化工学报 ›› 2025, Vol. 76 ›› Issue (3): 963-974.DOI: 10.11949/0438-1157.20240631
肖俊兵1( ), 邹博1, 任建地1, 刘昌会2(
), 邹博1, 任建地1, 刘昌会2( ), 贾传坤1(
), 贾传坤1( )
)
收稿日期:2024-06-07
修回日期:2024-10-10
出版日期:2025-03-25
发布日期:2025-03-28
通讯作者:
刘昌会,贾传坤
作者简介:肖俊兵(1988—),男,博士,讲师,xjb1th@163.com
基金资助:
Junbing XIAO1( ), Bo ZOU1, Jiandi REN1, Changhui LIU2(
), Bo ZOU1, Jiandi REN1, Changhui LIU2( ), Chuankun JIA1(
), Chuankun JIA1( )
)
Received:2024-06-07
Revised:2024-10-10
Online:2025-03-25
Published:2025-03-28
Contact:
Changhui LIU, Chuankun JIA
摘要:
熔盐储热技术广泛应用于太阳能光热发电、电力调峰、可再生能源消纳等领域,其关键是熔盐。基于相图热力学计算设计NaCl-KCl-ZnCl2、NaCl-KCl-CaCl2熔盐,所设计NaCl-KCl-ZnCl2熔盐熔点比目前商用Solar Salt盐的熔点低了36.6℃,NaCl-KCl-CaCl2熔盐最高工作温度达747.5℃,超过下一代光热发电所需熔盐最低工作温度。结合红外成像技术与数字图像处理技术分析丝瓜络碳材料(CLSF)对NaCl-KCl-CaCl2熔盐瞬态热响应性能的影响。与三元熔盐相比,NaCl-KCl-CaCl2/CLSF复合熔盐熔化焓最大降幅为27.09%,热导率最大增幅为60.03%,相同温度范围内加热时间和冷却时间分别最大减少了62.50%和39.13%。CLSF在复合熔盐内部形成了高效导热通道,显著提升复合熔盐储热性能,未影响三元熔盐的相变行为和热稳定性。可见,NaCl-KCl-CaCl2/CLSF复合熔盐具有良好导热性能、热稳定性和储热性能,具有广阔的应用前景。
中图分类号:
肖俊兵, 邹博, 任建地, 刘昌会, 贾传坤. 基于相图分析的氯化物复合熔盐储热性能研究[J]. 化工学报, 2025, 76(3): 963-974.
Junbing XIAO, Bo ZOU, Jiandi REN, Changhui LIU, Chuankun JIA. Research on heat storage performance of chloride composite molten salt based on phase diagram analysis[J]. CIESC Journal, 2025, 76(3): 963-974.
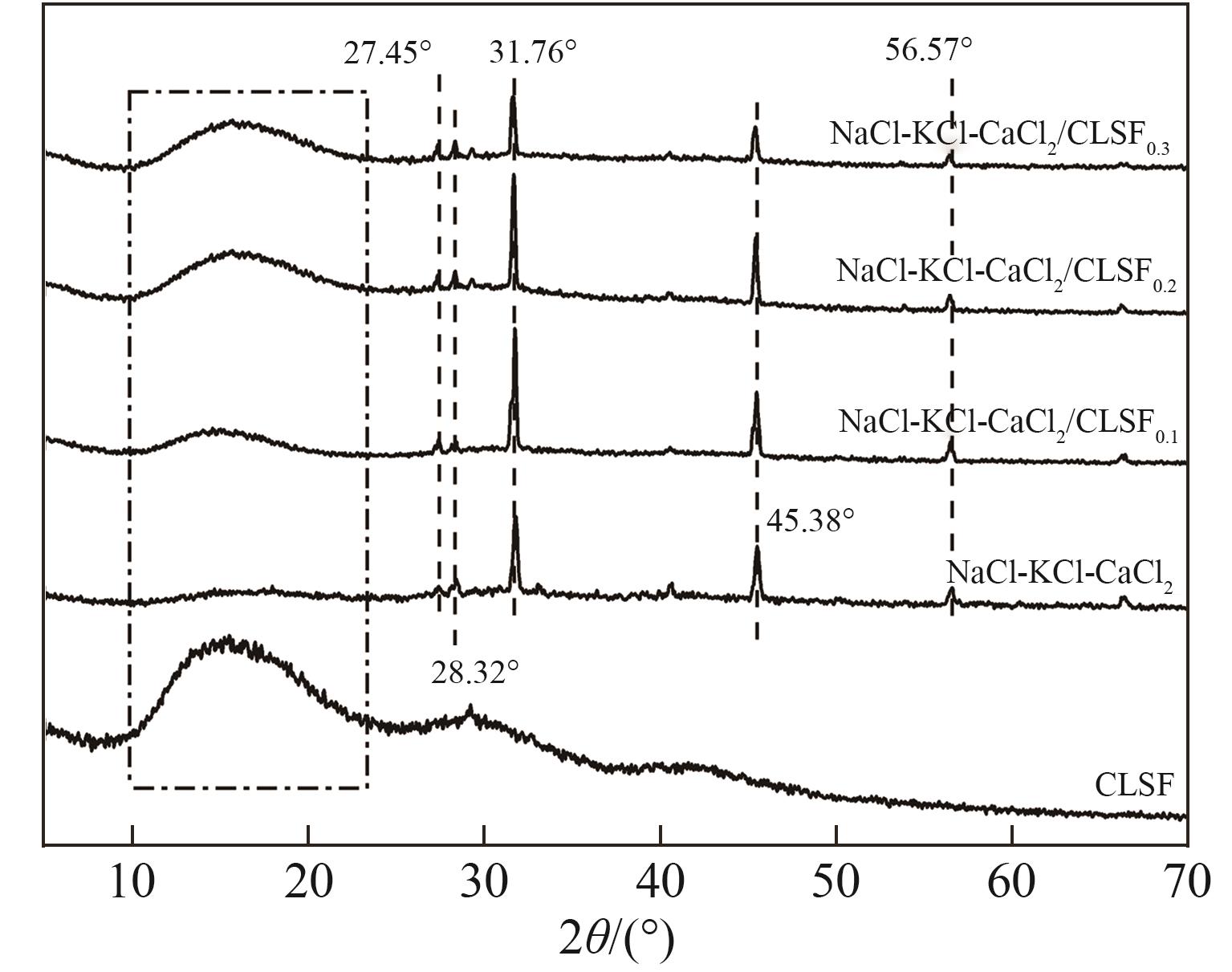
图5 NaCl-KCl-CaCl2熔盐及NaCl-KCl-CaCl2/CLSF复合熔盐的XRD谱图
Fig.5 XRD patterns of NaCl-KCl-CaCl2 ternary molten salt and NaCl-KCl-CaCl2/CLSF composite molten salt
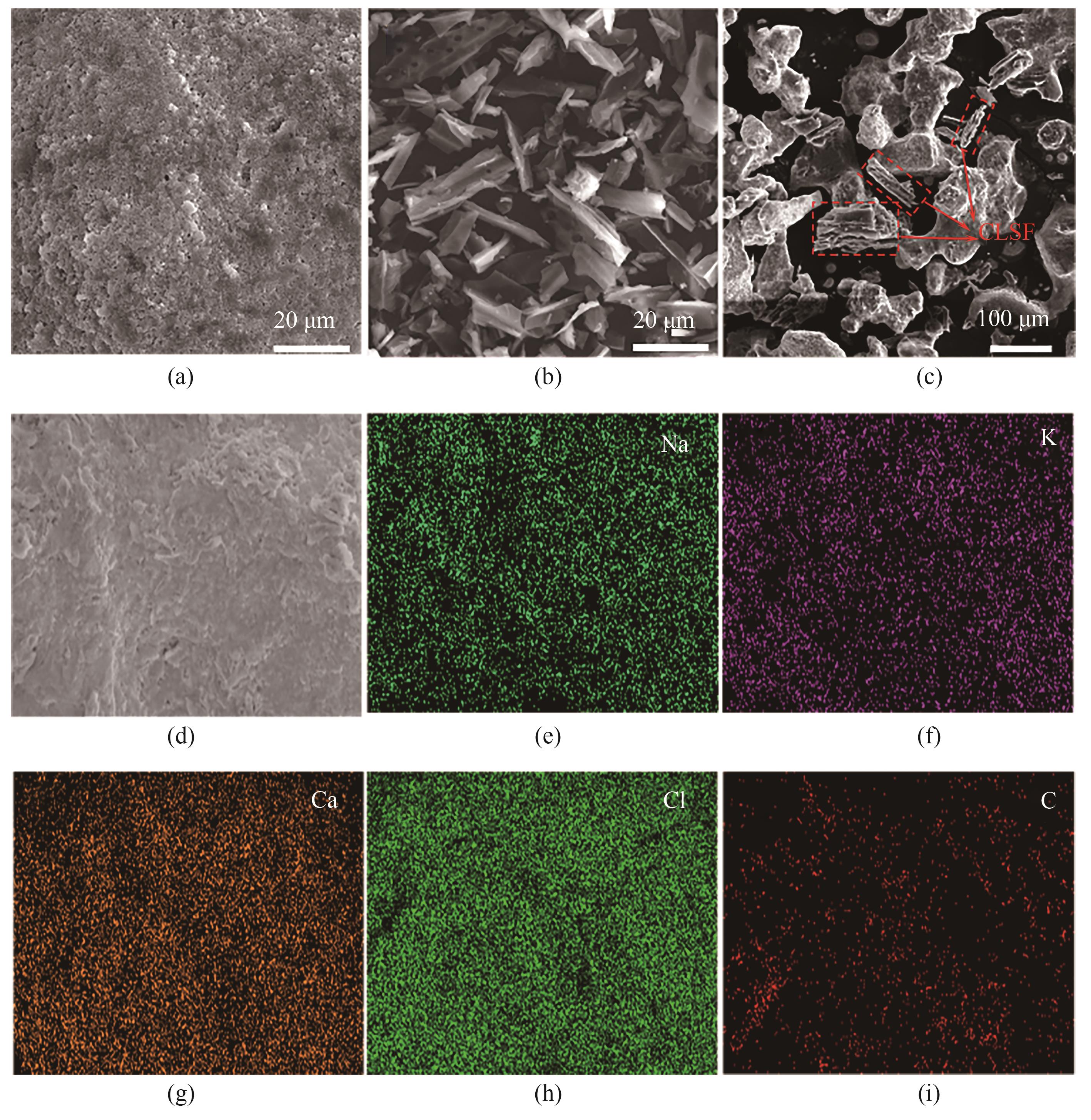
图6 微观形貌图像:(a)NaCl-KCl-CaCl2熔盐、(b)CLSF、(c)NaCl-KCl-CaCl2/CLSF0.3复合熔盐的SEM图;(d)~(i)NaCl-KCl-CaCl2/CLSF0.3复合熔盐的EDS能谱图
Fig.6 Microtopography images: SEM images of (a) NaCl-KCl-CaCl2 ternary molten salt, (b) CLSF, (c) NaCl-KCl-CaCl2/CLSF0.3 composite molten salt; (d)—(i) EDS energy spectra of NaCl-KCl-CaCl2/CLSF0.3 composite molten salt
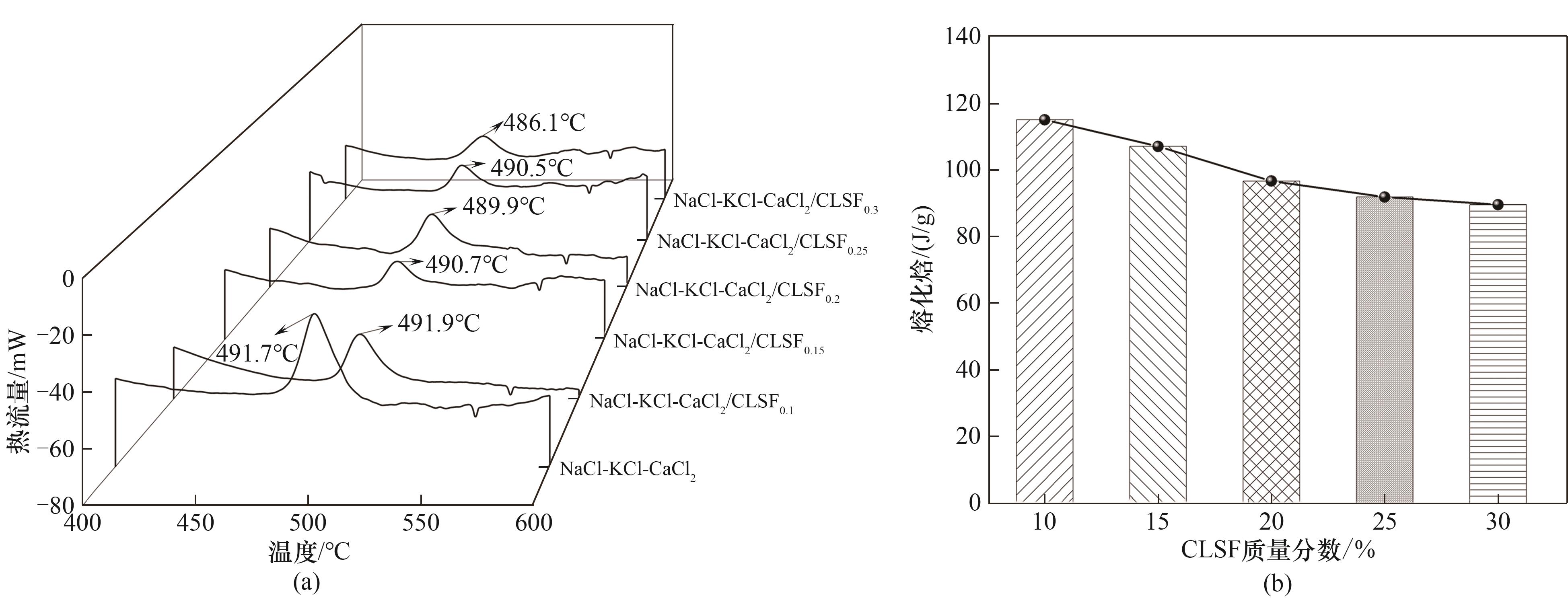
图7 熔化焓结果:(a) NaCl-KCl-CaCl2/CLSF复合熔盐的DSC曲线;(b) 复合熔盐熔化焓随CLSF质量分数的变化
Fig.7 Result of melting enthalpy: (a) DSC curves of NaCl-KCl-CaCl2/CLSF composite molten salts; (b) variation of melting enthalpy of composite molten salts with CLSF mass fraction
