化工学报 ›› 2022, Vol. 73 ›› Issue (9): 3787-3801.DOI: 10.11949/0438-1157.20220472
邵健( ), 冯军宗(
), 冯军宗( ), 柳凤琦, 姜勇刚, 李良军, 冯坚
), 柳凤琦, 姜勇刚, 李良军, 冯坚
收稿日期:2022-03-21
修回日期:2022-06-29
出版日期:2022-09-05
发布日期:2022-10-09
通讯作者:
冯军宗
作者简介:邵健(1998—),男,硕士研究生,15951721090@163.com
基金资助:
Jian SHAO( ), Junzong FENG(
), Junzong FENG( ), Fengqi LIU, Yonggang JIANG, Liangjun LI, Jian FENG
), Fengqi LIU, Yonggang JIANG, Liangjun LI, Jian FENG
Received:2022-03-21
Revised:2022-06-29
Online:2022-09-05
Published:2022-10-09
Contact:
Junzong FENG
摘要:
炭微球具有化学稳定性好、电导率优良、比表面积大、孔结构丰富等优点,在吸附、催化等领域具有广阔的应用前景,引起了研究人员的广泛关注。酚醛基炭微球以酚醛树脂为前体,经高温炭化制备而成,这种制备方法工艺简单、对设备要求低、产率高,而且通过调整反应物和反应条件可实现对炭微球结构和功能性的精细调控,从而更好地满足实际应用的需求。概述了酚醛基炭微球的最新研究进展,分为小粒径炭微球制备、多孔炭微球制备和功能化炭微球制备三个方面,并介绍了酚醛基炭微球在储能、吸附和电催化领域的应用,最后对其未来发展方向进行了展望。
中图分类号:
邵健, 冯军宗, 柳凤琦, 姜勇刚, 李良军, 冯坚. 酚醛树脂基炭微球结构调控与功能化制备研究进展[J]. 化工学报, 2022, 73(9): 3787-3801.
Jian SHAO, Junzong FENG, Fengqi LIU, Yonggang JIANG, Liangjun LI, Jian FENG. Research progress on structural modulation and functionalized preparation of phenolic resin-based carbon microspheres[J]. CIESC Journal, 2022, 73(9): 3787-3801.
| 炭前体 | 溶剂 | 催化剂 | 添加剂 | 炭球粒径/nm | 比表面积/(m2/g) | 文献 |
|---|---|---|---|---|---|---|
| 间苯三酚、对苯二甲醛、间苯二酚、甲醛 | 水 | 氨水 | — | 30~90 | — | [ |
| 苯酚、甲醛 | 水 | — | F127 | 约110 | 219 | [ |
| 间苯三酚、对苯二胺、甲醛 | 水、乙醇 | 对苯二胺 | — | 79.2~137.0 | — | [ |
| 间苯三酚、甲醛 | 水 | 盐酸 | F108,F127,F86,P123② | 80~90 | — | [ |
| 间苯二酚 、甲醛 | 水、乙醇 | 乙二胺 | PVP③ | 60~875 | 516~1083 | [ |
| 间苯二酚、甲醛 | 水 | 乙二胺、氨水 | F127 | 约180 | 711 | [ |
| 苯酚、甲醛 | 水 | Bis-tris① | CTAB ④ | 86~205 | 441~1462 | [ |
| 苯酚、甲醛 | 水 | 氢氧化钠 | F127 | 约100 | 1602 | [ |
| 间苯二酚、甲醛 | 水、甲醇 | 氨水 | — | 160~1800 | — | [ |
| 间苯二酚、甲醛 | 水、乙醇 | 氨水 | F127 | 52 | 304~3259 | [ |
| 二羟基沙林、甲醛 | 水、甲醇 | 氨水 | F127 | 106~188 | 499~528 | [ |
| 三聚氰胺、甲醛 | 水 | 氢氧化钠 | F127 | 40~160 | 795~883 | [ |
表1 小粒径炭微球制备相关参数
Table 1 Parameters related to the preparation of small particle size carbon microspheres
| 炭前体 | 溶剂 | 催化剂 | 添加剂 | 炭球粒径/nm | 比表面积/(m2/g) | 文献 |
|---|---|---|---|---|---|---|
| 间苯三酚、对苯二甲醛、间苯二酚、甲醛 | 水 | 氨水 | — | 30~90 | — | [ |
| 苯酚、甲醛 | 水 | — | F127 | 约110 | 219 | [ |
| 间苯三酚、对苯二胺、甲醛 | 水、乙醇 | 对苯二胺 | — | 79.2~137.0 | — | [ |
| 间苯三酚、甲醛 | 水 | 盐酸 | F108,F127,F86,P123② | 80~90 | — | [ |
| 间苯二酚 、甲醛 | 水、乙醇 | 乙二胺 | PVP③ | 60~875 | 516~1083 | [ |
| 间苯二酚、甲醛 | 水 | 乙二胺、氨水 | F127 | 约180 | 711 | [ |
| 苯酚、甲醛 | 水 | Bis-tris① | CTAB ④ | 86~205 | 441~1462 | [ |
| 苯酚、甲醛 | 水 | 氢氧化钠 | F127 | 约100 | 1602 | [ |
| 间苯二酚、甲醛 | 水、甲醇 | 氨水 | — | 160~1800 | — | [ |
| 间苯二酚、甲醛 | 水、乙醇 | 氨水 | F127 | 52 | 304~3259 | [ |
| 二羟基沙林、甲醛 | 水、甲醇 | 氨水 | F127 | 106~188 | 499~528 | [ |
| 三聚氰胺、甲醛 | 水 | 氢氧化钠 | F127 | 40~160 | 795~883 | [ |

图2 “种子”合成策略制备聚合物纳米球的示意图(a);胶体种子(b)和聚合物微球(c)的TEM图像[19]
Fig.2 Schematic diagram of the “seed” synthesis strategy for the preparation of polymer nanospheres (a); TEM images of colloidal seeds (b) and polymer microspheres (c) [19]
| 添加剂 | 粒径/nm | 孔型 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 文献 |
|---|---|---|---|---|---|
| 正硅酸乙酯、硅溶胶 | 300~500 | 微孔、介孔 | 430~560 | 0.23~0.60 | [ |
| F127、CTAB | 40~750 | 大孔 | 67~1295 | 0.05~0.84 | [ |
| F127 | 约110 | 介孔 | 219 | 0.27 | [ |
| 聚乙二醇 | — | 微孔 | 556~625 | 0.25~0.26 | [ |
| — | 约850 | 超微孔 | 1113~1235 | 0.60~0.88 | [ |
