• •
韦正兵1( ), 徐世彪1, 潘美伊1, 尹飞龙1, 韦丹1, 贾洋刚1, 檀杰1, 冒爱琴1,2(
), 徐世彪1, 潘美伊1, 尹飞龙1, 韦丹1, 贾洋刚1, 檀杰1, 冒爱琴1,2( )
)
收稿日期:2025-10-16
修回日期:2025-12-03
出版日期:2025-12-26
通讯作者:
冒爱琴
作者简介:韦正兵(2001—),男,硕士研究生,18175312236@163.com
基金资助:
Zhengbing Wei1( ), Shibiao Xu1, Meiyi Pan1, Feilong Yin1, Dan Wei1, Yanggang Jia1, Jie Tan1, Aiqin Mao1,2(
), Shibiao Xu1, Meiyi Pan1, Feilong Yin1, Dan Wei1, Yanggang Jia1, Jie Tan1, Aiqin Mao1,2( )
)
Received:2025-10-16
Revised:2025-12-03
Online:2025-12-26
Contact:
Aiqin Mao
摘要:
为解决高熵氧化物(HEOs)在储能领域面临的本征电导率低、锂离子传输动力学缓慢等问题,本研究采用溶液燃烧法制备了不同 Li⁺掺杂量的钙钛矿型La (CoCrFeMnNiLix)₁/(5+x) O3(x=0、0.2、0.4、0.6)HEOs负极材料,通过调控晶格畸变与氧空位等本征缺陷,实现储锂性能的显著提升。电化学测试表明,Li⁺掺杂可有效优化材料的电化学性能,其中Li0.4电极展示了卓越的倍率性能和循环稳定性,在 200 mA g-1 电流密度下循环 150 圈后放电比容量达 549.1 mAh g-1,较未掺杂提升55.3%;1000 mA g-1 下循环 500 圈,仍保持 503.6 mAh g-1的可逆比容量(未掺杂样品为440 mAh g-1)。其优异性能可归因于晶格畸变与高浓度氧空位的耦合调控作用,一方面优化电子/离子传输路径,增加反应活性位点;另一方面显著提升锂离子扩散速率与表面赝电容贡献率,缓解了材料储锂过程中的动力学限制。
中图分类号:
韦正兵, 徐世彪, 潘美伊, 尹飞龙, 韦丹, 贾洋刚, 檀杰, 冒爱琴. Li+掺杂诱导缺陷工程增强钙钛矿型高熵氧化物储锂性能[J]. 化工学报, DOI: 10.11949/0438-1157.20251156.
Zhengbing Wei, Shibiao Xu, Meiyi Pan, Feilong Yin, Dan Wei, Yanggang Jia, Jie Tan, Aiqin Mao. Li+ doping induced defect engineering for enhanced lithium storage performance in perovskite-type high-entropy oxides[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251156.