| 样品 | 熔点/℃ | 熔化焓测量值/(J/g) | 熔化焓计算值/(J/g) | 偏差/% |
|---|---|---|---|---|
| NaCl-KCl-CaCl2 | 491.7 | 132.5 | 132.5 | — |
| NaCl-KCl-CaCl2/CLSF0.1 | 491.9 | 115.0 | 119.3 | 3.6 |
| NaCl-KCl-CaCl2/CLSF0.15 | 490.7 | 107.0 | 112.6 | 5.0 |
| NaCl-KCl-CaCl2/CLSF0.2 | 489.9 | 96.6 | 106 | 8.9 |
| NaCl-KCl-CaCl2/CLSF0.25 | 490.5 | 91.8 | 99.4 | 7.6 |
| NaCl-KCl-CaCl2/CLSF0.3 | 486.1 | 89.5 | 92.8 | 3.4 |
表1 纯盐和复合材料的熔点和熔化焓
Table 1 Melting point and melting enthalpy of pure salts and composites
| 样品 | 熔点/℃ | 熔化焓测量值/(J/g) | 熔化焓计算值/(J/g) | 偏差/% |
|---|---|---|---|---|
| NaCl-KCl-CaCl2 | 491.7 | 132.5 | 132.5 | — |
| NaCl-KCl-CaCl2/CLSF0.1 | 491.9 | 115.0 | 119.3 | 3.6 |
| NaCl-KCl-CaCl2/CLSF0.15 | 490.7 | 107.0 | 112.6 | 5.0 |
| NaCl-KCl-CaCl2/CLSF0.2 | 489.9 | 96.6 | 106 | 8.9 |
| NaCl-KCl-CaCl2/CLSF0.25 | 490.5 | 91.8 | 99.4 | 7.6 |
| NaCl-KCl-CaCl2/CLSF0.3 | 486.1 | 89.5 | 92.8 | 3.4 |
| 样品组成 | 分解温度/℃ | 质量损失/% |
|---|---|---|
| NaCl-KCl-CaCl2 | 747.50 | 1.0 |
| NaCl-KCl-CaCl2/CLSF0.1 | 745.23 | 1.6 |
| NaCl-KCl-CaCl2/CLSF0.15 | 742.37 | 1.2 |
| NaCl-KCl-CaCl2/CLSF0.2 | 748.41 | 1.4 |
| NaCl-KCl-CaCl2/CLSF0.25 | 742.49 | 1.8 |
| NaCl-KCl-CaCl2/CLSF0.3 | 746.89 | 2.1 |
表2 NaCl-KCl-CaCl2/CLSF复合熔盐的TGA结果
Table 2 TGA results of NaCl-KCl-CaCl2/CLSF composites
| 样品组成 | 分解温度/℃ | 质量损失/% |
|---|---|---|
| NaCl-KCl-CaCl2 | 747.50 | 1.0 |
| NaCl-KCl-CaCl2/CLSF0.1 | 745.23 | 1.6 |
| NaCl-KCl-CaCl2/CLSF0.15 | 742.37 | 1.2 |
| NaCl-KCl-CaCl2/CLSF0.2 | 748.41 | 1.4 |
| NaCl-KCl-CaCl2/CLSF0.25 | 742.49 | 1.8 |
| NaCl-KCl-CaCl2/CLSF0.3 | 746.89 | 2.1 |
| 复合材料 | 添加剂质量分数/% | 热导率/(W/(m·K)) | 热导率增加分数/% | 文献 |
|---|---|---|---|---|
| NaCl-KCl-MgCl2/Al2O3 | 0.7 | — | 62.59 | [ |
| NaNO3-NaNO2-KNO2-LiNO3/MgO | 40 | 0.41 | — | [ |
| NaCl-KCl-MgCl2/CuO | 0.2 | 0.340 | 15.85 | [ |
| 0.7 | 0.385 | 31.40 | ||
| 2 | 0.409 | 39.55 | ||
| NaCl-KCl-LiCl/CuO | 1 | 0.483 | 3.20 | [ |
| 3 | 0.529 | 13.03 | ||
| 5 | 0.571 | 22.01 | ||
| NaNO3-KNO3/MgO | 0.125 | 0.851 | 9.24 | [ |
| 0.25 | 0.883 | 13.36 | ||