| F127 | 约300 | 微孔 | 409 | 0.25 | [ |
| 正硅酸乙酯 | 100~500 | 大孔 | — | — | [ |
| PVP | 约260 | 大孔 | — | — | [ |
| F108、F127、F86、P123 | 80~90 | 介孔 | — | — | [ |
| PVP | 60~875 | 微孔 | 516~1083 | 0.28~0.82 | [ |
| F127 | 约180 | 介孔 | 711 | — | [ |
| CTAB | 86~205 | 微孔、介孔 | 441~1462 | 0.56~1.00 | [ |
| F127 | 约100 | 介孔 | 1602 | 2.09 | [ |
| — | 约600 | 微孔 | 836 | 0.40 | [ |
| F127 | 50~700 | 微孔 | 304~3259 | 0.34~2.44 | [ |
| F127 | 40~160 | 介孔 | 795~883 | — | [ |
| CTAB、正硅酸乙酯 | 320~400 | 微孔、介孔 | 550~1261 | 0.53~1.05 | [ |
| 聚乙二醇 | 300~1000 | 微孔、介孔 | 1101~1835 | 0.28~0.48 | [ |
表2 多孔炭微球制备相关参数
Table 2 Parameters related to the preparation of porous carbon microspheres
| 添加剂 | 粒径/nm | 孔型 | 比表面积/(m2/g) | 孔体积/(cm3/g) | 文献 |
|---|---|---|---|---|---|
| 正硅酸乙酯、硅溶胶 | 300~500 | 微孔、介孔 | 430~560 | 0.23~0.60 | [ |
| F127、CTAB | 40~750 | 大孔 | 67~1295 | 0.05~0.84 | [ |
| F127 | 约110 | 介孔 | 219 | 0.27 | [ |
| 聚乙二醇 | — | 微孔 | 556~625 | 0.25~0.26 | [ |
| — | 约850 | 超微孔 | 1113~1235 | 0.60~0.88 | [ |
| F127 | 约300 | 微孔 | 409 | 0.25 | [ |
| 正硅酸乙酯 | 100~500 | 大孔 | — | — | [ |
| PVP | 约260 | 大孔 | — | — | [ |
| F108、F127、F86、P123 | 80~90 | 介孔 | — | — | [ |
| PVP | 60~875 | 微孔 | 516~1083 | 0.28~0.82 | [ |
| F127 | 约180 | 介孔 | 711 | — | [ |
| CTAB | 86~205 | 微孔、介孔 | 441~1462 | 0.56~1.00 | [ |
| F127 | 约100 | 介孔 | 1602 | 2.09 | [ |
| — | 约600 | 微孔 | 836 | 0.40 | [ |
| F127 | 50~700 | 微孔 | 304~3259 | 0.34~2.44 | [ |
| F127 | 40~160 | 介孔 | 795~883 | — | [ |
| CTAB、正硅酸乙酯 | 320~400 | 微孔、介孔 | 550~1261 | 0.53~1.05 | [ |
| 聚乙二醇 | 300~1000 | 微孔、介孔 | 1101~1835 | 0.28~0.48 | [ |
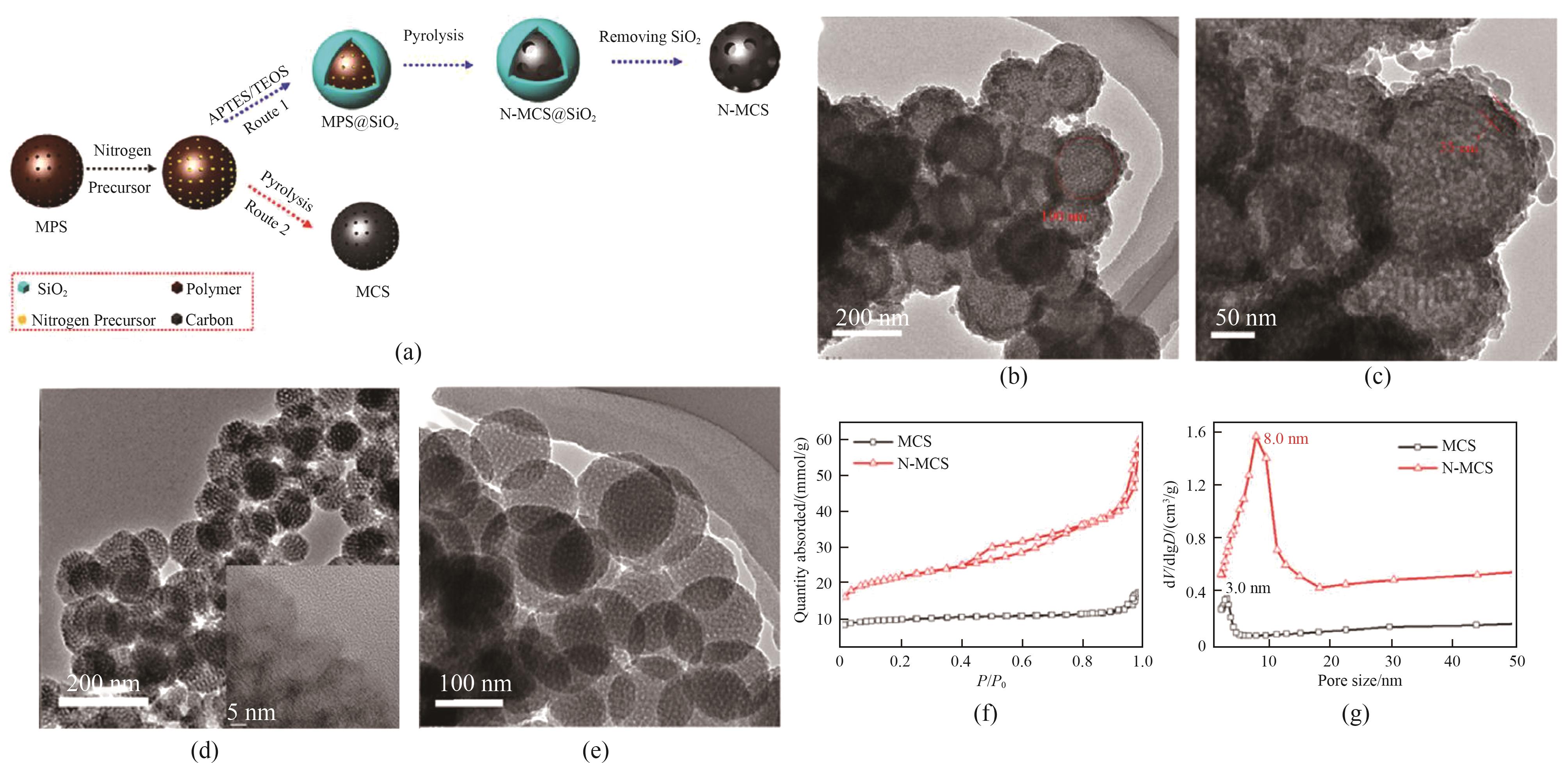
图6 受限热解制备介孔炭球示意图(a);SiO2包裹酚醛微球的TEM图像[(b),(c)];介孔炭球的TEM图像[(d),(e)];受限热解和直接热解得到炭球的氮气吸附-脱附等温线和孔径分布曲线[(f),(g)][24]
Fig.6 Schematic diagram of mesoporous carbon spheres prepared by restricted pyrolysis(a); TEM images of MPS@SiO2[(b), (c)]; TEM images of mesoporous carbon spheres[(d), (e)]; Nitrogen adsorption-desorption isotherms and pore size distribution curves of carbon spheres obtained by restricted pyrolysis and direct pyrolysis[(f), (g)][24]
| 炭前体 | 添加剂 | 催化剂 | 粒径/nm | 功能化措施 | 氮掺杂量 | 文献 |
|---|---|---|---|---|---|---|
| 间苯二酚、甲醛 | F127 | 氨水 | — | 负载Ag | — | [ |
| 间苯二酚、甲醛 | F127 | 氨水 | 150~900 | 负载Pt | — | [ |
| 氨基苯酚、甲醛 | — | 氨水 | 80~2500 | 负载Pt | — | [ |
| 苯酚、甲醛 | F127 | 氢氧化钠 | 约110 | 负载Fe | — | [ |
| 单宁酸、甲醛 | — | 氨水 | 60~2100 | 负载Fe | — | [ |
| 间苯三酚、对苯二胺、甲醛 | — | 对苯二胺 | 79.2~137 | 掺N | 9.77%(质量分数) | [ |
| 氨基苯酚、六氯甲烷 | F127 | 氨水 | 约300 | 掺N | 5.31%(质量分数) | [ |
| 间苯二酚、甲醛 | PVP | 氨水 | 约260 | 掺N | 1.5%(原子分数) | [ |
| 单宁酸、甲醛 | F127 | 氨水 | 约300 | 负载Co、Fe、Ni 等 | — | [ |
| 间苯二酚 、甲醛 | PVP | 乙二胺 | 60~875 | 掺N | 5%(质量分数) | [ |
| 苯酚、甲醛 | CTAB | Bis-tris | 86~205 | 掺N | 2.32%(原子分数) | [ |
| 苯酚、甲醛 | F127 | 氢氧化钠 | 约100 | 掺N | — | [ |
| 间苯二酚、HMTA | — | HMTA | 约800 | 掺N | 2.05%(原子分数) | [ |
| 二羟基沙林、甲醛 | F127 | 氨水 | 106~188 | 掺N | 35%(质量分数) | [ |
| 三聚氰胺、甲醛 | F127 | 氢氧化钠 | 40~160 | 掺N | 15.6%(质量分数) | [ |
| 间苯二酚、甲醛 | PEG | 氨水、乙二胺或己二胺 | 300~1000 | 掺N | — | [ |
表3 功能化炭微球制备相关参数
Table 3 Relevant parameters for the preparation of functionalized carbon microspheres
| 炭前体 | 添加剂 | 催化剂 | 粒径/nm | 功能化措施 | 氮掺杂量 | 文献 |
|---|---|---|---|---|---|---|
| 间苯二酚、甲醛 | F127 | 氨水 | — | 负载Ag | — | [ |
