化工学报 ›› 2024, Vol. 75 ›› Issue (2): 685-694.DOI: 10.11949/0438-1157.20231212
收稿日期:2023-11-21
修回日期:2024-01-05
出版日期:2024-02-25
发布日期:2024-04-10
通讯作者:
易清风,李广
作者简介:尹玉华(2001—),女,本科,2941952904@qq.com基金资助:
Yuhua YIN( ), Can FANG(
), Can FANG( ), Qingfeng YI(
), Qingfeng YI( ), Guang LI(
), Guang LI( )
)
Received:2023-11-21
Revised:2024-01-05
Online:2024-02-25
Published:2024-04-10
Contact:
Qingfeng YI, Guang LI
摘要:
金属-空气电池(MABs)具有成本低、环境友好、比功率和比能量高等优点,而其中的铁-空气电池(IAB)更具有资源丰富以及高比能量等特点,但IAB在充放电过程中存在的铁电极钝化、腐蚀、自放电、析氢反应以及体积变化等问题制约着其发展。考虑到在铁负极制备过程中通常使用乙炔黑作为导电与支撑活性物质的作用,为了考察其他碳材料对铁电极电化学性能的影响,分别利用乙炔黑、石墨粉和碳纳米管制备了不同的铁电极,并与Pt/C空气电极组装成相应的铁-空气电池,对这些电池进行了不同电流密度下的充放电研究并测试了放电极化曲线。结果表明,碳纳米管修饰铁负极的电池整体性能优异,表现出较高的循环稳定性、较高的充放电电压效率以及功率密度。
中图分类号:
尹玉华, 方灿, 易清风, 李广. 不同碳导电剂对铁-空气电池性能的影响[J]. 化工学报, 2024, 75(2): 685-694.
Yuhua YIN, Can FANG, Qingfeng YI, Guang LI. Impact of different carbon conductive agents on performance of iron-air battery[J]. CIESC Journal, 2024, 75(2): 685-694.
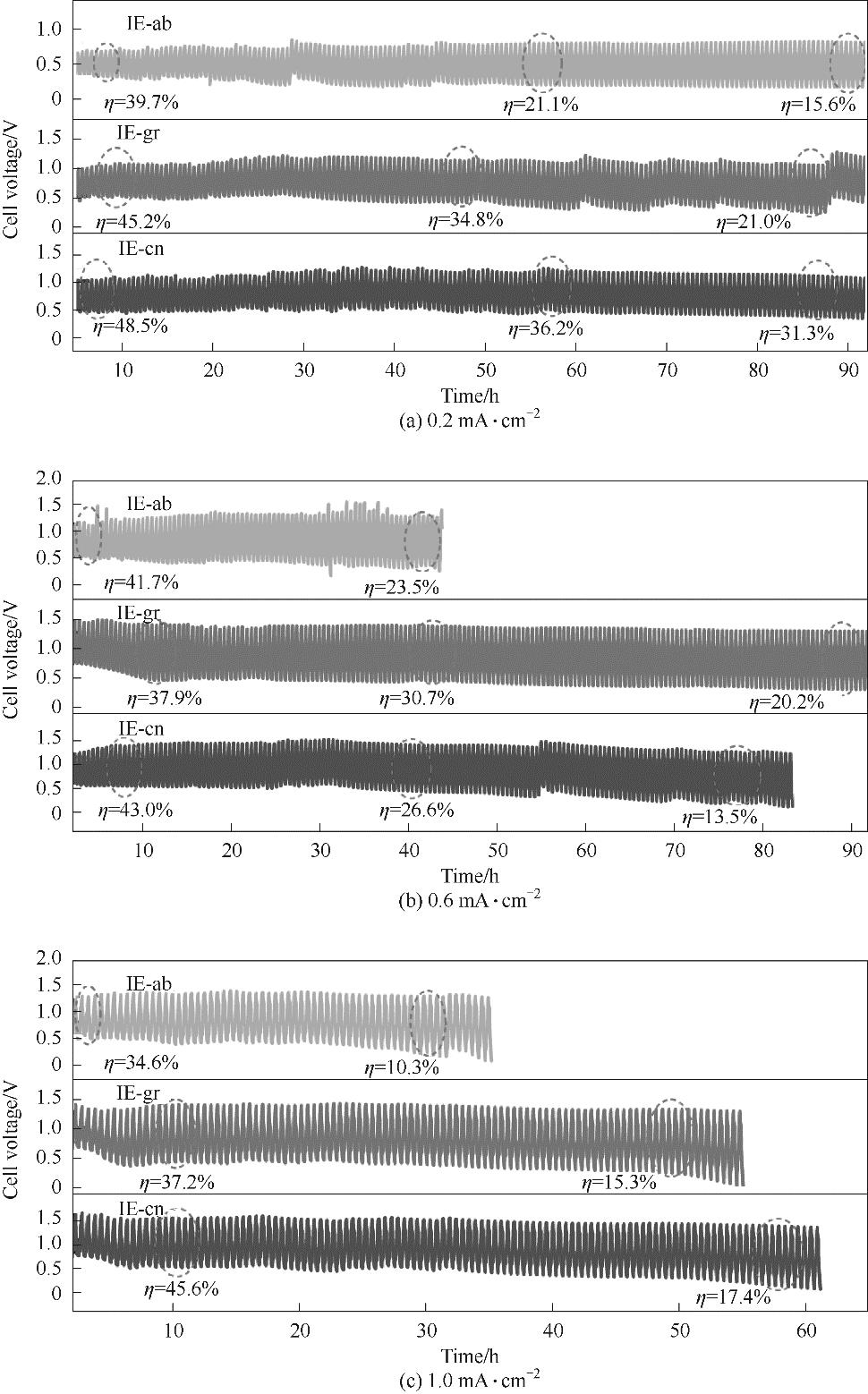
图1 IE-ab、IE-gr和IE-cn电池在4 mol·L-1 NH4Cl+1 mol·L-1 KCl溶液和不同电流密度下的恒流循环充放电曲线
Fig.1 Cycling discharge/charge performance of IE-ab, IE-gr and IE-cn battery in 4 mol·L-1 NH4Cl+1 mol·L-1 KCl electrolyte at 0.2, 0.6 and 1.0 mA·cm-2
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 180 | 0.40 | 40.5 | 0.65 | 16.1 |
| IE-gr | 180 | 0.57 | 44.9 | 0.83 | 20.9 |
| IE-cn | 180 | 0.50 | 49.3 | 0.76 | 31.7 |
表1 IE-ab、IE-gr和IE-cn电池在0.2 mA·cm-2恒流下的循环充放电测试结果