| 0.5 | 0.853 | 9.57 | ||
| 1 | 0.858 | 10.19 | ||
| 2 | 0.872 | 11.99 | ||
| NaNO3/SiC | 20 | 1.16 | 50 | [ |
| LiNO3-NaNO3-KNO3-Ca(NO3)2/CaSiO3 | 20 | 1.177 | — | [ |
| NaCl-Na2CO3-Na2SO4/graphene nanoplatelets | 0.2 | 1.21 | 11.53 | [ |
| 0.5 | 1.13 | 3.69 | ||
| 1 | 1.38 | 27.02 | ||
| 2 | 1.44 | 32.02 | ||
| NaNO3-KNO3/EG | 20 | — | 71.31 | [ |
| lauric acid/modified BN | 21.8 | 0.563 | 124.30 | [ |
| diglycidyl ether of bisphenol A/BN | 20 | 0.61 | — | [ |
| polyimide/BN | 30 | 0.696 | — | [ |
| poly (tetradecyl acrylate)/BN | 5 | 0.33 | 13.7 | [ |
| 10 | 0.41 | 41.4 | ||
| 15 | 0.46 | 58.6 | ||
| 20 | 0.62 | 113.8 | ||
| NaCl-KCl-CaCl2/CLSF | 10 | 0.3611 | 2.73 | 本文 |
| 15 | 0.3937 | 12.01 | ||
| 20 | 0.4207 | 19.69 | ||
| 25 | 0.4603 | 30.95 | ||
| 30 | 0.5625 | 60.03 |
表3 不同类型添加剂对复合材料导热性能的影响
Table 3 Effect of different types of additives on thermal conductivity of composite materials
| 复合材料 | 添加剂质量分数/% | 热导率/(W/(m·K)) | 热导率增加分数/% | 文献 |
|---|---|---|---|---|
| NaCl-KCl-MgCl2/Al2O3 | 0.7 | — | 62.59 | [ |
| NaNO3-NaNO2-KNO2-LiNO3/MgO | 40 | 0.41 | — | [ |
| NaCl-KCl-MgCl2/CuO | 0.2 | 0.340 | 15.85 | [ |
| 0.7 | 0.385 | 31.40 | ||
| 2 | 0.409 | 39.55 | ||
| NaCl-KCl-LiCl/CuO | 1 | 0.483 | 3.20 | [ |
| 3 | 0.529 | 13.03 | ||
| 5 | 0.571 | 22.01 | ||
| NaNO3-KNO3/MgO | 0.125 | 0.851 | 9.24 | [ |
| 0.25 | 0.883 | 13.36 | ||
| 0.5 | 0.853 | 9.57 | ||
| 1 | 0.858 | 10.19 | ||
| 2 | 0.872 | 11.99 | ||
| NaNO3/SiC | 20 | 1.16 | 50 | [ |
| LiNO3-NaNO3-KNO3-Ca(NO3)2/CaSiO3 | 20 | 1.177 | — | [ |
| NaCl-Na2CO3-Na2SO4/graphene nanoplatelets | 0.2 | 1.21 | 11.53 | [ |
| 0.5 | 1.13 | 3.69 | ||
| 1 | 1.38 | 27.02 | ||
| 2 | 1.44 | 32.02 | ||
| NaNO3-KNO3/EG | 20 | — | 71.31 | [ |
| lauric acid/modified BN | 21.8 | 0.563 | 124.30 | [ |
| diglycidyl ether of bisphenol A/BN | 20 | 0.61 | — | [ |
| polyimide/BN | 30 | 0.696 | — | [ |
| poly (tetradecyl acrylate)/BN | 5 | 0.33 | 13.7 | [ |
| 10 | 0.41 | 41.4 | ||
| 15 | 0.46 | 58.6 | ||
| 20 | 0.62 | 113.8 | ||
| NaCl-KCl-CaCl2/CLSF | 10 | 0.3611 | 2.73 | 本文 |
| 15 | 0.3937 | 12.01 | ||
