化工学报 ›› 2025, Vol. 76 ›› Issue (11): 6018-6026.DOI: 10.11949/0438-1157.20250262
• 能源和环境工程 • 上一篇
收稿日期:2025-03-17
修回日期:2025-06-25
出版日期:2025-11-25
发布日期:2025-12-19
通讯作者:
魏进家
作者简介:刘辉(1998—),男,博士研究生,lh3041594963@stu.xjtu.edu.cn
基金资助:Received:2025-03-17
Revised:2025-06-25
Online:2025-11-25
Published:2025-12-19
Contact:
Jinjia WEI
摘要:
使用安全无污染前体,通过改进的溶胶-凝胶法合成了Mn、Al共掺杂钙基储能材料,对材料的各项储能性能进行了研究,并考察了初步放大合成对材料性能的影响。结果表明,乙酸钙前体合成的共掺杂材料Ca100Mn15Al10-Ac在1000次储能循环中表现出优异的稳定性,性能仅衰减23.2%,且主要集中在前期,其平均光吸收率也达到70.1%,通过形貌分析发现,共掺杂材料在循环过程中演化出的稳定多孔结构有效抑制性能衰减。材料的初步放大合成以及添加微晶纤维素黏结剂不会对Ca100Mn15Al10-Ac的稳定性造成负面影响,有利于后续大规模制备。
中图分类号:
刘辉, 魏进家. Mn/Al改性的高稳定性钙基储能材料[J]. 化工学报, 2025, 76(11): 6018-6026.
Hui LIU, Jinjia WEI. Mn/Al modified calcium-based energy storage materials with high stability[J]. CIESC Journal, 2025, 76(11): 6018-6026.
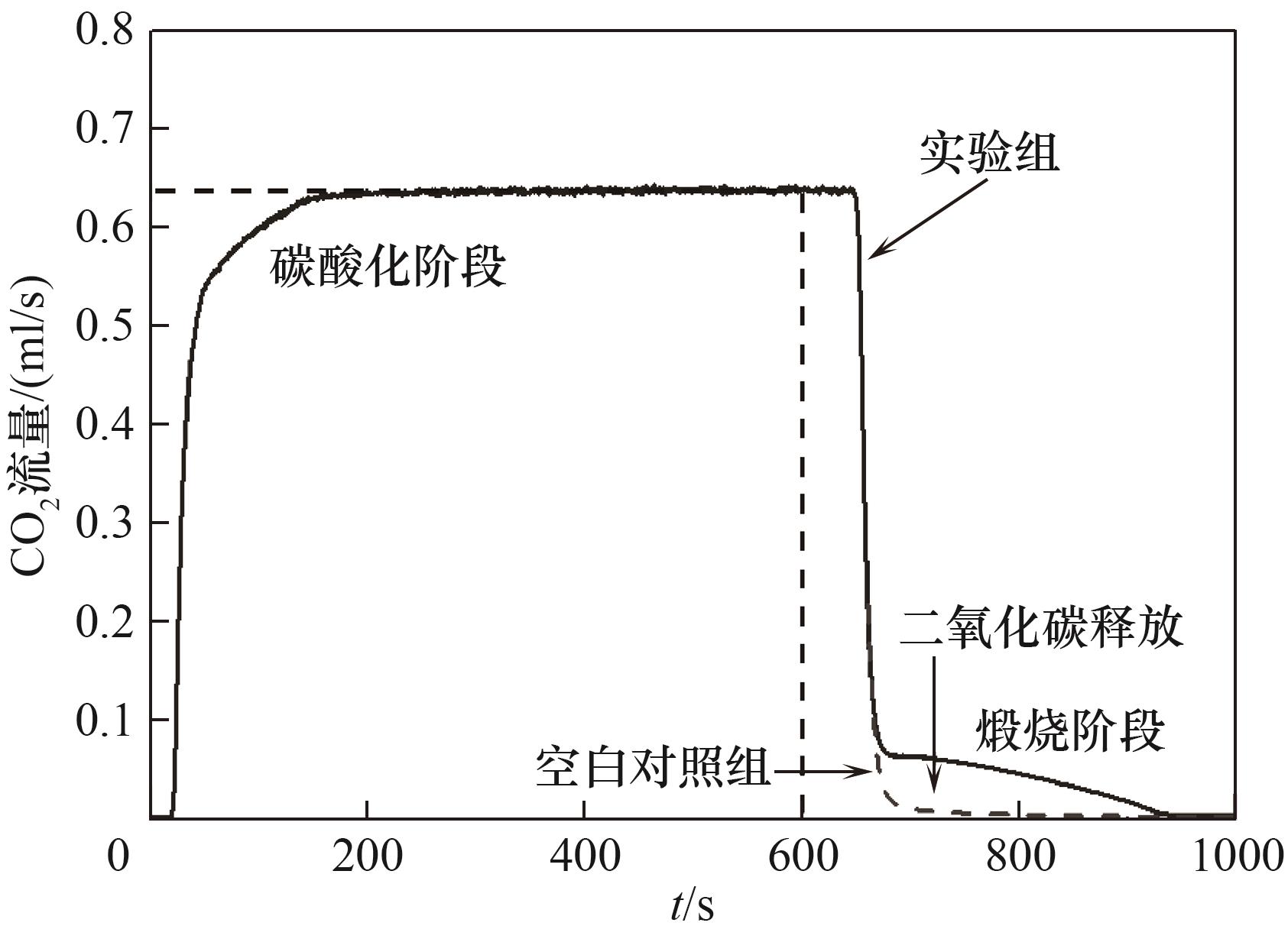
图2 循环中二氧化碳流量曲线(围成的面积为煅烧阶段的二氧化碳释放量)
Fig.2 CO2 flow rate in one energy storage cycle (the enclosed area is equal to the volume of carbon dioxide released in calcination process)
| 掺杂钙基材料 | 碳酸化温度/℃ | 碳酸化时间/min | 首次储能密度/(kJ/kg) | 循环次数/衰减率 | 文献 |
|---|---|---|---|---|---|
| CaCO3/Fe,Mn | 700 | 10 | 1500 | 60/3.3% | [ |
| CaO/Al,Mn,Fe,Li | 725 | 20 | 1746 | 60/4.26% | [ |
| CaCO3/Ti,Al,Mg | 750 | 10 | 1157 | 100/7% | [ |
| CaO/Mn,Mg | 800 | 10 | 1604 | 100/6.23% | [ |
| CaO/Al | 850 | 10 | 1496 | 100/32% | [ |
| CaO/Mn,Al | 800 | 10 | 1245.7 | 1000/23.2% | this study |
表1 各种用于储能的改性钙基材料对比
Table 1 Comparison of various modified calcium-based materials for energy storage
| 掺杂钙基材料 | 碳酸化温度/℃ | 碳酸化时间/min | 首次储能密度/(kJ/kg) | 循环次数/衰减率 | 文献 |
|---|---|---|---|---|---|