| 间苯二酚、甲醛 | F127 | 氨水 | 150~900 | 负载Pt | — | [ |
| 氨基苯酚、甲醛 | — | 氨水 | 80~2500 | 负载Pt | — | [ |
| 苯酚、甲醛 | F127 | 氢氧化钠 | 约110 | 负载Fe | — | [ |
| 单宁酸、甲醛 | — | 氨水 | 60~2100 | 负载Fe | — | [ |
| 间苯三酚、对苯二胺、甲醛 | — | 对苯二胺 | 79.2~137 | 掺N | 9.77%(质量分数) | [ |
| 氨基苯酚、六氯甲烷 | F127 | 氨水 | 约300 | 掺N | 5.31%(质量分数) | [ |
| 间苯二酚、甲醛 | PVP | 氨水 | 约260 | 掺N | 1.5%(原子分数) | [ |
| 单宁酸、甲醛 | F127 | 氨水 | 约300 | 负载Co、Fe、Ni 等 | — | [ |
| 间苯二酚 、甲醛 | PVP | 乙二胺 | 60~875 | 掺N | 5%(质量分数) | [ |
| 苯酚、甲醛 | CTAB | Bis-tris | 86~205 | 掺N | 2.32%(原子分数) | [ |
| 苯酚、甲醛 | F127 | 氢氧化钠 | 约100 | 掺N | — | [ |
| 间苯二酚、HMTA | — | HMTA | 约800 | 掺N | 2.05%(原子分数) | [ |
| 二羟基沙林、甲醛 | F127 | 氨水 | 106~188 | 掺N | 35%(质量分数) | [ |
| 三聚氰胺、甲醛 | F127 | 氢氧化钠 | 40~160 | 掺N | 15.6%(质量分数) | [ |
| 间苯二酚、甲醛 | PEG | 氨水、乙二胺或己二胺 | 300~1000 | 掺N | — | [ |
| 电极材料 | 比表面积/(m2/g) | 比电容/(F/g) | 电解液 | 文献 |
|---|---|---|---|---|
| 酚醛基炭微球 | 409 | 288 (0.1 A/g) | 6 mol/L KOH | [ |
| 173 (0.5 A/g) | 6 mol/L KOH | [ | ||
| 1602 | 326 (1.0 A/g) | 6 mol/L KOH | [ | |
| 201 (0.5 A/g) | 6 mol/L KOH | [ | ||
| 836 | 282 (0.5 A/g) | 6 mol/L KOH | [ | |
| 3259 | 225 (0.5 A/g) | 6 mol/L KOH | [ | |
| 1835 | 234 (1.0 A/g) | 6 mol/L KOH | [ | |
| 泡沫炭 | 1286 | 227 (1.0 A/g) | 6 mol/L KOH | [ |
| 石墨烯 | 2582 | 186 (1.0 A/g) | 6 mol/L KOH | [ |
表4 各种炭材料电化学性能的比较
Table 4 Comparison of electrochemical performance of various carbon materials
| 电极材料 | 比表面积/(m2/g) | 比电容/(F/g) | 电解液 | 文献 |
|---|---|---|---|---|
| 酚醛基炭微球 | 409 | 288 (0.1 A/g) | 6 mol/L KOH | [ |
| 173 (0.5 A/g) | 6 mol/L KOH | [ | ||
| 1602 | 326 (1.0 A/g) | 6 mol/L KOH | [ | |
| 201 (0.5 A/g) | 6 mol/L KOH | [ | ||
| 836 | 282 (0.5 A/g) | 6 mol/L KOH | [ | |
| 3259 | 225 (0.5 A/g) | 6 mol/L KOH | [ | |
| 1835 | 234 (1.0 A/g) | 6 mol/L KOH | [ | |
| 泡沫炭 | 1286 | 227 (1.0 A/g) | 6 mol/L KOH | [ |
| 石墨烯 | 2582 | 186 (1.0 A/g) | 6 mol/L KOH | [ |

图8 不同文献中超级电容器循环稳定性(a)和不同电流密度下的面积比电容(b)的比较
Fig.8 Comparison of cycling stability (a) and area specific capacitance at different current densities (b) of supercapacitors in different literature

图9 DHCSs/RGO制备示意图(a);DHCSs/RGO活化前循环性能(b);DHCSs/RGO活化后循环性能(c)[57]Cycling performance after DHCSs/RGO activation (c)[57]
Fig.9 Schematic diagram of DHCSs/RGO preparation (a); Cycling performance before DHCSs/RGO activation (b);
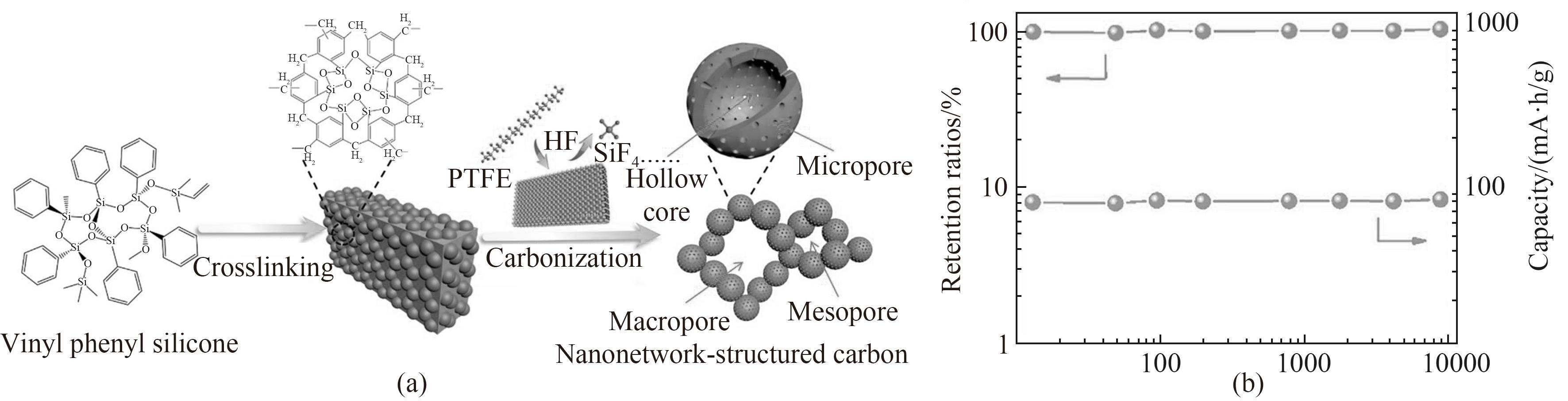
图10 NNSC制备示意图(a);循环9000次时,NNSC的容量保留率和容量(b)[60]
Fig.10 Schematic diagram of NNSC preparation (a); Capacity retention and capacity of NNSC at 9000 cycles (b)[60]

图11 不同文献中吸附材料CO2吸附性能的对比(1 bar=100 kPa)
Fig.11 Comparison of CO2 adsorption performance of different adsorbent materials in the literatures (1 bar=100 kPa)

图12 TAF-C@Fe制备示意图(a);不同电极的CV曲线(b);添加0.5 mol/L甲醇后TAF-C@Fe和Pt/C的CV曲线(c)[45]
Fig.12 Schematic diagram of TAF-C@Fe preparation (a); CV curves of different electrodes (b); CV curves of TAF-C@Fe and Pt/C after adding 0.5 mol/L methanol (c) [45]
| 1 | Xu K L, Liu J, Yan Z X, et al. Synthesis and use of hollow carbon spheres for electric double-layer capacitors[J]. New Carbon Materials, 2021, 36(4): 794-809. |
| 2 | Qiu P P, Ma B, Hung C T, et al. Spherical mesoporous materials from single to multilevel architectures[J]. Accounts of Chemical Research, 2019, 52(10): 2928-2938. |