| 20 | 0.4207 | 19.69 | ||
| 25 | 0.4603 | 30.95 | ||
| 30 | 0.5625 | 60.03 |
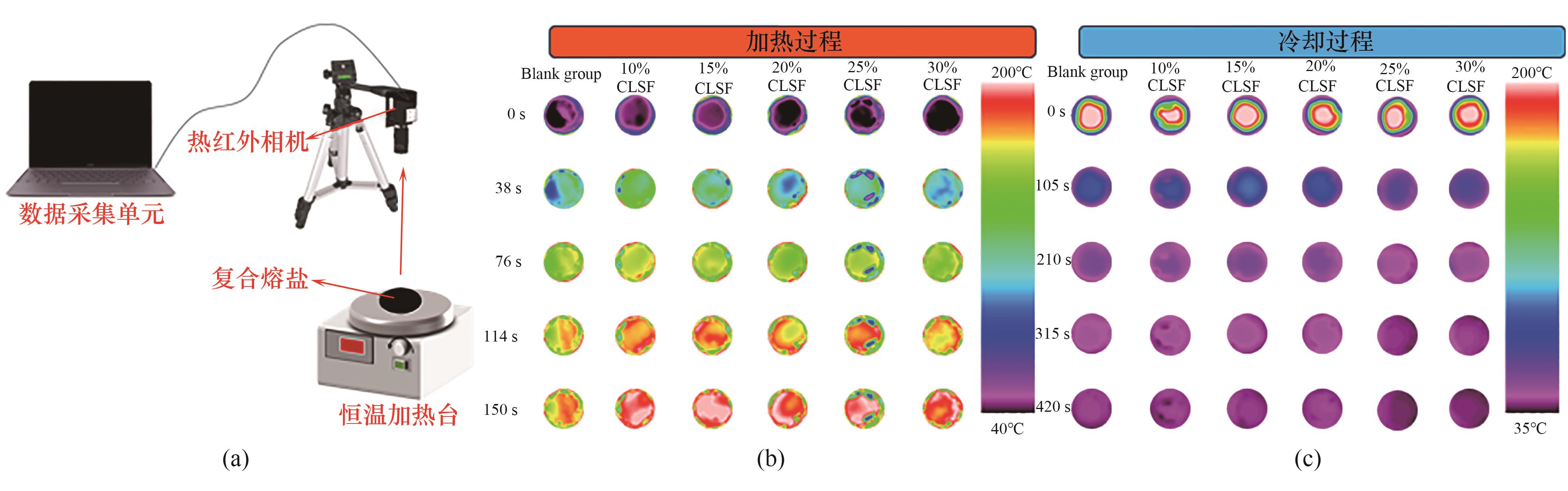
图10 瞬态热响应性能分析:(a)热响应性能测量平台示意图;(b)加热和(c)冷却过程中样品的红外热像图
Fig.10 Transient thermal response performance analysis: (a) schematic diagram of the thermal response performance measurement platform; infrared thermograms of the sample during (b) heating and (c) cooling processes
| 1 | Lewis N S. Research opportunities to advance solar energy utilization[J]. Science, 2016, 351(6271): aad1920. |
| 2 | 郭学伯, 范良迟, 许浈婧, 等. 助力节能降碳的相变储热材料研究和应用进展[J]. 发电技术, 2023, 44(2): 201-212. |
| Guo X B, Fan L C, Xu Z J, et al. Research and application progress of phase change thermal energy storage materials for energy saving and carbon reduction[J]. Power Generation Technology, 2023, 44(2): 201-212. | |
| 3 | Skrbek K, Bartůněk V, Sedmidubský D. Molten salt-based nanocomposites for thermal energy storage: materials, preparation techniques and properties[J]. Renewable and Sustainable Energy Reviews, 2022, 164: 112548. |
| 4 | Xiao X, Jia H W, Wen D S, et al. Thermal performance analysis of a solar energy storage unit encapsulated with HITEC salt/copper foam/nanoparticles composite[J]. Energy, 2020, 192: 116593. |
| 5 | Ding W J, Bauer T. Progress in research and development of molten chloride salt technology for next generation concentrated solar power plants[J]. Engineering, 2021, 7(3): 334-347. |
| 6 | 魏小兰, 谢佩, 张雪钏, 等. 氯化物熔盐材料的制备及其热物理性质研究[J]. 化工学报, 2020, 71(5): 2423-2431. |
| Wei X L, Xie P, Zhang X C, et al. Research on preparation and thermodynamic properties of chloride molten salt materials[J]. CIESC Journal, 2020, 71(5): 2423-2431. | |
| 7 | Wu S, Peng H, Xie L D. Design and investigation of the novel ZnCl2 based ternary chloride salts with low-temperature for sensible energy storage[J]. Applied Thermal Engineering, 2020, 171: 114917. |
| 8 | 涂易, 王文磊, 王军涛, 等. 高温熔盐储能材料的模型计算方法研究[J]. 材料导报, 2014, 28(6): 136-140, 148. |
| Tu Y, Wang W L, Wang J T, et al. Study on model calculation methods of high temperature fused salt energy storage material[J]. Materials Review, 2014, 28(6): 136-140, 148. | |