| CaCO3/Fe,Mn | 700 | 10 | 1500 | 60/3.3% | [ |
| CaO/Al,Mn,Fe,Li | 725 | 20 | 1746 | 60/4.26% | [ |
| CaCO3/Ti,Al,Mg | 750 | 10 | 1157 | 100/7% | [ |
| CaO/Mn,Mg | 800 | 10 | 1604 | 100/6.23% | [ |
| CaO/Al | 850 | 10 | 1496 | 100/32% | [ |
| CaO/Mn,Al | 800 | 10 | 1245.7 | 1000/23.2% | this study |
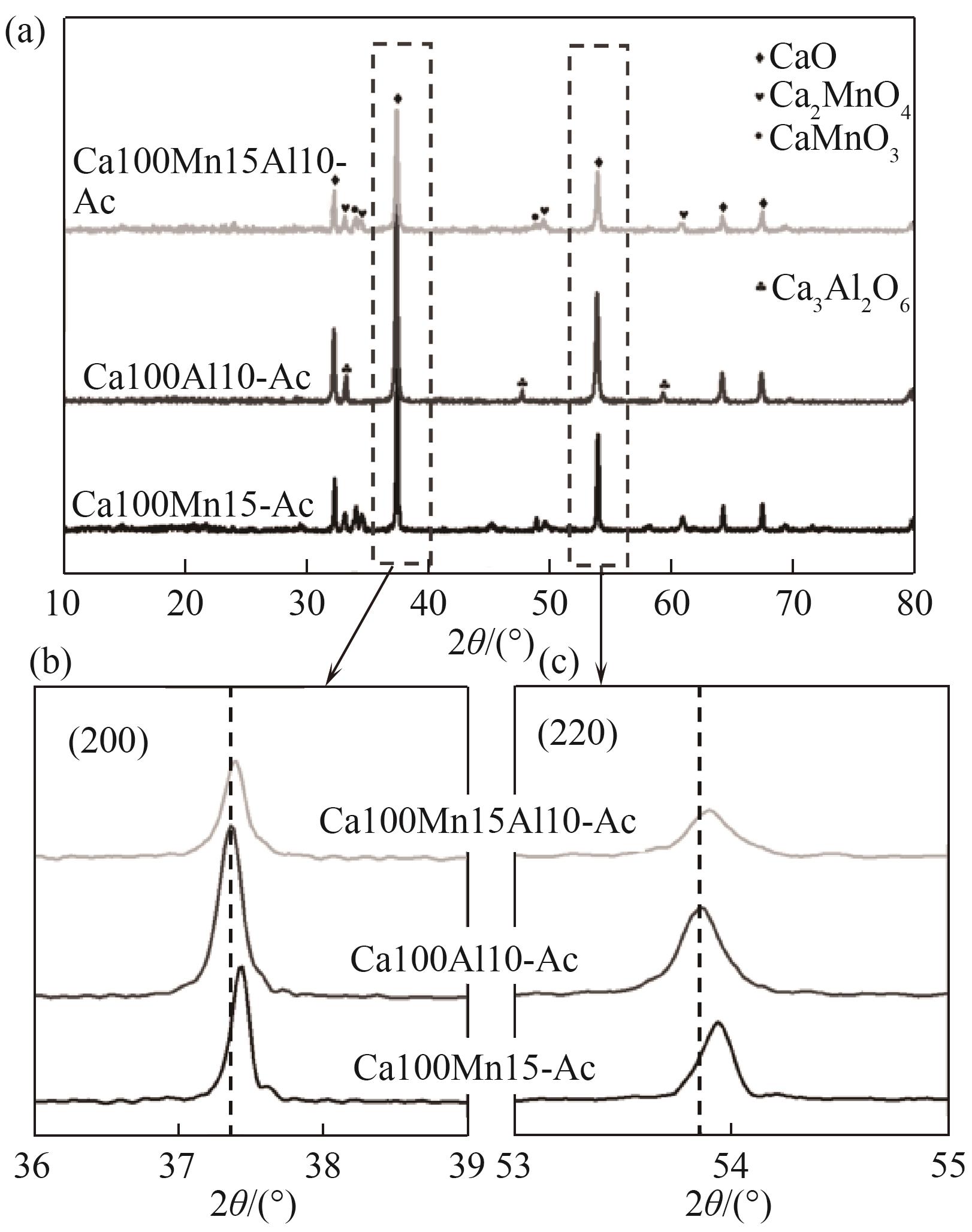
图5 各样品的XRD谱图:(a)10°~80°全谱图;(b)和(c)主峰放大图
Fig.5 XRD patterns of various samples: (a) patterns ranged from 10° to 80°; (b) and (c) the magnified pattern of the CaO peaks [(200) and (220)]
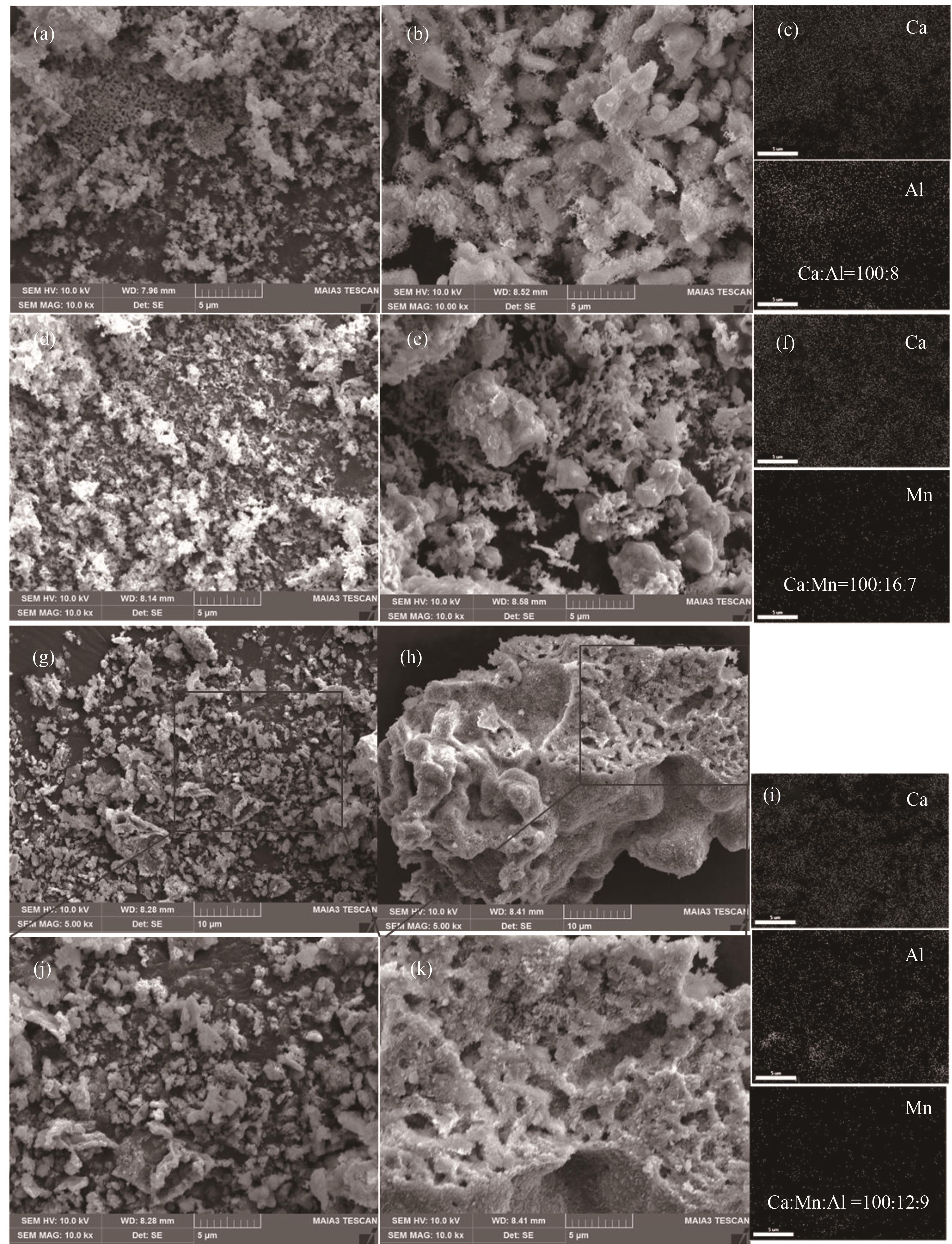
图7 各样品扫描电镜图:(a)新鲜Ca100Al10-Ac样品;(b)100次循环后Ca100Al10-Ac样品;(c)新鲜Ca100Al10-Ac样品EDS谱图;(d)新鲜Ca100Mn15-Ac样品;(e)100次循环后Ca100Mn15-Ac样品;(f)新鲜Ca100Mn15-Ac样品EDS谱图;(g)、(j)新鲜Ca100Mn15Al10-Ac样品;(h)、(k)1000次循环后Ca100Mn15Al10-Ac样品;(i)新鲜Ca100Mn15Al10-Ac样品EDS谱图
Fig.7 SEM images and corresponding EDS images of various samples: (a) fresh Ca100Al10-Ac; (b) 100th cycled Ca100Al10-Ac; (c) EDS images of fresh Ca100Al10-Ac; (d) fresh Ca100Mn15-Ac; (e) 100th cycled Ca100Mn15-Ac; (f) EDS images of fresh Ca100Mn15-Ac; (g),(j) fresh Ca100Mn15Al10-Ac; (h),(k) Ca100Mn15Al10-Ac after 1000 cycles; (i) EDS images of fresh Ca100Mn15Al10-Ac
| [1] | Leonard M D, Michaelides E E, Michaelides D N. Energy storage needs for the substitution of fossil fuel power plants with renewables[J]. Renewable Energy, 2020, 145: 951-962. |
| [2] | Lv J Q, Xie J F, Mohamed A G A, et al. Solar utilization beyond photosynthesis[J]. Nature Reviews Chemistry, 2023, 7(2): 91-105. |
| [3] | Wang G, Zhang Z, Lin J Q. Multi-energy complementary power systems based on solar energy: a review[J]. Renewable and Sustainable Energy Reviews, 2024, 199: 114464. |