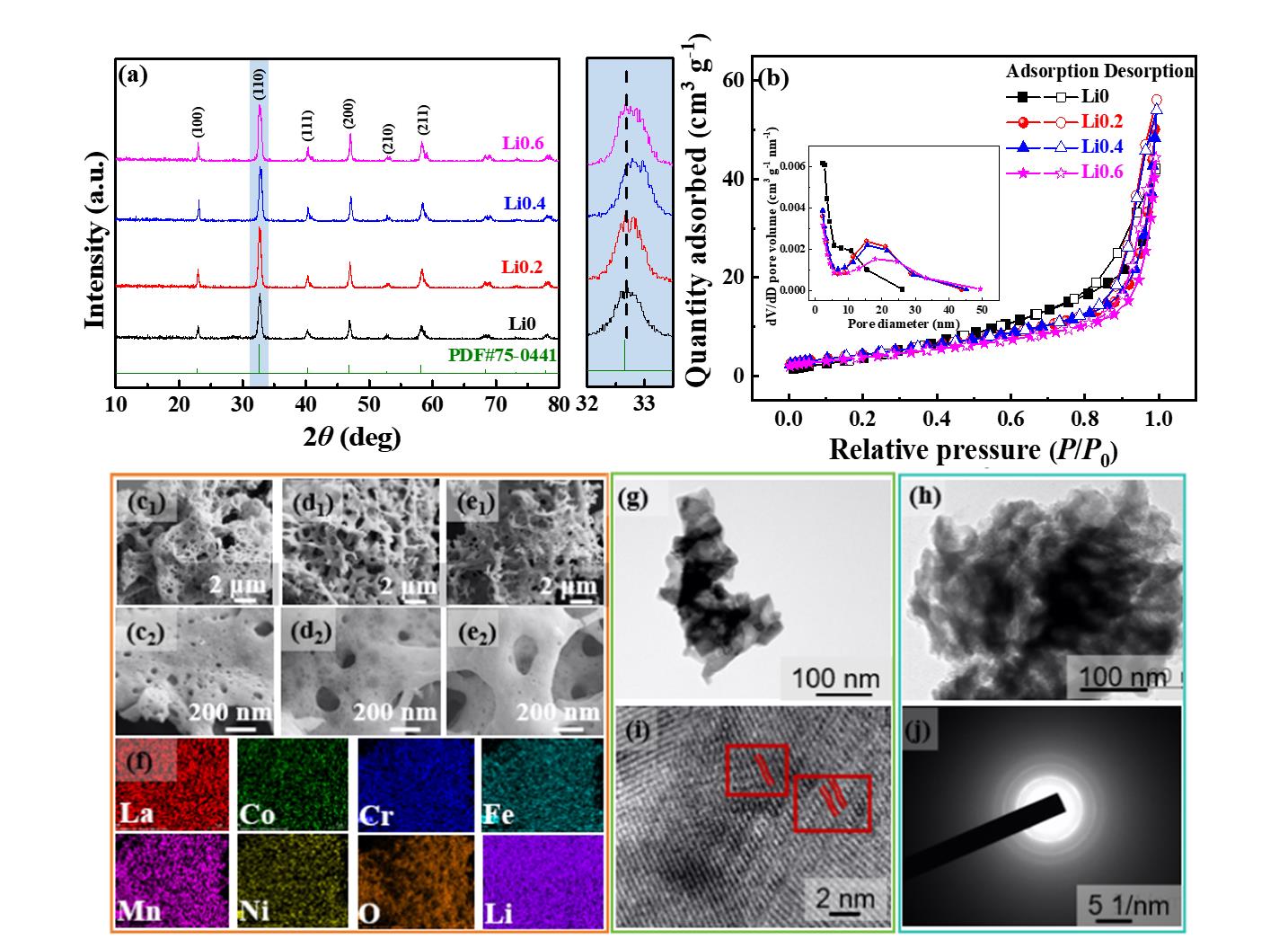
图1 (a)样品的XRD图(插图为局部放大图);(b)N2吸/脱附等温曲线及样品的孔径分布图;Li0(c1,c2)、Li0.4(d1,d2)和Li0.6(e1,e2)样品的SEM图;(f)Li0.4样品的EDS mapping图;Li0.4样品循环前的TEM图像(g)和循环150圈后的TEM图像(h);循环前的高分辨率TEM(HRTEM)图像(i)和选区电子衍射(SAED)图(j)
Fig. 1 (a) XRD pattern of the sample (with the inset showing a locally magnified view); (b) N2 adsorption-desorption isotherm and pore size distribution curve of the sample; SEM images of Li0 (c1, c2), Li0.4 (d1, d2), and Li0.6 (e1, e2) samples; (f) EDS mapping images of the Li0.4 sample; TEM images of the Li0.4 sample before cycling (g) and after 150 cycles (h); High-resolution TEM (HRTEM) image before cycling (i) and selected area electron diffraction (SAED) pattern (j)
| Sample | SBET (m2 g-1) | VBJH (cm3 g-1) | Daver. (nm) | Dmost/nm |
|---|---|---|---|---|
| Li0 | 18.337 | 0.063 | 13.849 | 3.367 |
| Li0.2 | 16.739 | 0.079 | 18.861 | 2.780 |
| Li0.4 | 16.719 | 0.076 | 18.235 | 2.539 |
| Li0.6 | 14.445 | 0.065 | 18.121 | 2.764 |
表1 BET比表面积(SBET)、BJH吸附累积孔隙体积(VBJH)、平均孔径(Daver.)和最可几孔径(Dmost)
Table 1 BET specific surface areas (SBET), BJH adsorption cumulative pore volumes (VBJH), and average pore diameters (Daver.)