| 3 | Sivaranjanee R, Kumar P S. A review on cleaner approach for effective separation of toxic pollutants from wastewater using carbon Sphere’s as adsorbent: preparation, activation and applications[J]. Journal of Cleaner Production, 2021, 291: 125911. |
| 4 | Tian H J, Wang T Y, Zhang F, et al. Tunable porous carbon spheres for high-performance rechargeable batteries[J]. Journal of Materials Chemistry A, 2018, 6(27): 12816-12841. |
| 5 | Liu T, Zhang L Y, Cheng B, et al. Hollow carbon spheres and their hybrid nanomaterials in electrochemical energy storage[J]. Advanced Energy Materials, 2019, 9(17): 1803900. |
| 6 | 巩玉同. 生物质水热炭微球的可控合成表征及应用研究[D]. 杭州: 浙江大学, 2015. |
| Gong Y T. Synthsis, characterization and applications of carbon spheres based on hydrothermal carbonization process[D]. Hangzhou: Zhejiang University, 2015. | |
| 7 | 曾宪阳. 淀粉基炭微球的制备及其在吸附和光催化中的应用研究[D]. 大连: 大连工业大学, 2020. |
| Zeng X Y. Preparation of starch based carbon microsphere and its application in adsorption and photocatalysis[D]. Dalian: Dalian Polytechnic University, 2020. | |
| 8 | 王泉高. 多孔纳米炭球的可控制备[D]. 大连: 大连理工大学, 2021. |
| Wang Q G. Controllable synthesis of porous carbon nanospheres[D]. Dalian: Dalian University of Technology, 2021. | |
| 9 | Xu H L, Yin X W, Li M H, et al. Mesoporous carbon hollow microspheres with red blood cell like morphology for efficient microwave absorption at elevated temperature[J]. Carbon, 2018, 132: 343-351. |
| 10 | 逯英英. 酚醛树脂基球形炭的制备及脱硫脱氮性能研究[D]. 成都:西南石油大学, 2017. |
| Lu Y Y. Preparation of phenolic resin-based spherical carbon and study of desulfurization and denitrogenation performance[D]. Chengdu: Southwest Petroleum University, 2017. | |
| 11 | Wang T, Okejiri F, Qiao Z N, et al. Tailoring polymer colloids derived porous carbon spheres based on specific chemical reactions[J]. Advanced Materials (Deerfield Beach, Fla.), 2020, 32(44): e2002475. |
| 12 | Wu H X, Qin Y M, Zong S, et al. Porous yolk-shell-structured carbon nanospheres for electrochemical energy storage[J]. Journal of Materials Science: Materials in Electronics, 2020, 31(16): 13321-13329. |
| 13 | Du J, Zhang Y, Wu H X, et al. N-doped hollow mesoporous carbon spheres by improved dissolution-capture for supercapacitors[J]. Carbon, 2020, 156: 523-528. |
| 14 | Zhao X, Zhang M, Sun X D, et al. Comprehensive understanding of the formation process on monodisperse resorcinol-formaldehyde polymer and carbon spheres and their use as substrates for surface-enhanced Raman spectroscopy[J]. Applied Surface Science, 2020, 506: 144591. |
| 15 | Zhao J M, Gilani M R H S, Liu Z Y, et al. Facile surfactant-free synthesis of polybenzoxazine-based polymer and nitrogen-doped carbon nanospheres[J]. Polymer Chemistry, 2018, 9(33): 4324-4331. |
| 16 | 李长东. 酚醛基炭球及氮掺杂多孔石墨化炭球的制备及电化学性能研究[D]. 北京: 北京化工大学, 2021. |
| Li C D. Preparation and electrochemical performance of phenolic-based carbon spheres and nitrogen-doped porous graphitized carbon spheres[D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| 17 | Hu L J, Qian Z X, Gao W, et al. Nanoengineering of uniform and monodisperse mesoporous carbon nanospheres mediated by long hydrophilic chains of triblock copolymers[J]. Journal of Materials Science, 2020, 55(5): 2052-2067. |