Table 1 Cycling discharge/charge results of IE-ab, IE-gr and IE-cn battery at 0.2 mA·cm-2
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 180 | 0.40 | 40.5 | 0.65 | 16.1 |
| IE-gr | 180 | 0.57 | 44.9 | 0.83 | 20.9 |
| IE-cn | 180 | 0.50 | 49.3 | 0.76 | 31.7 |
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 67 | 0.85 | 34.7 | 1.13 | 10.4 |
| IE-gr | 107 | 0.81 | 37.2 | 1.11 | 15.0 |
| IE-cn | 119 | 0.82 | 45.6 | 1.09 | 17.4 |
表2 IE-ab、IE-gr和IE-cn电池在1.0 mA·cm-2恒流下的循环充放电测试结果
Table 2 Cycling discharge/charge results of IE-ab, IE-gr and IE-cn battery at 1.0 mA·cm-2
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 67 | 0.85 | 34.7 | 1.13 | 10.4 |
| IE-gr | 107 | 0.81 | 37.2 | 1.11 | 15.0 |
| IE-cn | 119 | 0.82 | 45.6 | 1.09 | 17.4 |
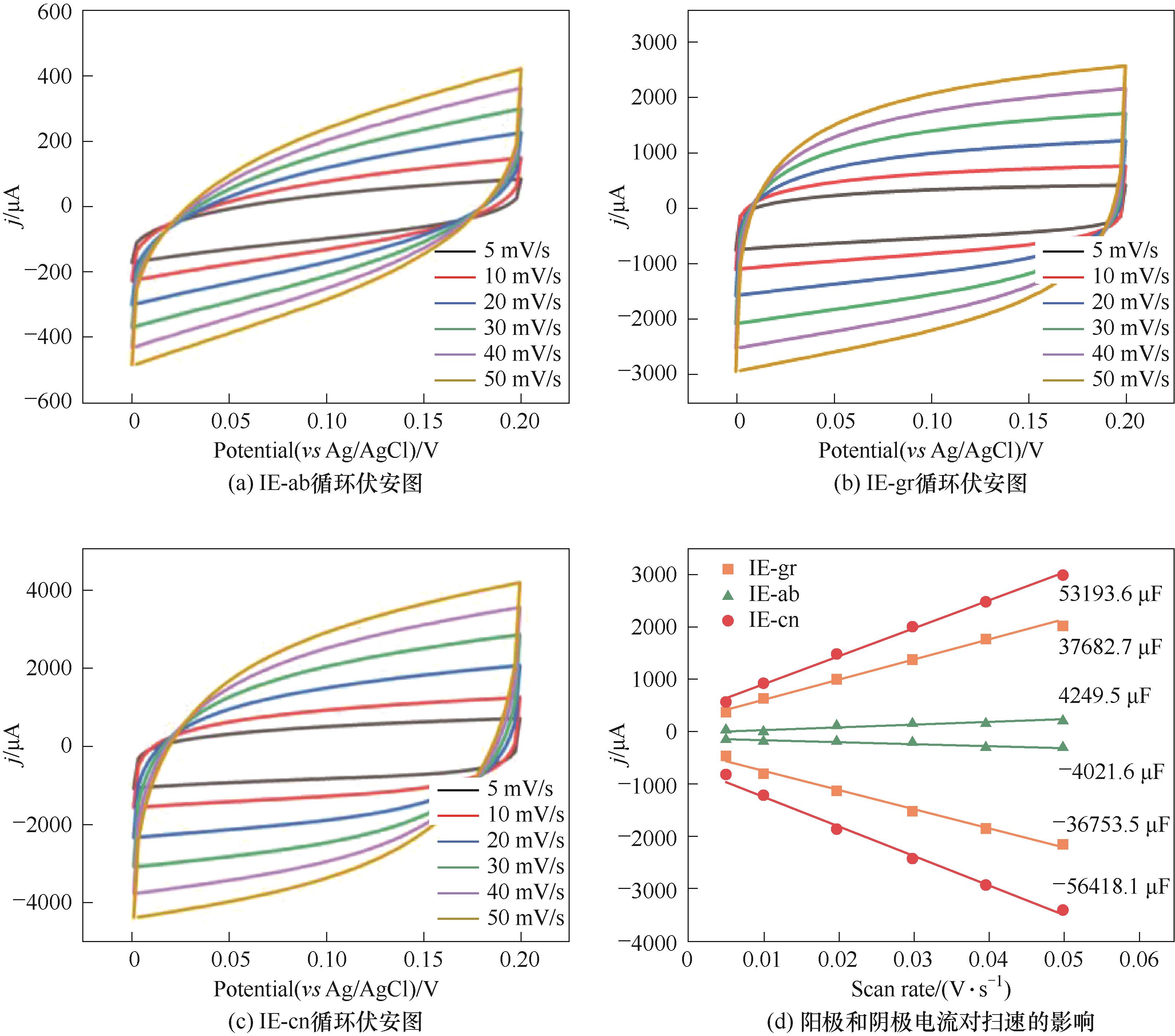
图2 4 mol·L-1 NH4Cl+1 mol·L-1 KCl溶液中,不同扫描速率下不同电极的循环伏安图以及在0.10 V (vs Ag/AgCl)下的阳极和阴极电流对扫速的影响
Fig.2 Cyclic voltammograms at different scan rates in 4 mol·L-1 NH4Cl+1 mol·L-1 KCl electrolyte, and the effect of the scan rate on the anodic and cathodic charging current taken from center of each CV at 0.10 V(vs Ag/AgCl)
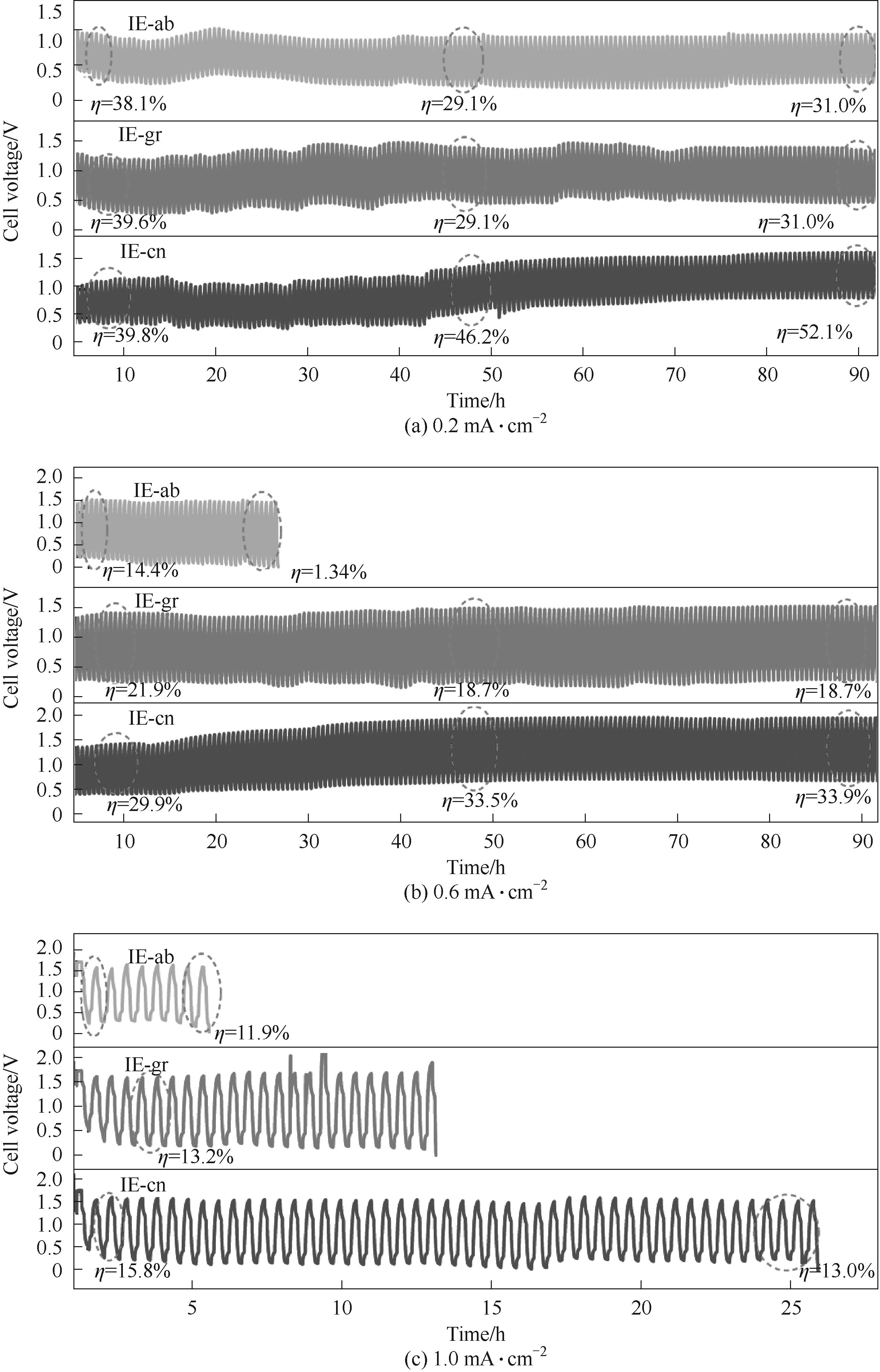
图3 IE-ab、IE-gr和IE-cn电池在0.5 mol·L-1 K2SO4溶液中,不同电流密度下的恒流循环充放电曲线
Fig.3 Cycling discharge/charge performance of IE-ab, IE-gr and IE-cn battery in 0.5 mol·L-1 K2SO4 electrolyte at 0.2, 0.6 and 1.0 mA·cm-2
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 51 | 1.31 | 14.5 | 1.47 | 1.4 |
