.1459 | D(7,1,6,28) 0.018 D(9,3,4,5) -179.895 D(5,4,12,13) -179.9181 D(14,17,19,23) -0.5538 |
| P5 |  | R(4,12) 1.3987 R(6,28) 1.4911 R(13,14) 1.3956 R(22,24) 1.3993 | A(6,1,7) 119.0185 A(3,4,12) 125.829 A(17,18,20) 119.1356 A(18,20,25) 119.5079 | D(7,1,2,3) -177.9088 D(8,2,3,9) 0.5803 D(4,5,10,11) -1.8132 D(14,13,15,16) -2.0753 |
| P6 |  | R(3,24) 1.4668 R(11,16) 1.4076 R(14,25) 1.4006 R(26,27) 1.3968 | A(5,4,20) 117.4778 A(12,11,16) 121.0413 A(14,15,22) 119.9191 A(26,28,29) 119.5608 | D(8,2,3,24) 0.9432 D(5,4,20,21) 177.2876 D(17,11,12,13) 179.3928 D(22,15,16,11) -177.754 |
表2 BPR热解反应路径中的反应物、重要的过渡态、中间体和产物的优化几何结构
Table 2 Optimized geometries of reactants, important transition states, intermediates and products in the pyrolysis reaction pathways of BPR
| Species | Structure parameter | |||
|---|---|---|---|---|
| Bond length/Å | Bond angle/(°) | Dihedral angle/(°) | ||
| R |  | R(3,4) 1.4129 R(3,24) 1.5263 R(6,10) 1.0856 R(11,16) 1.4053 | A(6,1,7) 120.5751 A(2,3,24) 119.9444 A(3,4,20) 123.7277 A(12,11,17) 119.7931 | D(7,1,6,10) -0.1032 D(8,2,3,4) -179.5135 D(24,3,4,5) -177.5334 D(4,3,24,25) 37.4906 |
| TS1a |  | R(1,7) 1.0847 R(2,8) 1.0872 R(3,21) 1.4603 R(11,16) 1.4039 | A(3,4,20) 108.8091 A(21,3,24) 89.6951 A(4,5,6) 116.4379 A(12,13,14) 118.4517 | D(2,3,4,20) -149.7406 D(4,3,24,26) 90.1564 D(21,4,5,9) -113.5019 D(15,16,24,3) 65.6825 |
| 1-i1 |  | R(2,8) 1.0868 R(3,21) 1.1056 R(3,24) 1.5676 R(6,10) 1.087 | A(3,2,8) 116.7196 A(4,3,21) 105.3531 A(21,3,24) 104.907 A(12,11,16) 121.5719 | D(7,1,2,3) 178.9128 D(8,2,3,4) -174.8452 D(21,3,4,20) -69.4398 D(21,3,24,26) 179.7412 |
| P4 |  | R(6,28) 1.4711 R(13,15) 1.3738 R(19,23) 1.0851 R(22,24) 1.3993 | A(6,1,7) 117.5481 A(2,3,9) 121.2674 A(13,14,17) 125.9009 A(17,18,20) 119.1459 | D(7,1,6,28) 0.018 D(9,3,4,5) -179.895 D(5,4,12,13) -179.9181 D(14,17,19,23) -0.5538 |
| P5 |  | R(4,12) 1.3987 R(6,28) 1.4911 R(13,14) 1.3956 R(22,24) 1.3993 | A(6,1,7) 119.0185 A(3,4,12) 125.829 A(17,18,20) 119.1356 A(18,20,25) 119.5079 | D(7,1,2,3) -177.9088 D(8,2,3,9) 0.5803 D(4,5,10,11) -1.8132 D(14,13,15,16) -2.0753 |
| P6 |  | R(3,24) 1.4668 R(11,16) 1.4076 R(14,25) 1.4006 R(26,27) 1.3968 | A(5,4,20) 117.4778 A(12,11,16) 121.0413 A(14,15,22) 119.9191 A(26,28,29) 119.5608 | D(8,2,3,24) 0.9432 D(5,4,20,21) 177.2876 D(17,11,12,13) 179.3928 D(22,15,16,11) -177.754 |
| 1 | 闫联生, 姚冬梅, 杨学军. 新型耐烧蚀材料研究[J]. 宇航材料工艺, 2002, 32(2): 29-31. |
| Yan L S, Yao D M, Yang X J. A study of new ablation resistant materials[J]. Aerospace Materials & Technology, 2002, 32(2): 29-31. | |
| 2 | 王子龙, 邢素丽, 尹昌平, 等. 高残炭耐烧蚀树脂基体研究进展[J]. 工程塑料应用, 2018, 46(8): 131-137. |
| Wang Z L, Xing S L, Yin C P, et al. Research progress of ablative resistant resin matrix with high carbonization rate[J]. Engineering Plastics Application, 2018, 46(8): 131-137. | |
| 3 | 陈鸯飞, 陈智琴, 肖绍懿, 等. 酚醛树脂热降解过程中的结构变化[J]. 热固性树脂, 2008, 23(4): 4-8, 12. |
| Chen Y F, Chen Z Q, Xiao S Y, et al. The structural changes of phenolic resin during thermal degradation[J]. Thermosetting Resin, 2008, 23(4): 4-8, 12. | |
| 4 | 王亚楠, 李兆, 曹静, 等. 酚醛树脂及含硼酚醛树脂热裂解和碳化研究进展[J]. 当代化工, 2021, 50(9): 2235-2241. |
| Wang Y N, Li Z, Cao J, et al. Research progress of pyrolysis and carbonization of phenolic resin and boron-containing phenolic resin[J]. Contemporary Chemical Industry, 2021, 50(9): 2235-2241. | |