| 9 | Xu X K, Dehghani G, Ning J X, et al. Basic properties of eutectic chloride salts NaCl-KCl-ZnCl2 and NaCl-KCl-MgCl2 as HTFs and thermal storage media measured using simultaneous DSC-TGA[J]. Solar Energy, 2018, 162: 431-441. |
| 10 | Bauer T, Odenthal C, Bonk A. Molten salt storage for power generation[J]. Chemie Ingenieur Technik, 2021, 93(4): 534-546. |
| 11 | Han D M, Lougou B G, Shuai Y, et al. Study of thermophysical properties of chloride salts doped with CuO nanoparticles for solar thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2022, 234: 111432. |
| 12 | Wang Q, Wu C L, Sun S P, et al. Comprehensive performance of composite phase change materials based on ternary eutectic chloride with CuO nanoparticles for thermal energy storage systems[J]. Solar Energy, 2023, 250: 324-334. |
| 13 | Liu J W, Wang Q H, Ling Z Y, et al. A novel process for preparing molten salt/expanded graphite composite phase change blocks with good uniformity and small volume expansion[J]. Solar Energy Materials and Solar Cells, 2017, 169: 280-286. |
| 14 | Tian H Q, Wang W L, Ding J, et al. Thermal conductivities and characteristics of ternary eutectic chloride/expanded graphite thermal energy storage composites[J]. Applied Energy, 2015, 148: 87-92. |
| 15 | Xiao J B, Zou B, Liu C H, et al. Carbonized loofah sponge fragments enhanced phase change thermal energy storage: preparation and thermophysical property analysis[J]. Applied Thermal Engineering, 2024, 242: 122505. |
| 16 | Xiao J B, Zou B, Zhong F F, et al. Phase change energy storage using boron nitride/carbonized loofah sponge[J]. Applied Thermal Engineering, 2024, 257: 124182. |
| 17 | Su H X, Guo X, Chen G L, et al. A novel honeycomb-like porous carbon from loofah sponge for form-stable phase change materials with high encapsulation capacity and reliability[J]. Materials Letters, 2022, 308: 131118. |