| 18 | Fan C Y, Ou M Y, Wei P, et al. Hard carbon spheres prepared by a modified Stöber method as anode material for high-performance potassium-ion batteries[J]. RSC Advances, 2021, 11(24): 14883-14890. |
| 19 | Qian J S, Liu M X, Gan L H, et al. A seeded synthetic strategy for uniform polymer and carbon nanospheres with tunable sizes for high performance electrochemical energy storage[J]. Chemical Communications (Cambridge, England), 2013, 49(29): 3043-3045. |
| 20 | 李淑慧. 纳米碳球的制备及其吸附性能的研究[D]. 石家庄: 河北科技大学, 2017. |
| Li S H. Study on preparation of nanospheres carbon materials and its adsorption properties[D]. Shijiazhuang: Hebei University of Science and Technology, 2017. | |
| 21 | Ghimire P P, Dassanayake A C, Wickramaratne N P, et al. Polyvinyl pyrrolidone-assisted synthesis of size-tunable polymer spheres at elevated temperature and their conversion to nitrogen-containing carbon spheres[J]. Journal of Colloid and Interface Science, 2019, 549: 162-170. |
| 22 | Wang Q G, He L, Zhao L Y, et al. Surface charge-driven nanoengineering of monodisperse carbon nanospheres with tunable surface roughness[J]. Advanced Functional Materials, 2020, 30(6): 1906117. |
| 23 | Li X Y, Song Y F, You L, et al. Synthesis of highly uniform N-doped porous carbon spheres derived from their phenolic-resin-based analogues for high performance supercapacitors[J]. Industrial & Engineering Chemistry Research, 2019, 58(8): 2933-2944. |
| 24 | Du J, Liu L, Yu Y F, et al. N-doped ordered mesoporous carbon spheres derived by confined pyrolysis for high supercapacitor performance[J]. Journal of Materials Science & Technology, 2019, 35(10): 2178-2186. |
| 25 | Tsai C Y, Tai H C, Su C A, et al. Activated microporous carbon nanospheres for use in supercapacitors[J]. ACS Applied Nano Materials, 2020, 3(10): 10380-10388. |
| 26 | Hosseinzadeh S T, Khorshidi A, Yaghoubi R, et al. Stöber synthesis of salen-formaldehyde resin polymer-and carbon spheres with high nitrogen content and application of the corresponding Mn-containing carbon spheres as efficient electrocatalysts for the oxygen reduction reaction[J]. RSC Advances, 2020, 10(46): 27575-27584. |
| 27 | Guo D Y, Fu Y B, Bu F X, et al. Monodisperse ultrahigh nitrogen-containing mesoporous carbon nanospheres from melamine-formaldehyde resin[J]. Small Methods, 2021, 5(5): 2001137. |
| 28 | Zhao J M, Niu W X, Zhang L, et al. A template-free and surfactant-free method for high-yield synthesis of highly monodisperse 3-aminophenol-formaldehyde resin and carbon nano/microspheres[J]. Macromolecules, 2013, 46(1): 140-145. |
| 29 | Liu J, Qiao S Z, Liu H, et al. Extension of the stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres[J]. Angewandte Chemie International Edition, 2011, 50(26): 5947-5951. |
| 30 | Wang J G, Liu H Z, Sun H H, et al. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors[J]. Carbon, 2018, 127: 85-92. |
| 31 | Du X, Yang H M, Zhang Y L, et al. Synthesis of size-controlled carbon microspheres from resorcinol/formaldehyde for high electrochemical performance[J]. New Carbon Materials, 2021, 36(3): 616-624. |
| 32 | Choma J, Jamioła D, Augustynek K, et al. New opportunities in Stöber synthesis: preparation of microporous and mesoporous carbon spheres[J]. Journal of Materials Chemistry, 2012, 22(25): 12636. |