| Sample | SBET (m2 g-1) | VBJH (cm3 g-1) | Daver. (nm) | Dmost/nm |
|---|---|---|---|---|
| Li0 | 18.337 | 0.063 | 13.849 | 3.367 |
| Li0.2 | 16.739 | 0.079 | 18.861 | 2.780 |
| Li0.4 | 16.719 | 0.076 | 18.235 | 2.539 |
| Li0.6 | 14.445 | 0.065 | 18.121 | 2.764 |
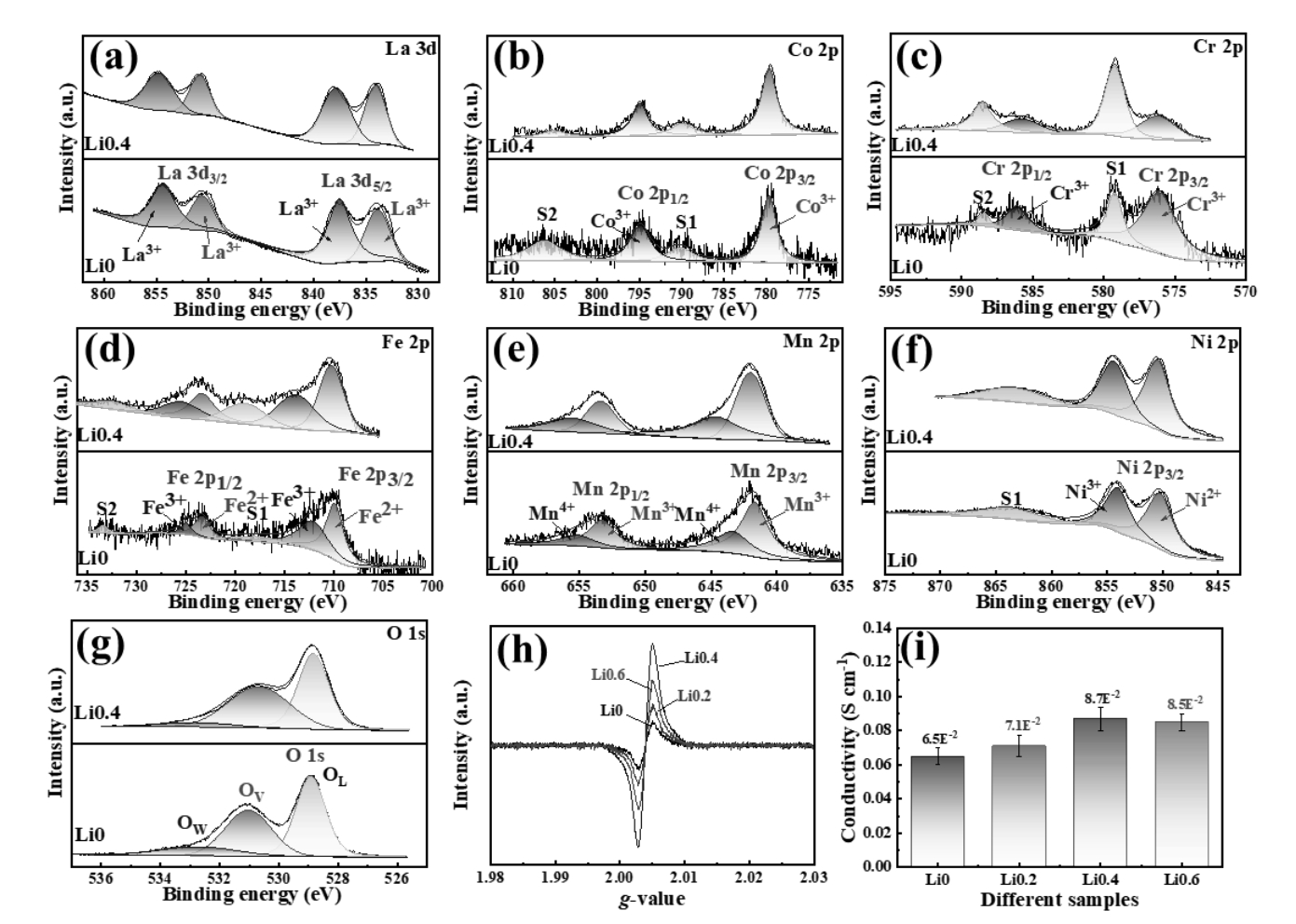
图2 (a-g)Li0和Li0.4样品中各元素的XPS能谱,(h)EPR光谱和(i)四探针电导率
Fig. 2 (a-g) XPS energy spectra of each element in Li0 and Li0.4 samples, (h) EPR spectra, and (i) Four-probes couctivity
| Samples | La3+ | Co3+ | Cr3+ | Fe2+/Fe3+ | Mn3+/Mn4+ | Ni2+/Ni3+ |
|---|---|---|---|---|---|---|
| Li0 | 1 | 1 | 1 | 57.2/42.8 | 66.3/33.7 | 57.8/42.2 |
| Li0.4 | 1 | 1 | 1 | 55.4/44.6 | 56.2/43.4 | 59.7/40.3 |
表2 XPS中阳离子价态浓度比
Table 2 Concentration ratios of cationic valence states in XPS
| Samples | La3+ | Co3+ | Cr3+ | Fe2+/Fe3+ | Mn3+/Mn4+ | Ni2+/Ni3+ |
|---|---|---|---|---|---|---|
| Li0 | 1 | 1 | 1 | 57.2/42.8 | 66.3/33.7 | 57.8/42.2 |
| Li0.4 | 1 | 1 | 1 | 55.4/44.6 | 56.2/43.4 | 59.7/40.3 |
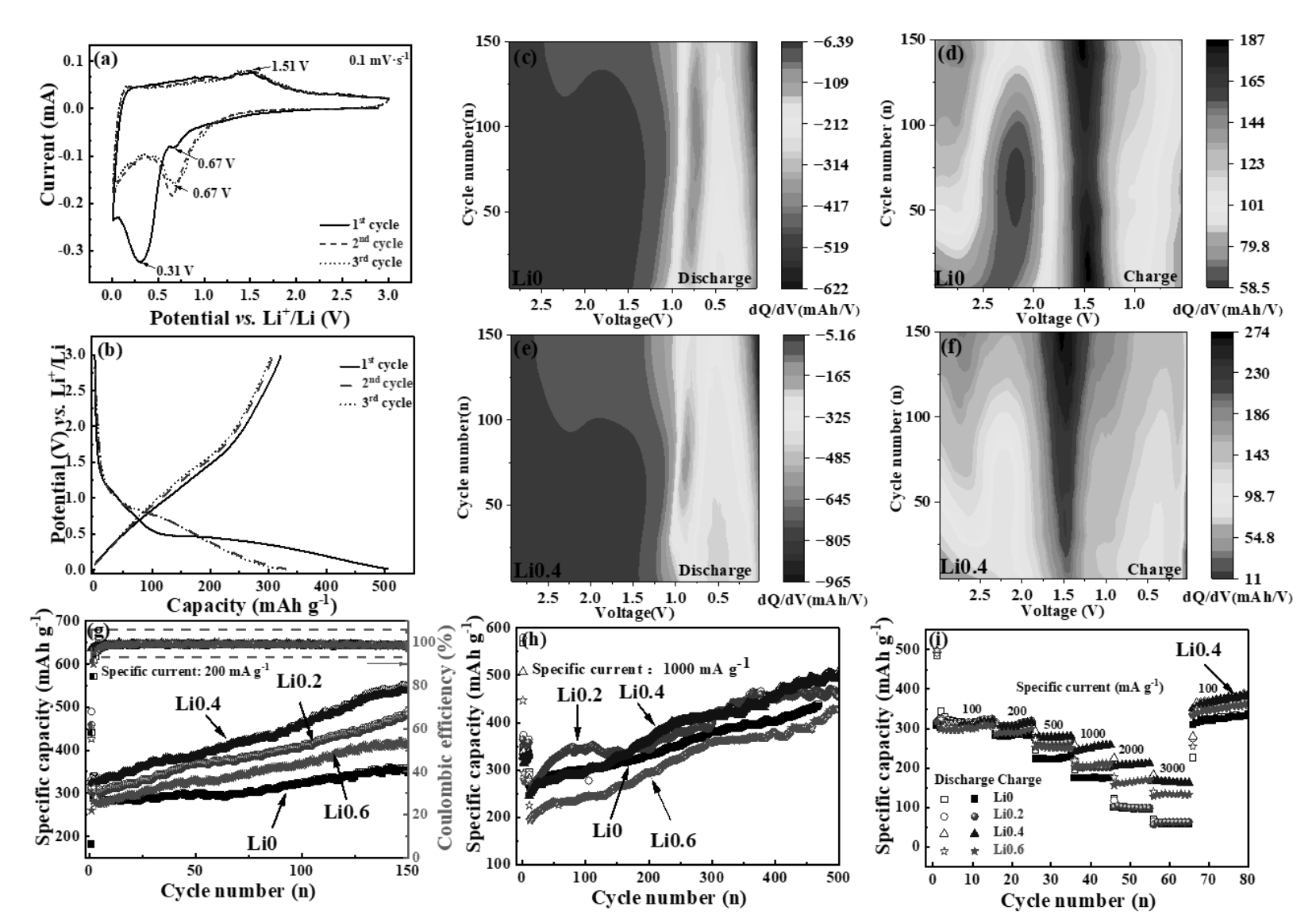
图3 (a)Li0.4电极的循环伏安曲线;(b)充放电曲线;(c-f)Li0和Li0.4充放电时的微分容量图;(g-i) 不同电流密度下的循环性能及倍率性能
Fig. 3 (a) CV curves of the Li0.4 electrode; (b) Galvanostatic charge-discharge profiles; (c-f) Differential capacity plots of Li0 and Li0.4 electrodes during charge-discharge processes; (g-i) Cycling performance and rate capability at different current densities
| Composition | Method | Cycling performance (mAh g-1 ) (cycles) | Ref. |
|---|---|---|---|
| La (CoCrFeMnNiLix)₁/(5+x) O3 | SCS | 549.1(150) at 0.2 A g-1; 503.6(500) at 1.0 A g-1 | This work |
| La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | SCS | 569 (500) at 0.2 A g-1; 325 (500) at 1.0 A g-1 | [ |
| Gd(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | SCS | 403 (500) at 0.2 A g-1 394 (500) at 1.0 A g-1 | [ |
| Li0.1(LiLaCaSrBa)Ti0.9Al0.1O3 | Mechanochemical | 57 (100) at 0.1 A g-1; | [ |
| LaCoO3/Co3O4-800 | Hydrothermal method | 550 (100) at 0.1 A g-1 | [ |
表3 钙钛矿型负极材料性能对比
Table 3 Performance comparison of perovskite-type anode materials
| Composition | Method | Cycling performance (mAh g-1 ) (cycles) | Ref. |
|---|---|---|---|
| La (CoCrFeMnNiLix)₁/(5+x) O3 | SCS | 549.1(150) at 0.2 A g-1; 503.6(500) at 1.0 A g-1 | This work |