| IE-gr | 180 | 1.06 | 22.3 | 1.26 | 18.6 |
| IE-cn | 180 | 0.94 | 29.7 | 1.27 | 33.8 |
表3 IE-ab、IE-gr和IE-cn电池在0.6 mA·cm-2恒流下的循环充放电测试结果
Table 3 Cycling discharge/charge results of IE-ab, IE-gr and IE-cn battery at 0.6 mA·cm-2
| Battery | Cycling number | Voltage gap of charge/ discharge at initial cycle/V | Voltage efficiency at initial cycle/% | Voltage gap of charge /discharge at last cycle/V | Voltage efficiency at last cycle/% |
|---|---|---|---|---|---|
| IE-ab | 51 | 1.31 | 14.5 | 1.47 | 1.4 |
| IE-gr | 180 | 1.06 | 22.3 | 1.26 | 18.6 |
| IE-cn | 180 | 0.94 | 29.7 | 1.27 | 33.8 |
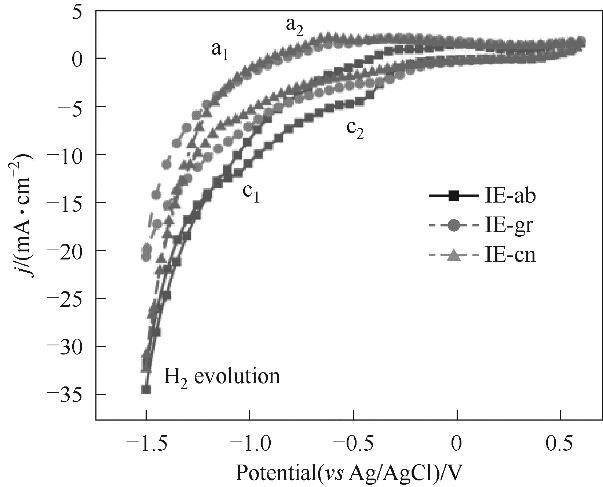
图4 在0.5 mol·L-1 K2SO4溶液和扫描速率10 mV·s-1时,IE-ab、IE-gr和IE-cn的循环伏安曲线
Fig.4 Cyclic voltammograms of IE-ab, IE-gr and IE-cn electrodes in 0.5 mol·L-1 K2SO4 at10 mV·s-1
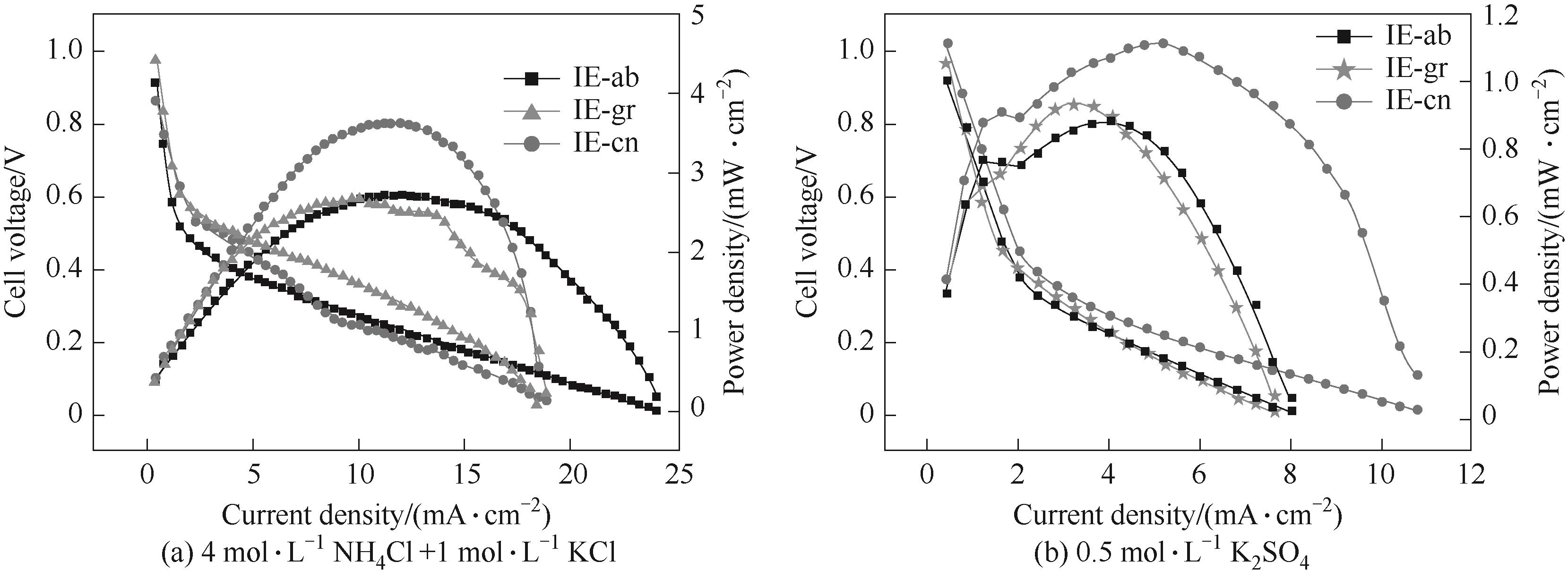
图5 在不同电解质溶液中,以不同碳材料为导电剂制备的铁-空气电池的极化曲线和功率密度曲线
Fig.5 Polarization and power density curves of iron-air batteries with different iron electrodes in 4 mol·L-1 NH4Cl+1 mol·L-1 KCl and 0.5 mol·L-1 K2SO4 electrolyte
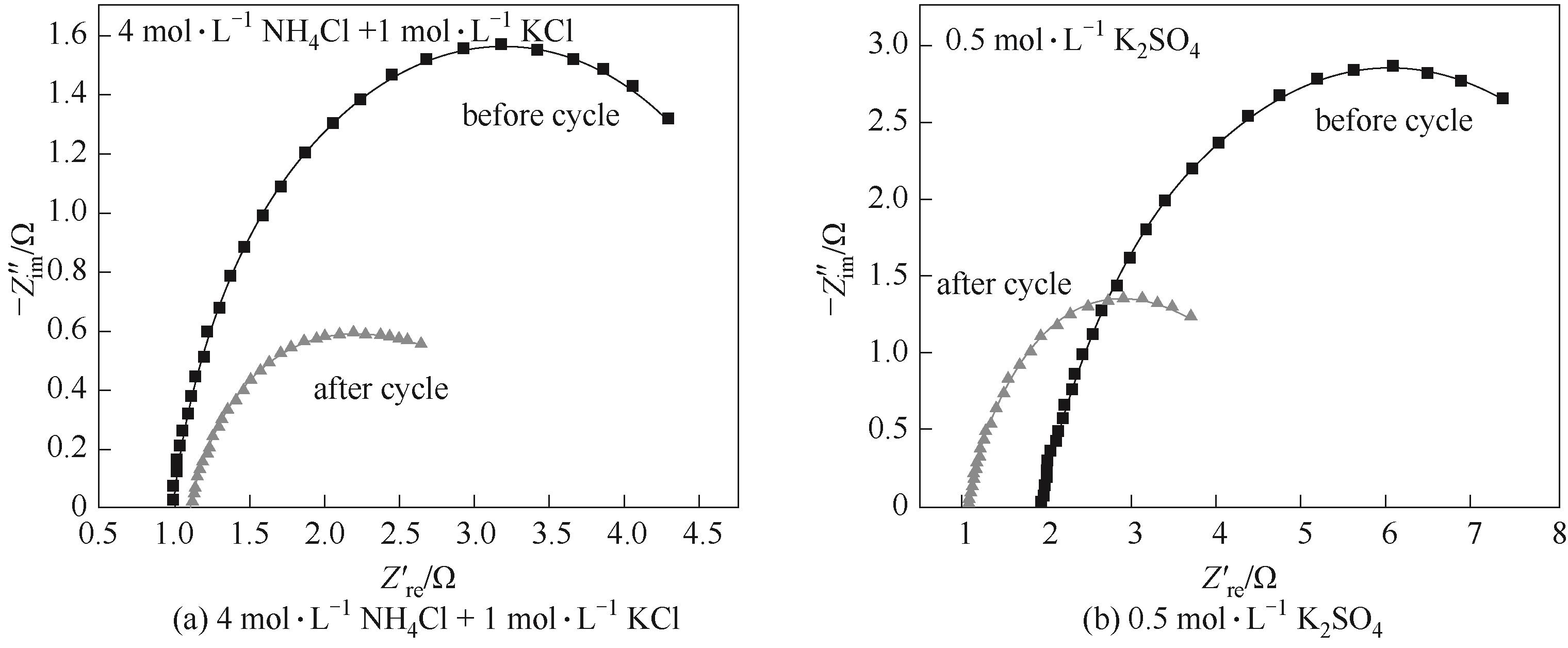
图6 在不同电解质溶液中,IE-cn电极在循环充放电10 h前后的交流阻抗谱
Fig.6 Electrochemical impedance spectra of IE-cn electrode before and after 10 h cycling charge/discharge in 4 mol·L-1 NH4Cl+1 mol·L-1 KCl and 0.5 mol·L-1 K2SO4 electrolyte
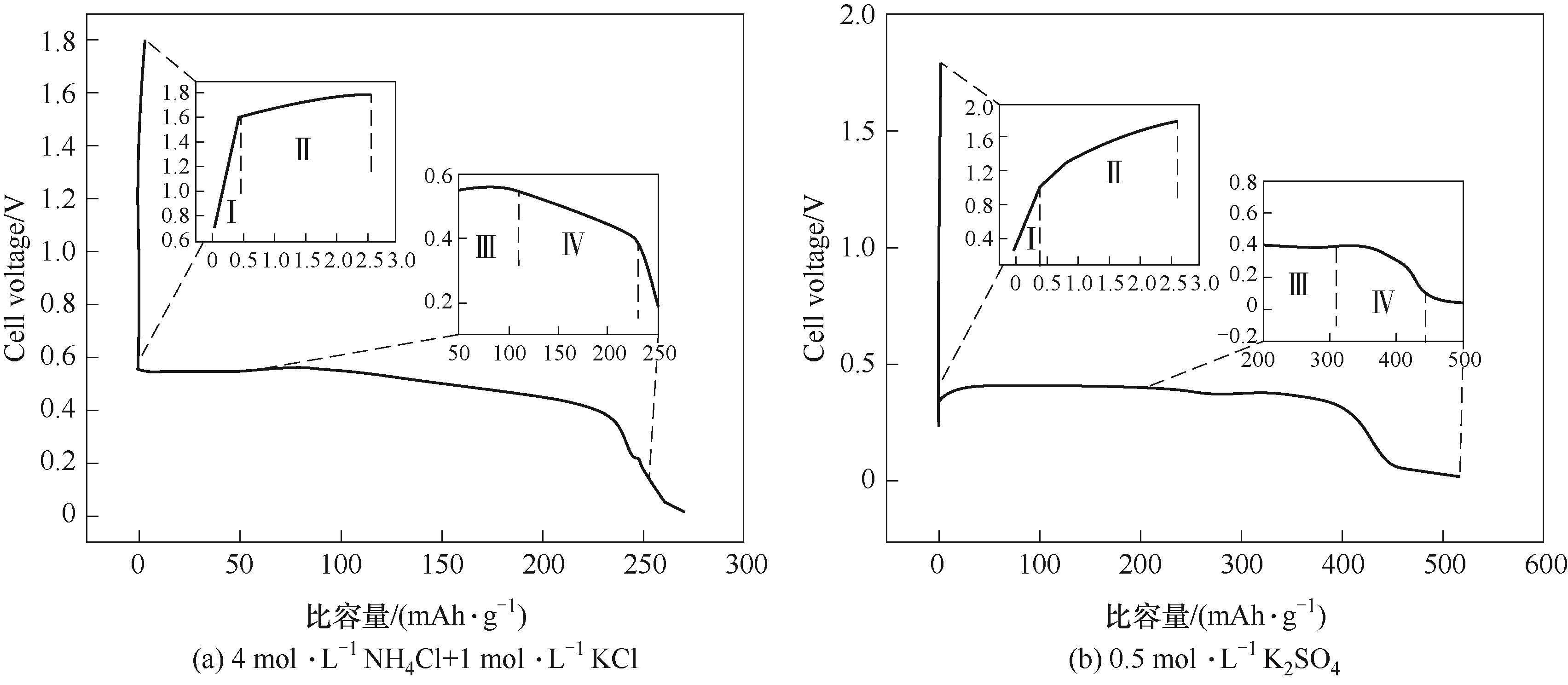
图7 IE-cn电极在不同电解质溶液中充电电流12 mA·cm-2、放电电流2 mA·cm-2时的充放电容量-电压曲线