| 33 | Tian H, Liang J, Liu J. Nanoengineering carbon spheres as nanoreactors for sustainable energy applications[J]. Advanced Materials, 2019, 31(50): 1903886. |
| 34 | Yang T Y, Liu J, Zhou R F, et al. N-doped mesoporous carbon spheres as the oxygen reduction reaction catalysts[J]. J.Mater. Chem. A, 2014, 2(42): 18139-18146. |
| 35 | Wang X, Zhou J, Xing W, et al. Resorcinol-formaldehyde resin-based porous carbon spheres with high CO2 capture capacities[J]. Journal of Energy Chemistry, 2017, 26(5): 1007-1013. |
| 36 | Yang J Y, Miao T, Zhang Z, et al. Nitrogen-doped hollow carbon spheres as a support for the synthesis of multifunctional composites[J]. Micro & Nano Letters, 2018, 13(4): 473-476. |
| 37 | Liu M, Yu Y F, Liu B B, et al. PVP-assisted synthesis of nitrogen-doped hollow carbon spheres for supercapacitors[J]. Journal of Alloys and Compounds, 2018, 768: 42-48. |
| 38 | 于强. 酚醛树脂碳及其复合材料的制备与电化学性能研究[D]. 武汉: 武汉理工大学, 2020. |
| Yu Q. Synthesis and electrochemical performance of phenolic resinderived carbon and its composites[D]. Wuhan: Wuhan University of Technology, 2020. | |
| 39 | Yu X F, Li W C, Hu Y R, et al. Sculpturing solid polymer spheres into internal gridded hollow carbon spheres under controlled pyrolysis micro-environment[J]. Nano Research, 2021, 14(5): 1565-1573. |
| 40 | 李雪娜. 空心碳球的可控制备及其在CO2吸附领域的研究[D]. 北京: 北京工业大学, 2016. |
| Li X N. Controlled preparation of hollow carbon spheres and there application in CO2 adsorption[D]. Beijing: Beijing University of Technology, 2016. | |
| 41 | Zhang D D, He C, Zhao J H, et al. Facile synthesis of hierarchical mesopore-rich activated carbon with excellent capacitive performance[J]. Journal of Colloid and Interface Science, 2019, 546: 101-112. |
| 42 | 张兴淼, 张威, 李伟. 碳基空心结构纳米材料的制备与应用[J]. 黑龙江大学工程学报, 2021, 12(3): 42-56. |
| Zhang X M, Zhang W, Li W. Synthesis and application of carbon-based hollow nanomaterials[J]. Journal of Engineering of Heilongjiang University, 2021, 12(3): 42-56. | |
| 43 | Jin C B, Shi P, Zhang X Q, et al. Advances in carbon materials for stable lithium metal batteries[J]. New Carbon Materials, 2022, 37(1): 1-24. |
| 44 | Yu X H, Yi J L, Zhang R L, et al. Hollow carbon spheres and their noble metal-free hybrids in catalysis[J]. Frontiers of Chemical Science and Engineering, 2021, 15(6): 1380-1407. |
| 45 | Liu M M, Cai C, Li J, et al. Stöber synthesis of tannic acid-formaldehyde resin polymer spheres and their derived carbon nanospheres and nanocomposites for oxygen reduction reaction[J]. Journal of Colloid and Interface Science, 2018, 528: 1-9. |
| 46 | Wei J, Wang G, Chen F, et al. Sol-gel synthesis of metal-phenolic coordination spheres and their derived carbon composites[J]. Angewandte Chemie International Edition, 2018, 57(31): 9838-9843. |
| 47 | Liu M, Liu L, Yu Y F, et al. Synthesis of nitrogen-doped carbon spheres using the modified Stöber method for supercapacitors[J]. Frontiers of Materials Science, 2019, 13(2): 156-164. |
| 48 | 戴兰轩. 铁(钴)基/空心介孔碳球复合材料的合成及其储锂性能研究[D]. 扬州: 扬州大学, 2020. |
| Dai L X. Synthesis of iron(cobalt)-based/C hollow mesoporous carbon nanospheres composites and their lithium storage performances[D]. Yangzhou: Yangzhou University, 2020. | |