| La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | SCS | 569 (500) at 0.2 A g-1; 325 (500) at 1.0 A g-1 | [ |
| Gd(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 | SCS | 403 (500) at 0.2 A g-1 394 (500) at 1.0 A g-1 | [ |
| Li0.1(LiLaCaSrBa)Ti0.9Al0.1O3 | Mechanochemical | 57 (100) at 0.1 A g-1; | [ |
| LaCoO3/Co3O4-800 | Hydrothermal method | 550 (100) at 0.1 A g-1 | [ |
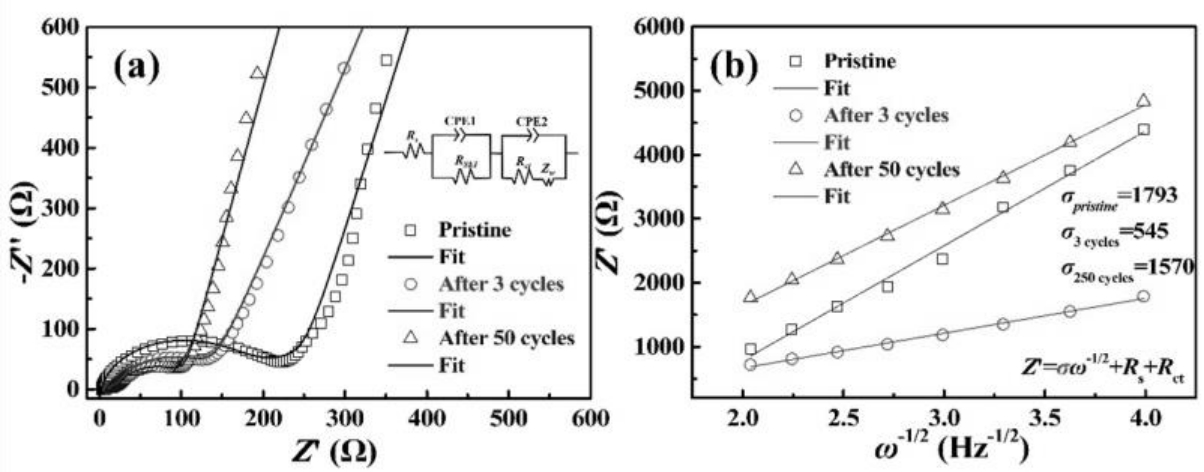
图4 (a)Li0.4电极在循环0, 3和50圈后的Nyquist图和等效电路 (b)在低频区域ω-1/2与Z'的关系图
Fig. 4 (a) Nyquist plots together with the equivalent circuit and (b) Plots of Z'vs. ω-1/2 of Li0.4 electrode before cycling, after 3 and 50 cycles
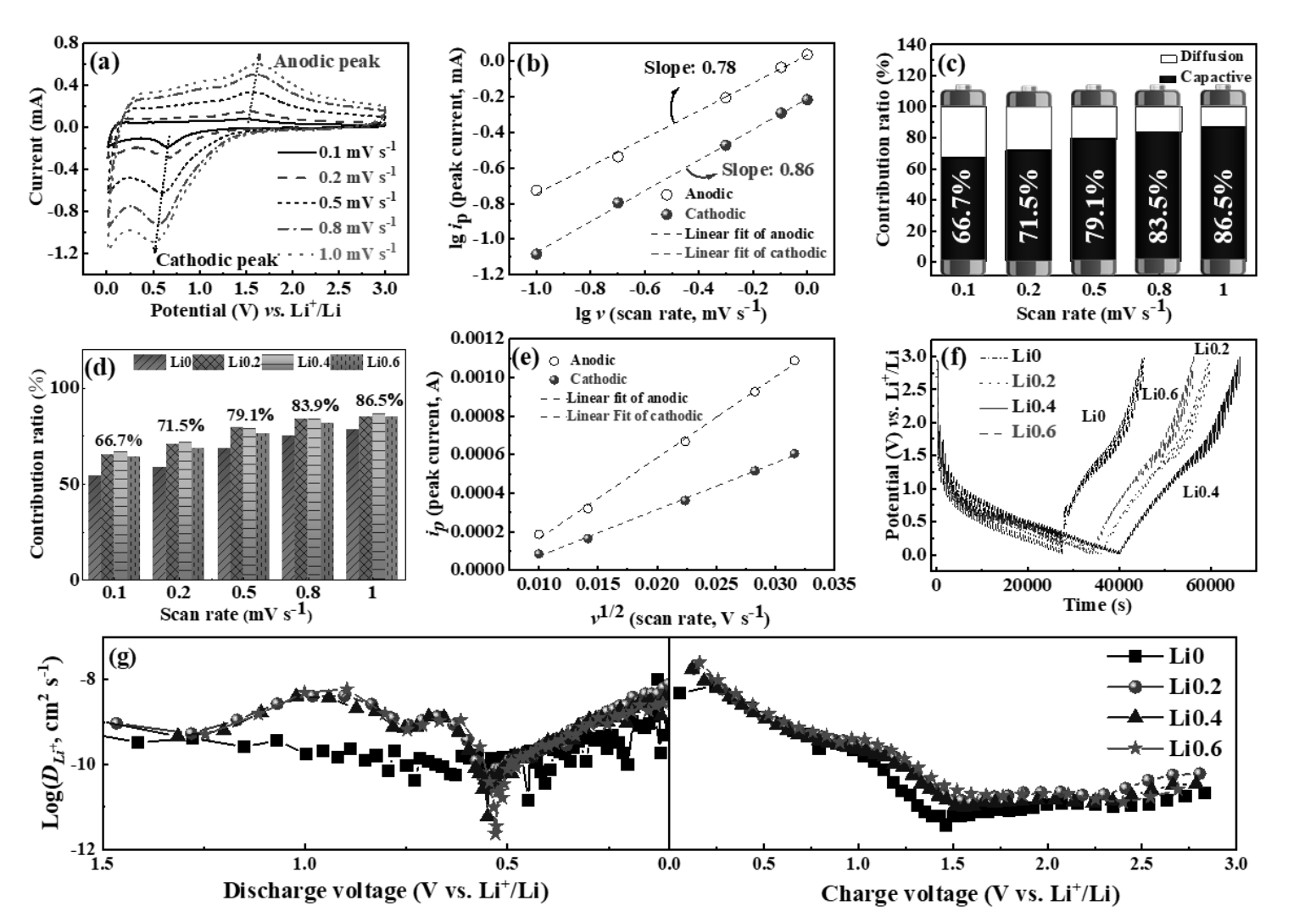
图5 (a)Li0.4电极在不同扫速下的CV曲线,(b)lg(ip)与lg(v)的关系曲线和(c)不同扫描速率下赝电容贡献率;(d)电极在不同扫描速率下赝电容贡献率汇总图,(e)ip与v1/2的关系图,(f)GITT测试过程中的充电/放电曲线和(g)充电/放电过程中的DLi+
Fig. 5 (a) CV curves of Li0.4 electrode at 0.1, 0.2, 0.5, 0.8, and 1.0 mV s-1 sweep rates, (b) lg(ip) vs. lg(v) curves and (c) Contribution ratios at different scan rates of the Li0.4 electrode; (d) Contribution ratios at different scan rates, (e) ipvsv1/2 curves of Li0.4 electrode, (f) Charge/discharge capacity curves during the GITT measurements and (g) DLi+ during the charge/discharge process of as-prepared electrodes
| Rs (Ω) | Rct (Ω) | σ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pristine | 3rd | 50th | Pristine | 3rd | 50th | Pristine | 3rd | 50th | |
| Li0 | 7.3 | 12.8 | 25.3 | 387.5 | 163.4 | 139.6 | 1299 | 1043 | 1722 |
| Li0.2 | 13.9 | 5.1 | 12.8 | 201.2 | 187.1 | 95.6 | 707 | 532 | 1369 |
| Li0.4 | 2.4 | 7.5 | 13.7 | 153.5 | 106.5 | 84.3 | 662 | 402 | 797 |
| Li0.6 | 10.9 | 10.4 | 15.1 | 339.3 | 88.2 | 90.1 | 883 | 440 | 1653 |
表4 电极循环0、3和50圈后的等效电路图参数
Table 4 Parameters of equivalent circuit diagrams of as-prepared electrodes before cycling, after 3 and 50 cycles
| Rs (Ω) | Rct (Ω) | σ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pristine | 3rd | 50th | Pristine | 3rd | 50th | Pristine | 3rd | 50th | |
| Li0 | 7.3 | 12.8 | 25.3 | 387.5 | 163.4 | 139.6 | 1299 | 1043 | 1722 |
| Li0.2 | 13.9 | 5.1 | 12.8 | 201.2 | 187.1 | 95.6 | 707 | 532 | 1369 |
| Li0.4 | 2.4 | 7.5 | 13.7 | 153.5 | 106.5 | 84.3 | 662 | 402 | 797 |
| Li0.6 | 10.9 | 10.4 | 15.1 | 339.3 | 88.2 | 90.1 | 883 | 440 | 1653 |