Fig.7 Dependence of charge capacity at 12 mA·cm-2/discharge capacity at 2 mA·cm-2 upon voltage for IE-cn electrode in different electrolytes
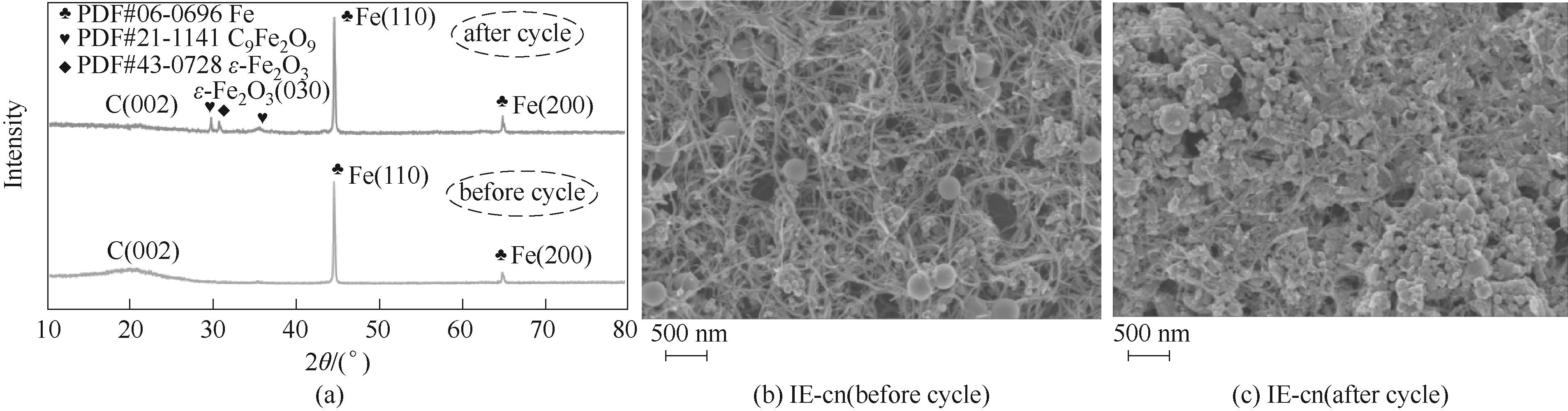
图8 在4 mol·L-1 NH4Cl+1 mol·L-1 KCl溶液中,IE-cn电极在循环充放电10 h前后的 XRD谱图(a)以及SEM图[(b), (c)]
Fig.8 X- ray diffraction spectra (a) and SEM images [(b), (c)] of IE-cn electrode before and after 10 h cycling charge/discharge in 4 mol·L-1 NH4Cl+1 mol·L-1 KCl electrolyte
| 1 | Villanueva-Martínez N I, Alegre C, Rubín J, et al. Investigation of the properties influencing the deactivation of iron electrodes in iron-air batteries[J]. Electrochimica Acta, 2023, 465: 142964. |
| 2 | Hang B T, Van Dang T, Van Quy N. Effect of the charging conditions on the cycle performance of Fe2O3/C composite anodes for iron-air batteries[J]. Journal of Electronic Materials, 2022, 51(5): 2168-2177. |
| 3 | Li Y G, Lu J. Metal-air batteries: will they be the future electrochemical energy storage device of choice?[J]. ACS Energy Letters, 2017, 2(6): 1370-1377. |
| 4 | 马洪运, 范永生, 王保国. 锌-空气电池电解液Zn2+浓度对析氢过程的影响 [J]. 化工学报, 2014, 65(7):2843-2848. |
| Ma H Y, Fan Y S, Wang B G. Effects of Zn2+ concentration upon hydrogen evolution reaction for zinc-air battery[J]. CIESC Journal, 2014, 65(7):2843-2848. | |
| 5 | Bui H T, Vu T M. Hydrothermal preparation of Fe2O3 nanoparticles for Fe-air battery anodes[J]. Journal of Electronic Materials, 2019, 48(11): 7123-7130. |