| 18 | Song J Y, He H F, Wang Y B, et al. Shape-stabilized phase change composites supported by biomass loofah sponge-derived microtubular carbon scaffold toward thermal energy storage and electric-to-thermal conversion[J]. Journal of Energy Storage, 2022, 56: 105891. |
| 19 | Tian H Q, Wang W L, Ding J, et al. Thermal performance and economic evaluation of NaCl-CaCl2 eutectic salt for high-temperature thermal energy storage[J]. Energy, 2021, 227: 120412. |
| 20 | Yin H Q, Wang Z R, Lai X, et al. Optimum design and key thermal property of NaCl-KCl-CaCl2 eutectic salt for ultra-high-temperature thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2022, 236: 111541. |
| 21 | Sang L X, Lv X Y, Wu Y T. NaNO3-KNO3-KCl/K2CO3 with the elevated working temperature for CSP application: phase diagram calculation and machine learning[J]. Solar Energy, 2023, 252: 322-329. |
| 22 | Ren N, Wu Y T, Ma C F, et al. Preparation and thermal properties of quaternary mixed nitrate with low melting point[J]. Solar Energy Materials and Solar Cells, 2014, 127: 6-13. |
| 23 | Mohan G, Venkataraman M, Gomez-Vidal J, et al. Assessment of a novel ternary eutectic chloride salt for next generation high-temperature sensible heat storage[J]. Energy Conversion and Management, 2018, 167: 156-164. |
| 24 | Li J Q, Qin Y, Shen J, et al. Evolution of carbon nanostructures during coal graphitization: insights from X-ray diffraction and high-resolution transmission electron microscopy[J]. Energy, 2024, 290: 130316. |
| 25 | Yan C, Liang J F, Zhong X B, et al. BN white graphene well-dispersed solar salt nanofluids with significant improved thermal properties for concentrated solar power plants[J]. Solar Energy Materials and Solar Cells, 2022, 245: 111875. |
| 26 | Kang Z Y, Huang S H, Liu X Y, et al. A novel high temperature eutectic salt and its composite with enhanced high conductivity[J]. Journal of Energy Storage, 2023, 59: 106409. |
| 27 | Zhang T Y, Wang T Y, Wang K C, et al. Development and characterization of NaCl-KCl/Kaolin composites for thermal energy storage[J]. Solar Energy, 2021, 227: 468-476. |