| [4] | Romero M, Steinfeld A. Concentrating solar thermal power and thermochemical fuels[J]. Energy & Environmental Science, 2012, 5(11): 9234-9245. |
| [5] | He Y L, Qiu Y, Wang K, et al. Perspective of concentrating solar power[J]. Energy, 2020, 198: 117373. |
| [6] | Raganati F, Chirone R, Ammendola P. Calcium-looping for thermochemical energy storage in concentrating solar power applications: evaluation of the effect of acoustic perturbation on the fluidized bed carbonation[J]. Chemical Engineering Journal, 2020, 392: 123658. |
| [7] | 凌祥, 宋丹阳, 陈晓轶, 等. 钙基热化学储能体系装备与系统研究进展[J]. 化工进展, 2021, 40(4): 1777-1796. |
| Ling X, Song D Y, Chen X Y, et al. Progress in equipment and systems for calcium-based thermochemical energy storage system[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 1777-1796. | |
| [8] | Tian X K, Guo S J, Lv X J, et al. Progress in multiscale research on calcium-looping for thermochemical energy storage: from materials to systems[J]. Progress in Energy and Combustion Science, 2025, 106: 101194. |
| [9] | Ortiz C, Chacartegui R, Valverde J M, et al. Power cycles integration in concentrated solar power plants with energy storage based on calcium looping[J]. Energy Conversion and Management, 2017, 149: 815-829. |
| [10] | Teng L, Xuan Y M, Da Y, et al. Modified Ca-looping materials for directly capturing solar energy and high-temperature storage[J]. Energy Storage Materials, 2020, 25: 836-845. |