| Samples | b (Cathodic) | b (Anodic) | DLi+/10-10 cm2 s-1 (Cathodic) | DLi+/10-10 cm2 s-1 (Anodic) |
|---|---|---|---|---|
| Li0 | 0.81 | 0.91 | 0.8 | 2.3 |
| Li0.2 | 0.85 | 0.78 | 1.8 | 6.2 |
| Li0.4 | 0.86 | 0.78 | 2.5 | 7.6 |
| Li0.6 | 0.86 | 0.76 | 2.7 | 6.7 |
表5 赝电容b值和循环伏安法DLi+
Table 5 The b value of pseudocapacitive and DLi+ obtained by cyclic voltammetry
| Samples | b (Cathodic) | b (Anodic) | DLi+/10-10 cm2 s-1 (Cathodic) | DLi+/10-10 cm2 s-1 (Anodic) |
|---|---|---|---|---|
| Li0 | 0.81 | 0.91 | 0.8 | 2.3 |
| Li0.2 | 0.85 | 0.78 | 1.8 | 6.2 |
| Li0.4 | 0.86 | 0.78 | 2.5 | 7.6 |
| Li0.6 | 0.86 | 0.76 | 2.7 | 6.7 |
| [1] | Bai Y H, Li J R, Lu H, et al. Ultrafast high-temperature sintering of high-entropy oxides with refined microstructure and superior lithium-ion storage performance[J]. Journal of Advanced Ceramics, 2023, 12(10): 1857-1871. |
| [2] | Ma J X, Liu T Y, Ye W H, et al. High-entropy perovskite oxides for energy materials: A review[J]. Journal of Energy Storage, 2024, 90: 111890. |
| [3] | Li Y N, Wang B B, Wang Y L, et al. Modulating crystal structure and lithium-ion storage performance of high-entropy oxide (CrMnFeCoNiZn)3O4 by single element extraction[J]. Composites Part B: Engineering, 2025, 294: 112175. |
| [4] | Ajayi S O, Dolla T H, Bello I T, et al. Recent developments strategies in high entropy modified lithium-rich layered oxides cathode for lithium-ion batteries[J]. Inorganic Chemistry Communications, 2025, 172: 113721. |
| [5] | Liu X F, Xing Y Y, Xu K, et al. Kinetically accelerated lithium storage in high-entropy (LiMgCoNiCuZn)O enabled by oxygen vacancies[J]. Small, 2022, 18(18): 2200524. |
| [6] | Sarkar A, Velasco L, Wang D, et al. High entropy oxides for reversible energy storage[J]. Nature Communications, 2018, 9: 3400. |
| [7] | Xiao B, Wu G, Wang T D, et al. High entropy oxides (FeNiCrMnX)3O4 (X=Zn, Mg) as anode materials for lithium ion batteries[J]. Ceramics International, 2021, 47(24): 33972-33977. |
| [8] | Patra J, Nguyen T X, Tsai C C, et al. Effects of elemental modulation on phase purity and electrochemical properties of co-free high-entropy spinel oxide anodes for lithium-ion batteries[J]. Advanced Functional Materials, 2022, 32(17): 2110992. |