| 6 | Manohar A K, Malkhandi S, Yang B, et al. A high-performance rechargeable iron electrode for large-scale battery-based energy storage[J]. Journal of the Electrochemical Society, 2012, 159(8): A1209-A1214. |
| 7 | Öjefors L, Carlsson L. An iron—air vehicle battery[J]. Journal of Power Sources, 1978, 2(3): 287-296. |
| 8 | McKerracher R D, Ponce de Leon C, Wills R G A, et al. A review of the iron-air secondary battery for energy storage[J]. ChemPlusChem, 2015, 80(2): 323-335. |
| 9 | Cheng F Y, Chen J. Metal-air batteries: from oxygen reduction electrochemistry to cathode catalysts[J]. Chemical Society Reviews, 2012, 41(6): 2172-2192. |
| 10 | Egashira M, Kushizaki J Y, Yoshimoto N, et al. The effect of dispersion of nano-carbon on electrochemical behavior of Fe/nano-carbon composite electrode[J]. Journal of Power Sources, 2008, 183(1): 399-402. |
| 11 | Weinrich H, Come J, Tempel H, et al. Understanding the nanoscale redox-behavior of iron-anodes for rechargeable iron-air batteries[J]. Nano Energy, 2017, 41: 706-716. |
| 12 | Figueredo-Rodríguez H A, McKerracher R D, Insausti M, et al. A rechargeable, aqueous iron air battery with nanostructured electrodes capable of high energy density operation[J]. Journal of the Electrochemical Society, 2017, 164(6): A1148-A1157. |
| 13 | Malkhandi S, Yang B, Manohar A K, et al. Self-assembled monolayers of n-alkanethiols suppress hydrogen evolution and increase the efficiency of rechargeable iron battery electrodes[J]. Journal of the American Chemical Society, 2013, 135(1): 347-353. |