| 5 | 高南, 张亚峰, 邝健政, 等. 改性酚醛树脂及其在防腐涂料中的应用[J]. 新型建筑材料, 2011, 38(2): 11-14, 18. |
| Gao N, Zhang Y F, Kuang J Z, et al. Modified phenolic resin and its application in anticorrosive paint[J]. New Building Materials, 2011, 38(2): 11-14, 18. | |
| 6 | 张力, 张以河, 姚亚琳, 等. 硼酚醛树脂及其复合材料的研究进展[J]. 玻璃钢/复合材料, 2018(3): 107-120. |
| Zhang L, Zhang Y H, Yao Y L, et al. Research progress in boron modified phenolic resin and its composites[J]. Fiber Reinforced Plastics/Composites, 2018(3): 107-120. | |
| 7 | Jiang H Y, Wang J G, Wu S Q, et al. The pyrolysis mechanism of phenol formaldehyde resin[J]. Polymer Degradation and Stability, 2012, 97(8): 1527-1533. |
| 8 | 陈治宇, 胡宏林, 余瑞莲, 等. 一种酚醛树脂模型化合物热解反应的密度泛函理论研究及实验验证[J]. 宇航材料工艺, 2018, 48(1): 30-36. |
| Chen Z Y, Hu H L, Yu R L, et al. Pyrolysis reactions of one model compound of phenolic resin using density functional theory and experimental validation[J]. Aerospace Materials & Technology, 2018, 48(1): 30-36. | |
| 9 | Abdalla M O, Ludwick A, Mitchell T. Boron-modified phenolic resins for high performance applications[J]. Polymer, 2003, 44(24): 7353-7359. |
| 10 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 11 | Trick K A, Saliba T E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite[J]. Carbon, 1995, 33(11): 1509-1515. |
| 12 | Chen Z Q, Chen Y F, Liu H B. Pyrolysis of phenolic resin by TG-MS and FTIR analysis[C]//Materials Engineering for Advanced Technologies. Trans Tech Publications, 2012:102-107. |
| 13 | Wong H W, Peck J, Bonomi R E, et al. Quantitative determination of species production from phenol-formaldehyde resin pyrolysis[J]. Polymer Degradation and Stability, 2015, 112: 122-131. |
| 14 | Bennett A, Payne D R, Court R W. Pyrolytic and elemental analysis of decomposition products from a phenolic resin[J]. Macromolecular Symposia, 2014, 339(1): 38-47. |
| 15 | Fitzer E, Schaefer W, Yamada S. The formation of glasslike carbon by pyrolysis of polyfurfuryl alcohol and phenolic resin[J]. Carbon, 1969, 7(6): 643-648. |
| 16 | 马伟. 酚醛树脂的热解研究[D]. 重庆: 重庆大学, 2007. |
| Ma W. Study on the pyrolysis of phenolic resin[D]. Chongqing: Chongqing University, 2007. | |
| 17 | Chattaraj P K, Sarkar U, Roy D R. Electrophilicity index[J]. Chemical Reviews, 2006, 106(6): 2065-2091. |
| 18 | 张晨. 类煤模型化合物热解过程中脱羧反应和C—C/C—O断键规律研究[D]. 徐州: 中国矿业大学, 2020. |
| Zhang C. Research on decarboxylation reaction and C—C/C—O breaking law of coal-like model compounds during pyrolysis[D]. Xuzhou: China University of Mining and Technology, 2020. | |
| 19 | Li M, Mo C H, Luo X, et al. Exploring key reaction sites and deep degradation mechanism of perfluorooctane sulfonate via peroxymonosulfate activation under electrocoagulation process[J]. Water Research, 2021, 207: 117849. |
| 20 | 渠艳飞, 关泽宇, 马邕文, 等. 基于硫酸自由基氧化降解邻苯二甲酸二丁酯的密度泛函理论研究[J]. 环境化学, 2017, 36(9): 1896-1905. |
| Qu Y F, Guan Z Y, Ma Y W, et al. A theoretical investigation on the degradation of dibutyl phthalate based on sulfate radicals oxidation: a DFT study[J]. Environmental Chemistry, 2017, 36(9): 1896-1905. | |
| 21 | Shen Q R, Fu Z W, Li R, et al. A study on the pyrolysis mechanism of a β-O-4 lignin dimer model compound using DFT combined with Py-GC/MS[J]. Journal of Thermal Analysis and Calorimetry, 2021, 146(4): 1751-1761. |