| 28 | Han D M, Guene Lougou B, Xu Y T, et al. Thermal properties characterization of chloride salts/nanoparticles composite phase change material for high-temperature thermal energy storage[J]. Applied Energy, 2020, 264: 114674. |
| 29 | 许荣玉, 陆海涛, 郭荷渡, 等. 低熔点四元硝酸盐基定型复合相变材料的制备与研究[J]. 储能科学与技术, 2024, 13(5): 1451-1459. |
| Xu R Y, Lu H T, Guo H D, et al. Form-stable quaternary nitrate salt-based composite phase change material with low melting temperature for low-mediumtemperature thermal energy storage[J]. Energy Storage Science and Technology, 2024, 13(5): 1451-1459. | |
| 30 | Saranprabhu M K, Rajan K S. Magnesium oxide nanoparticles dispersed solar salt with improved solid phase thermal conductivity and specific heat for latent heat thermal energy storage[J]. Renewable Energy, 2019, 141: 451-459. |
| 31 | Jiang F, Ling X, Zhang L L, et al. Improved thermal conductivity of form-stable NaNO3: using the skeleton of porous ceramic modified by SiC[J]. Solar Energy Materials and Solar Cells, 2021, 231: 111310. |
| 32 | Jiang Z, Leng G H, Ye F, et al. Form-stable LiNO3-NaNO3-KNO3-Ca(NO3)2/calcium silicate composite phase change material (PCM) for mid-low temperature thermal energy storage[J]. Energy Conversion and Management, 2015, 106: 165-172. |
| 33 | Liu X Y, Kang Z Y, Zhao J H, et al. Preparation and thermal property characterization of NaCl-Na2CO3-Na2SO4 eutectic salt mixed with carbon nanomaterials for heat storage[J]. Solar Energy Materials and Solar Cells, 2023, 251: 112173. |
| 34 | Yu Q, Lu Y W, Zhang C C, et al. Preparation and thermal properties of novel eutectic salt/nano-SiO2/expanded graphite composite for thermal energy storage[J]. Solar Energy Materials and Solar Cells, 2020, 215: 110590. |
| 35 | Wang X, Cheng Q J, Wu M M, et al. Thermal properties optimization of lauric acid as phase change material with modified boron nitride nanosheets-sodium sulfate for thermal energy storage[J]. Journal of Energy Storage, 2023, 61: 106781. |
| 36 | Isarn I, Massagués L, Ramis X, et al. New BN-epoxy composites obtained by thermal latent cationic curing with enhanced thermal conductivity[J]. Composites Part A: Applied Science and Manufacturing, 2017, 103: 35-47. |