| [11] | 郑玉圆, 葛志伟, 韩翔宇, 等. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| Zheng Y Y, Ge Z W, Han X Y, et al. Progress and prospect of medium and high temperature thermochemical energy storage of calcium-based materials[J]. CIESC Journal, 2023, 74(8): 3171-3192. | |
| [12] | Tian X K, Lin S C, Yan J, et al. Sintering mechanism of calcium oxide/calcium carbonate during thermochemical heat storage process[J]. Chemical Engineering Journal, 2022, 428: 131229. |
| [13] | Wang J F, Xiong W, Ding Z X, et al. Enhancing the stability of CaO-based looping materials in thermochemical energy storage by codoping Y and Mg[J]. ACS Applied Energy Materials, 2024, 7(24): 12165-12173. |
| [14] | Wang K, Gu F, Clough P T, et al. Porous MgO-stabilized CaO-based powders/pellets via a citric acid-based carbon template for thermochemical energy storage in concentrated solar power plants[J]. Chemical Engineering Journal, 2020, 390: 124163. |
| [15] | Huang X K, Ma X T, Li J, et al. Enhancement effects of hydrolysable/soluble Al-type dopants on the efficiency of CaO/CaCO3 thermochemical energy storage[J]. Chemical Engineering Journal, 2024, 490: 151555. |
| [16] | Han R, Gao J H, Wei S Y, et al. Development of dense Ca-based, Al-stabilized composites with high volumetric energy density for thermochemical energy storage of concentrated solar power[J]. Energy Conversion and Management, 2020, 221: 113201. |
| [17] | Hu Y C, He W Z, Cao J X, et al. Decorating CaO with dark Ca2MnO4 for direct solar thermal conversion and stable thermochemical energy storage[J]. Solar Energy Materials and Solar Cells, 2022, 248: 111977. |