| 22 | 张洋, 陈世荣. 羟基自由基与苯酚反应机理的量子化学研究[J]. 陕西师范大学学报(自然科学版), 2008, 36(5): 58-61. |
| Zhang Y, Chen S R. Theoretical study on the reaction of hydroxyl radical with phenol[J]. Journal of Shaanxi Normal University (Natural Science Edition), 2008, 36(5): 58-61. | |
| 23 | Ouchi K, Honda H. Pyrolysis of coal(Ⅰ): Thermal cracking of phenolformaldehyde resins taken as coal models[J]. Fuel, 1959, 38:429-443. |
| 24 | Jackson W M, Conley R T. High temperature oxidative degradation of phenol-formaldehyde polycondensates[J]. Journal of Applied Polymer Science, 1964, 8(5): 2163-2193. |
| 25 | 姚灿, 田红, 黄章俊, 等. 基于量子化学的玉米秸秆热解机理的模拟计算[J]. 西北大学学报(自然科学版), 2019, 49(1): 122-131. |
| Yao C, Tian H, Huang Z J, et al. Theoretical study on pyrolysis mechanism of corn stalk based on quantum chemistry[J]. Journal of Northwest University (Natural Science Edition), 2019, 49(1): 122-131. | |
| 26 | Tang B, Wang Y T, Peng X L, et al. Efficient predictions of Gibbs free energy for the gases CO, BF, and gaseous BBr[J]. Journal of Molecular Structure, 2020, 1199: 126958. |
| 27 | 梁韬. 基于Py-GC/MS的半纤维素热裂解机理研究[D]. 杭州: 浙江大学, 2013. |
| Liang T. Mechanism research of hemicellulose pvrolvsis based on Py-GC/MS[D]. Hangzhou: Zhejiang University, 2013. | |
| 28 | 张亚运. 木质纤维素热化学转化机理及裂解气体CO2和H2吸附分离的分子模拟研究[D]. 重庆: 重庆大学, 2017. |
| Zhang Y Y. The mechanism of lignin cellulose thermal conversion and adsorption and separation of pyrolytic gases CO2 and H2 by molecular simulation[D]. Chongqing: Chongqing University, 2017. | |
| 29 | Costa L, di Montelera L R, Camino G, et al. Structure-charring relationship in phenol-formaldehyde type resins[J]. Polymer Degradation and Stability, 1997, 56(1): 23-35. |
| 30 | Wang S J, Wang Y, Bian C, et al. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: the effect of phenyl borates on the char formation[J]. Applied Surface Science, 2015, 331: 519-529. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 徐文杰, 贾献峰, 王际童, 乔文明, 凌立成, 王任平, 余子舰, 张寅旭. 有机硅/酚醛杂化气凝胶的制备和性能研究[J]. 化工学报, 2023, 74(8): 3572-3583. |
| [3] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [4] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [5] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [6] | 陈晨, 杨倩, 陈云, 张睿, 刘冬. 不同氧浓度下煤挥发分燃烧的化学动力学研究[J]. 化工学报, 2022, 73(9): 4133-4146. |
| [7] | 郝泽光, 张乾, 高增林, 张宏文, 彭泽宇, 杨凯, 梁丽彤, 黄伟. 生物质与催化裂化油浆共热解协同作用研究[J]. 化工学报, 2022, 73(9): 4070-4078. |
| [8] | 邵健, 冯军宗, 柳凤琦, 姜勇刚, 李良军, 冯坚. 酚醛树脂基炭微球结构调控与功能化制备研究进展[J]. 化工学报, 2022, 73(9): 3787-3801. |
| [9] | 肖皓宇, 杨海平, 张雄, 陈应泉, 王贤华, 陈汉平. 塑料催化热解制备高附加值产品的研究进展[J]. 化工学报, 2022, 73(8): 3461-3471. |
| [10] | 唐恺鸿, 何晓峰, 徐桂秋, 于洋, 刘啸凤, 葛铁军, 张爱玲. 酚醛泡沫的燃烧行为及阻燃研究进展[J]. 化工学报, 2022, 73(8): 3483-3500. |
| [11] | 刘晓涯, 王金超, 刘莹, 马敬环. 水合肼制氢纳米催化剂改性制备及机理研究进展[J]. 化工学报, 2022, 73(7): 2819-2834. |
| [12] | 陈永安, 周安宁, 李云龙, 石智伟, 贺新福, 焦卫红. 磁性MgFe2O4及其核壳催化剂制备与煤热解性能研究[J]. 化工学报, 2022, 73(7): 3026-3037. |
| [13] | 陈玉弓, 陈昊, 黄耀松. 基于分子反应动力学模拟的六甲基二硅氧烷热解机理研究[J]. 化工学报, 2022, 73(7): 2844-2857. |
| [14] | 郑默, 李晓霞. ReaxFF MD模拟揭示的煤热解挥发分自由基反应的竞争与协调[J]. 化工学报, 2022, 73(6): 2732-2741. |
| [15] | 陈冠益, 童图军, 李瑞, 王燕杉, 颜蓓蓓, 李宁, 侯立安. 热解时间对污泥生物炭活化过硫酸盐的影响研究[J]. 化工学报, 2022, 73(5): 2111-2119. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号