| [9] | Nguyen T X, Tsai C C, Patra J, et al. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in Lithium-ion batteries[J]. Chemical Engineering Journal, 2022, 430: 132658. |
| [10] | 徐昊宇, 王睿, 康巧玲, 等. 高熵氧化物的设计及其在锂离子电池中的应用研究进展[J]. 无机化学学报, 2023, 39(12): 2241-2255. |
| Xu H Y, Wang R, Kang Q L, et al. Research progress on design of high entropy oxides and their applications in lithium-ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2023, 39(12): 2241-2255. | |
| [11] | Yan S X, Luo S H, Yang L, et al. Novel P2-type layered medium-entropy ceramics oxide as cathode material for sodium-ion batteries[J]. Journal of Advanced Ceramics, 2022, 11(1): 158-171. |
| [12] | Chen S J, Bao M F, Jia Y G, et al. Lattice distortion induced rock salt high-entropy oxide for high-rate lithium-ion storage[J]. Journal of Alloys and Compounds, 2024, 990: 174480. |
| [13] | Jia Y G, Chen S J, Shao X, et al. Synergetic effect of lattice distortion and oxygen vacancies on high-rate lithium-ion storage in high-entropy perovskite oxides[J]. Journal of Advanced Ceramics, 2023, 12(6): 1214-1227.[LinkOut] |
| [14] | Yang X B, Wang H Q, Song Y Y, et al. Low-temperature synthesis of a porous high-entropy transition-metal oxide as an anode for high-performance lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(23): 26873-26881. |
| [15] | Du W Q, Zheng Y Q, Liu X Y, et al. Oxygen-enriched vacancy spinel MFe2O4/carbon (M = Ni, Mn, Co) derived from metal-organic frameworks toward boosting lithium storage[J]. Chemical Engineering Journal, 2023, 451: 138626. |
| [16] | Yan L, Zong L S, Zhang Z J, et al. Oxygen vacancies activated porous MnO/graphene submicron needle arrays for high-capacity lithium-ion batteries[J]. Carbon, 2022, 190: 402-411. |
| [17] | Lökçü E, Toparli Ç, Anik M. Electrochemical performance of (MgCoNiZn)1– x Li x O high-entropy oxides in lithium-Ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(21): 23860-23866. |
| [18] | Feng D Y, Dong Y B, Zhang L L, et al. Holey lamellar high-entropy oxide as an ultra-high-activity heterogeneous catalyst for solvent-free aerobic oxidation of benzyl alcohol[J]. Angewandte Chemie (International Ed. in English), 2020, 59(44): 19503-19509. |