| [18] | Guo H X, Kou X C, Zhao Y J, et al. Effect of synergistic interaction between Ce and Mn on the CO2 capture of calcium-based sorbent: textural properties, electron donation, and oxygen vacancy[J]. Chemical Engineering Journal, 2018, 334: 237-246. |
| [19] | Chen X B, Tang Y T, Ke C C, et al. CO2 capture by double metal modified CaO-based sorbents from pyrolysis gases[J]. Chinese Journal of Chemical Engineering, 2022, 43: 40-49. |
| [20] | Jiang T, Zhang H, Zhao Y J, et al. Kilogram-scale production and pelletization of Al-promoted CaO-based sorbent for CO2 capture[J]. Fuel, 2021, 301: 121049. |
| [21] | Sun H, Li Y J, Yan X Y, et al. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure[J]. Applied Energy, 2020, 263: 114650. |
| [22] | Li C L, Li Y J, Zhang C X, et al. CaO/CaCO3 thermochemical energy storage performance of high-alumina granule stabilized papermaking soda residue[J]. Fuel Processing Technology, 2022, 237: 107444. |
| [23] | Gao C Y, Zhang Y, Liu X L, et al. A dual modification method to prepare carbide slag into highly active CaO-based solar energy storage materials[J]. Industrial & Engineering Chemistry Research, 2024, 63(1): 769-779. |
| [24] | Kim S M, Kierzkowska A M, Broda M, et al. Sol-gel synthesis of MgAl2O4-stabilized CaO for CO2 capture[J]. Energy Procedia, 2017, 114: 220-229. |
| [25] | Luo T, Luo C, Shi Z W, et al. Optimization of sol-gel combustion synthesis for calcium looping CO2 sorbents (part Ⅰ): Effects of sol-gel preparation and combustion conditions[J]. Separation and Purification Technology, 2022, 292: 121081. |
| [26] | Song C, Liu X L, Zheng H B, et al. Decomposition kinetics of Al- and Fe-doped calcium carbonate particles with improved solar absorbance and cycle stability[J]. Chemical Engineering Journal, 2021, 406: 126282. |