| 49 | Wang G, Qin J, Zhao Y X, et al. Nanoporous carbon spheres derived from metal-phenolic coordination polymers for supercapacitor and biosensor[J]. Journal of Colloid and Interface Science, 2019, 544: 241-248. |
| 50 | Zhang C W, Song Y, Xu L B, et al. In situ encapsulation of Co/Co3O4 nanoparticles in nitrogen-doped hierarchically ordered porous carbon as high performance anode for lithium-ion batteries[J]. Chemical Engineering Journal, 2020, 380: 122545. |
| 51 | Du J, Zong S, Zhang Y, et al. Co-assembly strategy for uniform and tunable hollow carbon spheres with supercapacitor application[J]. Journal of Colloid and Interface Science, 2020, 565: 245-253. |
| 52 | Luo X Y, Chen Y, Mo Y. A review of charge storage in porous carbon-based supercapacitors[J]. New Carbon Materials, 2021, 36(1): 49-68. |
| 53 | 任子君. 改性碳气凝胶的超级电容器性能研究[D]. 西安: 西北大学, 2017. |
| Ren Z J. Study on modification of carbon aerogel and performance of supercapacitor[D]. Xi’an: Northwest University, 2017. | |
| 54 | 樊泽文. 碳基电极的设计合成及柔性超级电容器的构筑[D]. 太原: 太原理工大学, 2021. |
| Fan Z W. Design and synthesis of carbon-based electrodes and construction of flexible supercapacitors[D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| 55 | You B, Jiang J, Fan S. Three-dimensional hierarchically porous all-carbon foams for supercapacitor[J]. ACS Applied Materials & Interfaces, 2014, 6(17): 15302-15308. |
| 56 | Xu J, Tan Z, Zeng W, et al. A hierarchical carbon derived from sponge-templated activation of graphene oxide for high-performance supercapacitor electrodes[J]. Advanced Materials, 2016, 28(26): 5222-5228. |
| 57 | Zhang Y Q, Ma Q, Wang S L, et al. Poly(vinyl alcohol)-assisted fabrication of hollow carbon spheres/reduced graphene oxide nanocomposites for high-performance lithium-ion battery anodes[J]. ACS Nano, 2018, 12(5): 4824-4834. |
| 58 | Wang S, Li Y Y, Ma F T, et al. Phenolic resin-based carbon microspheres for potassium ion storage[J]. Applied Surface Science, 2020, 506: 144805. |
| 59 | 索莉瑶. 球形钠离子电池负极材料的制备及其储能性能研究[D]. 南京: 南京邮电大学, 2020. |
| Suo L Y. Preparation of spherical anode material for sodium ion battery and their applications to energy storage[D]. Nanjing: Nanjing University of Posts and Telecommunications, 2020. | |
| 60 | Zhang W C, Lan C W, Xie X H, et al. Facile construction of hollow carbon nanosphere-interconnected network for advanced sodium-ion battery anode[J]. Journal of Colloid and Interface Science, 2019, 546: 53-59. |
| 61 | Hu T, Li Y M, Gao W, et al. Engineering of rich nitrogen-doped and magnetic mesoporous carbon nanospheres with predictable size uniformity for acid dye molecules adsorption[J]. Microporous and Mesoporous Materials, 2019, 279: 234-244. |
| 62 | Zhang J C, Qin L, Yang Y Z, et al. Porous carbon nanospheres aerogel based molecularly imprinted polymer for efficient phenol adsorption and removal from wastewater[J]. Separation and Purification Technology, 2021, 274: 119029. |
| 63 | 张宾朋. 木质素碳材料的微观形貌调控及其在吸附和光催化领域的应用[D]. 广州: 华南理工大学, 2020. |
| Zhang B P. Micromorphological control of lignin-derived carbon material and its applications in adsorption and photocatalysis[D]. Guangzhou: South China University of Technology, 2020. | |