| 37 | Gu J W, Lv Z Y, Wu Y L, et al. Dielectric thermally conductive boron nitride/polyimide composites with outstanding thermal stabilities via in situ polymerization-electrospinning-hot press method[J]. Composites Part A: Applied Science and Manufacturing, 2017, 94: 209-216. |
| 38 | Li S Q, Wang H X, Mao H Q, et al. Enhanced thermal management performance of comb-like polymer/boron nitride composite phase change materials for the thermoregulated fabric application[J]. Journal of Energy Storage, 2021, 40: 102826. |
| [1] | 李远华, 凌思棋, 封科军, 冯颖, 郭于菁, 谢世桓. 基于cMOFs的固定化脂肪酶微反应器的构筑及其扁桃酸催化应用[J]. 化工学报, 2025, 76(3): 1170-1179. |
| [2] | 张亦鸣, 杨鹏, 纪献兵, 任纪星, 张磊, 苗政. 多回路平板式环路热管热性能[J]. 化工学报, 2025, 76(3): 1018-1028. |
| [3] | 张履胜, 王治红, 柳青, 李雪雯, 谭仁敏. 液-液相变吸收剂捕集二氧化碳研究进展[J]. 化工学报, 2025, 76(3): 933-950. |
| [4] | 高波, 王佳琪, 刘志亮, 赵玄烈, 葛坤. 海上风电制氢系统建模及热力学与经济学分析[J]. 化工学报, 2025, 76(3): 1207-1220. |
| [5] | 肖俊兵, 钟湘宇, 任建地, 钟芳芳, 刘昌会, 贾传坤. 基于生物碳材料强化的硬脂酸相变材料储热性能研究[J]. 化工学报, 2025, 76(3): 1312-1322. |
| [6] | 刘彦贝, 王若名, 刘娟, Raza Taimoor, 陆玉正, Raza Rizwan, 朱斌, 李松波, 安胜利, 云斯宁. CeO2@La0.6Sr0.4Co0.2Fe0.8O3-δ 电解质的制备及半导体离子燃料电池性能研究[J]. 化工学报, 2025, 76(3): 1353-1362. |
| [7] | 张先开, 王博宇, 郭亚丽, 沈胜强. 水平圆管降膜蒸发式冷凝器热力性能计算分析[J]. 化工学报, 2025, 76(3): 995-1005. |
| [8] | 李文宝, 胡锦鹏, 杜淼, 潘鹏举, 单国荣. 强韧P(SBMA-co-AAc)/SiO2复合水凝胶海洋防污减阻涂层[J]. 化工学报, 2025, 76(2): 787-796. |
| [9] | 黄鑫, 李逸龙, 李卫东, 施鸿翔, 尹鹏博, 李臻超, 滕霖, 江莉龙. 液氨-成品油混合体系相平衡及减压相变规律研究[J]. 化工学报, 2025, 76(1): 71-80. |
| [10] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [11] | 唐溯, 郑子鏖, 魏翰泽, 许晓玲, 翟晓强. PMMA/PEG600/CNT复合定型相变材料制备与导热强化[J]. 化工学报, 2024, 75(S1): 309-320. |
| [12] | 汪张洲, 唐天琪, 夏嘉俊, 何玉荣. 基于复合相变材料的电池热管理性能模拟[J]. 化工学报, 2024, 75(S1): 329-338. |
| [13] | 秦思宇, 刘艺佳, 杨佳成, 佟薇, 金立文, 孟祥兆. 受限蒸汽腔内气液两相传热特性研究[J]. 化工学报, 2024, 75(S1): 47-55. |
| [14] | 杜得辉, 冯威, 张江辉, 项燕龙, 乔高攀, 李蔚. 微型翅片疏水复合强化管管内流动沸腾换热预测模型[J]. 化工学报, 2024, 75(S1): 95-107. |
| [15] | 吴学红, 韦新, 侯加文, 吕财, 刘勇, 刘鹤, 常志娟. 热解法制备碳纳米管及其在散热涂层中的应用研究[J]. 化工学报, 2024, 75(9): 3360-3368. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号