| [27] | Angeli S D, Martavaltzi C S, Lemonidou A A. Development of a novel-synthesized Ca-based CO2 sorbent for multicycle operation: parametric study of sorption[J]. Fuel, 2014, 127: 62-69. |
| [28] | Chen H C, Zhang P P, Duan Y F, et al. Reactivity enhancement of calcium based sorbents by doped with metal oxides through the sol-gel process[J]. Applied Energy, 2016, 162: 390-400. |
| [29] | Liu X L, Yuan C J, Zheng H B, et al. Synergy of Li2CO3 promoters and Al-Mn-Fe stabilizers in CaCO3 pellets enables efficient direct solar-driven thermochemical energy storage[J]. Materials Today Energy, 2022, 30: 101174. |
| [30] | Carrillo A J, González-Aguilar J, Romero M, et al. Solar energy on demand: a review on high temperature thermochemical heat storage systems and materials[J]. Chemical Reviews, 2019, 119(7): 4777-4816. |
| [31] | Tian X K, Lin S C, Yan J, et al. Improved durability in thermochemical energy storage using Ti/Al/Mg co-doped calcium-based composites with hierarchical meso/micro pore structures[J]. Chemical Engineering Journal, 2022, 450: 138142. |
| [32] | Liu H, Li Y Z, Wei J J. High performance Mn/Mg co-modified calcium-based material via EDTA chelating agent for effective solar energy storage[J]. Chemical Engineering Journal, 2024, 480: 147892. |
| [33] | Koirala R, Reddy G K, Smirniotis P G. Single nozzle flame-made highly durable metal doped Ca-based sorbents for CO2 capture at high temperature[J]. Energy & Fuels, 2012, 26(5): 3103-3109. |