| 64 | Xiong W, Zhang P, Liu S T, et al. Catalyst-free synthesis of phenolic-resin-based carbon nanospheres for simultaneous electrochemical detection of Cu (Ⅱ) and Hg (Ⅱ)[J]. Diamond and Related Materials, 2021, 111: 108170. |
| 65 | Liu X N, Fu J Y, Tang Y W, et al. Mg-coordinated self-assembly of MgO-doped ordered mesoporous carbons for selective recovery of phosphorus from aqueous solutions[J]. Chemical Engineering Journal, 2021, 406: 126748. |
| 66 | 周亚兰, 罗路, 范毜仔, 等. 单宁改性酚醛基炭气凝胶的制备及其CO2吸附性能[J]. 复合材料学报, 2022, 39:1-10. |
| Zhou Y L, Luo L, Fan N Z, et al. Preparation and CO2 adsorption properties of tannin modified phenolic based carbon aerogels[J]. Acta Mater. Compos. Sin., 2022, 39:1-10. | |
| 67 | 刘沈芳. 生物质基氮掺杂多孔炭用于CO2吸附和超级电容器电极的研究[D]. 金华: 浙江师范大学, 2021. |
| Liu S F. Nitrogen-doped prous carbons derived from biomass for CO2 adsorption and suspercapacitor electodes[D]. Jinhua: Zhejiang Normal University, 2021. | |
| 68 | 安晓银, 鲁金明, 刘毅, 等. 双金属MOF-74的合成及其气体吸附分离性能研究[J]. 化工新型材料, 2020, 48(4): 185-190. |
| An X Y, Lu J M, Liu Y, et al. Synthesis of bimetallic MOF-74 and its gas adsorption and separation properties[J]. New Chemical Materials, 2020, 48(4): 185-190. | |
| 69 | Feng J Z, Su B L, Xia H S, et al. Printed aerogels: chemistry, processing, and applications[J]. Chemical Society Reviews, 2021, 50(6): 3842-3888. |
| 70 | 罗燚, 冯军宗, 冯坚, 等. 新型碳材料质子交换膜燃料电池Pt催化剂载体的研究进展[J]. 无机材料学报, 2020, 35(4): 407-415. |
| Luo Y, Feng J Z, Feng J, et al. Research progress on advanced carbon materials as Pt support for proton exchange membrane fuel cells[J]. Journal of Inorganic Materials, 2020, 35(4): 407-415. | |
| 71 | Wan X K, Wu H B, Guan B Y, et al. Confining sub-nanometer Pt clusters in hollow mesoporous carbon spheres for boosting hydrogen evolution activity[J]. Advanced Materials, 2020, 32(7): 1901349. |
| 72 | Gan T, Lv Z, Sun J Y, et al. Preparation of graphene oxide-wrapped carbon sphere@silver spheres for high performance chlorinated phenols sensor[J]. Journal of Hazardous Materials, 2016, 302: 188-197. |
| 73 | Saida T, Sakakibara K, Igami R, et al. Synthesis of a Pt/carbon-sphere catalyst and evaluation of its oxygen reduction reaction activity in acidic environments[J]. Energy & Fuels, 2022, 36(2): 1027-1033. |
| 74 | 程晓昆, 张越, 吕海军, 等. 多孔碳纳米材料构建抗肿瘤药物靶向传递系统的研究进展[J]. 无机材料学报, 2021, 36(1): 9-24. |
| Cheng X K, Zhang Y, Lu H J, et al. Porous carbon nanomaterials based tumor targeting drug delivery system: a review[J]. Journal of Inorganic Materials, 2021, 36(1): 9-24. | |
| 75 | Chen G, Yi Z, Chen X, et al. Polymerization-induced self-assembly of tea polyphenols into open-mouthed nanoparticles for active delivery systems and stable carbon bowls[J]. ACS Applied Nano Materials, 2021, 4(12): 13510-13522. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [8] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [9] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [10] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [11] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [12] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [15] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号