| [19] | Hu Q L, Yue B, Shao H Y, et al. Facile syntheses of perovskite type LaMO3 (M=Fe, Co, Ni) nanofibers for high performance supercapacitor electrodes and lithium-ion battery anodes[J]. Journal of Alloys and Compounds, 2021, 852: 157002. |
| [20] | Tian K H, Duan C Q, Ma Q, et al. High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X = Fe, Cr) for high-performance anode of Li-ion batteries[J]. Rare Metals, 2022, 41(4): 1265-1275. |
| [21] | Wei S, Wan C C, Zhang L Y, et al. N-doped and oxygen vacancy-rich NiCo2O4 nanograss for supercapacitor electrode[J]. Chemical Engineering Journal, 2022, 429: 132242. |
| [22] | Chen Z H, Lu L, Gao Y, et al. Effects of F-doping on the electrochemical performance of Na₂Ti₃O₇ as an anode for sodium-ion batteries[J]. Materials, 2018, 11(11): 2206. |
| [23] | Zhang Z Q, Lu S Y, Huang G, et al. Carbon-coated Li4Ti5O12 optimized by fluorine regulation strategy for high-rate lithium-ion batteries with mixed diffusion and capacitive effects[J]. Carbon, 2024, 221: 118885.[LinkOut] |
| [24] | Wang K, Hua W B, Huang X H, et al. Synergy of cations in high entropy oxide lithium ion battery anode[J]. Nature Communications, 2023, 14: 1487. |
| [25] | Zheng Y, Wu X, Lan X X, et al. A Spinel (FeNiCrMnMgAl)3O4 High Entropy Oxide as a Cycling Stable Anode Material for Li-Ion Batteries[J]. Processes, 2022, 10(1): 49. |
| [26] | Zhang Q Y, Zhang C L, Li B, et al. Preparation and electrochemical properties of Ca-doped Li4Ti5O12 as anode materials in lithium-ion battery[J]. Electrochimica Acta, 2013, 98: 146-152. |
| [27] | Zhang N, Liu E Q, Chen H W, et al. High-performance of LaCoO3/Co3O4 nanocrystal as anode for lithium-ion batteries[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 628: 127265. |
| [28] | 王朋朋, 贾洋刚, 邵霞, 等. K+掺杂尖晶石型(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4高熵氧化物负极材料制备与储锂性能研究[J]. 化工学报, 2022, 73(12): 5625-5637. |
| Wang P P, Jia Y G, Shao X, et al. Preparation and lithium storage performance of K+-doped spinel (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 high-entropy oxide anode materials[J]. CIESC Journal, 2022, 73(12): 5625-5637. | |
| [29] | Wang J B, Cui Y Y, Wang Q S, et al. Lithium containing layered high entropy oxide structures[J]. Scientific Reports, 2020, 10: 18430. |