| 14 | McKerracher R D, Figueredo-Rodriguez H A, Alegre C, et al. Improving the stability and discharge capacity of nanostructured Fe2O3/C anodes for iron-air batteries and investigation of 1-octhanethiol as an electrolyte additive[J]. Electrochimica Acta, 2019, 318: 625-634. |
| 15 | 贾玉龙, 王菁, 桂裕鹏, 等. 导电剂梯度化分布对锂离子电池性能的影响[J]. 电源技术, 2023, 47(1): 37-40. |
| Jia Y L, Wang J, Gui Y P, et al. Influence of gradient distribution of conductive agent on performance of lithium ion battery[J]. Chinese Journal of Power Sources, 2023, 47(1): 37-40. | |
| 16 | 张琦钰, 高利军, 苏宇航, 等. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 47(7): 2753-2772. |
| Zhang Q Y, Gao L J, Su Y H, et al. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide[J]. CIESC Journal, 2023, 47(7): 2753-2772. | |
| 17 | Yu X W, Manthiram A. A voltage-enhanced, low-cost aqueous iron-air battery enabled with a mediator-ion solid electrolyte[J]. ACS Energy Letters, 2017, 2(5): 1050-1055. |
| 18 | Li L J, Manthiram A. Long-life, high-voltage acidic Zn-air batteries[J]. Advanced Energy Materials, 2016, 6(5): 1502054. |
| 19 | Lim H K, Lim H D, Park K Y, et al. Toward a lithium-“air” battery: the effect of CO2 on the chemistry of a lithium-oxygen cell[J]. Journal of the American Chemical Society, 2013, 135(26): 9733-9742. |
| 20 | Xu S M, Lau S, Archer L A. CO2 and ambient air in metal-oxygen batteries: steps towards reality[J]. Inorganic Chemistry Frontiers, 2015, 2(12): 1070-1079. |