| [34] | Zhou Z M, Qi Y, Xie M M, et al. Synthesis of CaO-based sorbents through incorporation of alumina/aluminate and their CO2 capture performance[J]. Chemical Engineering Science, 2012, 74: 172-180. |
| [35] | Torma A J, Li W B, Zhang H, et al. Interstitial nature of Mn2+ doping in 2D perovskites[J]. ACS Nano, 2021, 15(12): 20550-20561. |
| [1] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [2] | 孔俊龙, 毕扬, 赵耀, 代彦军. 储能电池直冷热管理系统的模拟实验[J]. 化工学报, 2025, 76(S1): 289-296. |
| [3] | 吴梓航, 徐震原, 游锦方, 潘权稳, 王如竹. 基于吸附式储冷技术的深井钻探设备冷却系统[J]. 化工学报, 2025, 76(S1): 309-317. |
| [4] | 肖鑫, 杨耿, 王云峰. 基于TRNSYS的太阳能梯级蓄热热泵系统模拟[J]. 化工学报, 2025, 76(S1): 393-400. |
| [5] | 黄国瑞, 赵耀, 谢明熹, 陈尔健, 代彦军. 一种新型数据中心余热回收系统实验与分析[J]. 化工学报, 2025, 76(S1): 409-417. |
| [6] | 刘辉, 王佳, 赵晶, 李传常, 吕又付. 大容量储能电池产热行为特性及容量衰减研究[J]. 化工学报, 2025, 76(9): 4903-4912. |
| [7] | 张淇栋, 艾立强, 马原, 吴胜宝, 王磊, 厉彦忠. 基于一维漂移流模型的低温管路预冷过程两相流动与换热特性研究[J]. 化工学报, 2025, 76(8): 3842-3852. |
| [8] | 史松伟, 赵诚, 刘帅, 应雨轩, 严密. 富铁飞灰耦合Fe-Zn/Al2O3脱除沼气H2S研究[J]. 化工学报, 2025, 76(8): 4239-4247. |
| [9] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [10] | 董泽明, 娄聚伟, 王楠, 陈良奇, 王江峰, 赵攀. 含余热回收的超临界压缩二氧化碳储能系统热力学特性研究[J]. 化工学报, 2025, 76(7): 3477-3486. |
| [11] | 唐羽丰, 陶春珲, 王永正, 李印辉, 段然, 赵泽一, 马和平. 超高比表面积碳基多孔吸附剂制备及其Kr气存储性能研究[J]. 化工学报, 2025, 76(7): 3339-3349. |
| [12] | 杨盛华, 孙阳杰, 薛晓君, 米杰, 王建成, 冯宇. 缺陷型金属氧化物脱除气体污染物研究进展[J]. 化工学报, 2025, 76(6): 2469-2482. |
| [13] | 杨浩杰, 刘春雨, 李雪娇, 于亮, 吕兴才. 低旋流配置下氨-甲烷-空气预混旋流火焰稳定性和排放特性[J]. 化工学报, 2025, 76(6): 3029-3040. |
| [14] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [15] | 彭新艳, 刘云鸿, 陈凌宇, 韦跃兰, 陈淑琴, 胡柱东. 小分子外交联法制备超高交联聚苯乙烯血液灌流吸附剂[J]. 化工学报, 2025, 76(6): 3093-3103. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