| [30] | Castelli G F, Dörfler W. The numerical study of a microscale model for lithium-ion batteries[J]. Computers & Mathematics with Applications, 2019, 77(6): 1527-1540. |
| [31] | Xiang H Z, Xie H X, Chen Y X, et al. Porous spinel-type (Al0.2CoCrFeMnNi)0.58O4- δ high-entropy oxide as a novel high-performance anode material for lithium-ion batteries[J]. Journal of Materials Science, 2021, 56(13):8127-8142. |
| [32] | Chen H, Qiu N, Wu B Z, et al. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries[J]. RSC Advances, 2020, 10(16): 9736-9744.[LinkOut] |
| [33] | Chen H, Qiu N, Wu B Z, et al. Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties[J]. RSC Advances, 2019, 9(50): 28908-28915. |
| [34] | Lv H L, Wang X J, Yu Y, et al. RGO-Coated MOF-Derived In2Se3 as a High-Performance Anode for Sodium-Ion Batteries[J]. Acta Phys -Chim Sin, 2023, 39(3): 2210014. |
| [35] | Chen S J, Bao M F, Jia Y G, et al. Boosting high-rate Li-ion storage properties by La(III) ion doping in spinel (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 high-entropy oxide anode[J]. Journal of Advanced Ceramics, 2024, 13(6): 769-779. |
| [1] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [2] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [3] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [4] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [5] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [6] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [7] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [8] | 王珺仪, 夏章讯, 景粉宁, 王素力. 基于重整气的高温聚合物电解质膜燃料电池电化学阻抗谱弛豫时间分布研究[J]. 化工学报, 2025, 76(7): 3509-3520. |
| [9] | 孙国庆, 李海波, 丁志阳, 郭文辉, 徐浩, 赵艳侠. 硅基负极材料的研究进展[J]. 化工学报, 2025, 76(7): 3197-3211. |
| [10] | 高凤凤, 程慧峰, 杨博, 郝晓刚. 电驱动NiFeMn LDH/CNTs/PVDF膜电极选择性提取钨酸根离子[J]. 化工学报, 2025, 76(7): 3350-3360. |
| [11] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| [12] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [13] | 陈培强, 郑群, 姜玉廷, 熊春华, 陈今茂, 王旭东, 黄龙, 阮曼, 徐万里. 电液流量及电流密度对海水激活电池输出特性的影响[J]. 化工学报, 2025, 76(7): 3235-3245. |
| [14] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [15] | 康佳, 刘欢, 李海燕, 罗茂亮, 姚洪. 宽温区HCl/NaOH热介质中碳钢腐蚀行为及涂层性能研究[J]. 化工学报, 2025, 76(6): 2872-2885. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号