| 21 | Chen A L, Yi Q F, Sheng K, et al. Mesoporous N-P codoped carbon nanosheets as superior cathodic catalysts of neutral metal-air batteries[J]. Langmuir, 2021, 37(43): 12616-12628. |
| 22 | Yu L, Yi Q F, Yang X K, et al. An easy synthesis of Ni-Co doped hollow C-N tubular nanocomposites as excellent cathodic catalysts of alkaline and neutral zinc-air batteries[J]. Science China Materials, 2019, 62(9): 1251-1264. |
| 23 | Yang X K, Yi Q F, Sheng K, et al. CoNi-doped C-N/CNT nanocomposites as cathodic catalysts of neutral Zn-air battery[J]. Ionics, 2019, 25(10): 4817-4830. |
| 24 | Trasatti S, Petrii O A. Real surface area measurements in electrochemistry[J]. Pure and Applied Chemistry, 1991, 63(5): 711-734. |
| 25 | Bu K, Wang J T. Topological states in the polymerized carbon nanotubes[J]. Physics Letters A, 2023, 480: 128936. |
| 26 | Arroyo-Gascón O, Fernández-Perea R, Morell E S, et al. Universality of moiré physics in collapsed chiral carbon nanotubes[J]. Carbon, 2023, 205: 394-401. |
| 27 | Deng X S, Kang N, Zhang Z Y. Carbon-based cryoelectronics: graphene and carbon nanotube[J]. Chip, 2023, 2(4): 100064. |
| 28 | Roy A, Gupta K K, Naskar S, et al. Compound influence of topological defects and heteroatomic inclusions on the mechanical properties of SWCNTs[J]. Materials Today Communications, 2021, 26: 102021. |
| 29 | Fang C, Tang X M, Wang J Y, et al. Performance of iron-air battery with iron nanoparticle-encapsulated C-N composite electrode[J]. Frontiers in Energy, 2023, 17(6): 0913. |
| 30 | Trinh T A, Bui T H. α-Fe2O3 urchins synthesized by a facile hydrothermal route as an anode for an Fe-air battery[J]. Journal of Materials Engineering and Performance, 2020, 29(2): 1245-1252. |
| 31 | Tan W K, Asami K, Maegawa K, et al. Formation of Fe-embedded graphitic carbon network composites as anode materials for rechargeable Fe-air batteries[J]. Energy Storage, 2020, 2(6): e196. |
| 32 | Frausto C, Avila-García A. Pyrrole-added Fe2O3 films by ultrasonic spray pyrolisis[J]. Superficies y VacíO, 2009, 22(4): 15-19. |
| [1] | 刘昌会, 肖桐, 刘庆祎, 耿龙, 赵佳腾. 多孔二氧化钛强化的相变材料储热机理研究[J]. 化工学报, 2024, 75(2): 706-714. |
| [2] | 曹宇, 张国辉, 高昂, 杜心宇, 周静, 蔡永茂, 余璇, 于晓明. 二维MXene材料在太阳能电池和金属离子电池中的研究进展[J]. 化工学报, 2024, 75(2): 412-428. |
| [3] | 闻文, 王慧艳, 周静红, 曹约强, 周兴贵. 石墨负极颗粒对锂离子电池容量衰减及SEI膜生长影响的模拟研究[J]. 化工学报, 2024, 75(1): 366-376. |
| [4] | 齐元帅, 彭文朝, 李阳, 张凤宝, 范晓彬. 电化学脱盐机理及相关研究进展[J]. 化工学报, 2024, 75(1): 171-189. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [7] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [8] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [9] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [10] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [11] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [12] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [15] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号