化工学报 ›› 2025, Vol. 76 ›› Issue (6): 2872-2885.DOI: 10.11949/0438-1157.20241260
收稿日期:2024-11-08
修回日期:2024-11-28
出版日期:2025-06-25
发布日期:2025-07-09
通讯作者:
刘欢
作者简介:康佳(2001—),男,硕士研究生,m202371219@hust.edu.cn
基金资助:
Jia KANG( ), Huan LIU(
), Huan LIU( ), Haiyan LI, Maoliang LUO, Hong YAO
), Haiyan LI, Maoliang LUO, Hong YAO
Received:2024-11-08
Revised:2024-11-28
Online:2025-06-25
Published:2025-07-09
Contact:
Huan LIU
摘要:
针对氯碱生产精馏与固碱工段的腐蚀问题,研究了Q235碳钢在盐酸、氢氧化钠介质中的腐蚀行为,以及两种掺杂型环氧树脂有机涂层(EP-SiTiMg及EP-SiAlCa)的腐蚀防护性能与作用机制。结果表明,HCl浓度和温度升高均会增大Q235的析氢反应速率,加剧点蚀。在NaOH碱性环境中,当温度≤90℃时,碳钢腐蚀初期表面会形成钝化膜,腐蚀速率略有减小;当温度进一步升高,钝化膜逐步溶解,腐蚀速率加快至0.522 mg/(cm2·h),达到严重腐蚀标准。EP-SiTiMg涂层在酸性环境中防护性能优异,150℃时仍可保持4×109 Ω·cm2以上的高阻抗值,适合精馏工段长期应用;EP-SiAlCa涂层在<150℃的碱性环境中防护效果更好,腐蚀速率较碳钢降低52%以上,适合在相应作业温度范围的固碱工段使用。当温度达150℃时,两种涂层防护效果均小幅下降,涂层电阻均保持在104 Ω·cm2左右,但仍对碳钢有防护效果。
中图分类号:
康佳, 刘欢, 李海燕, 罗茂亮, 姚洪. 宽温区HCl/NaOH热介质中碳钢腐蚀行为及涂层性能研究[J]. 化工学报, 2025, 76(6): 2872-2885.
Jia KANG, Huan LIU, Haiyan LI, Maoliang LUO, Hong YAO. Corrosion behavior and coating performance of carbon steel in HCl/NaOH thermal medium in wide temperature zone[J]. CIESC Journal, 2025, 76(6): 2872-2885.
| 温度/℃ | 腐蚀速率KL/(mg/(cm2·h)) | |
|---|---|---|
| HCl | NaOH | |
| 30 | -0.308 | -0.009 |
| 60 | -0.825 | -0.044 |
| 90 | -6.852 | -0.045 |
| 120 | -11.262 | -0.135 |
| 150 | -21.721 | -0.522 |
表1 Q235不同温度下的腐蚀速率
Table 1 Corrosion rate constants of Q235 at different temperatures
| 温度/℃ | 腐蚀速率KL/(mg/(cm2·h)) | |
|---|---|---|
| HCl | NaOH | |
| 30 | -0.308 | -0.009 |
| 60 | -0.825 | -0.044 |
| 90 | -6.852 | -0.045 |
| 120 | -11.262 | -0.135 |
| 150 | -21.721 | -0.522 |
| 温度/℃ | 腐蚀速率KL/(mg/(cm2·h)) | |||
|---|---|---|---|---|
| HCl | NaOH | |||
| EP-SiAlCa | EP-SiTiMg | EP-SiAlCa | EP-SiTiMg | |
| 30 | -0.053 | -0.043 | -0.034 | -0.048 |
| 60 | -0.233 | -0.109 | -0.034 | -0.066 |
| 90 | -0.253 | -0.146 | -0.042 | -0.065 |
| 120 | -0.348 | -0.198 | -0.071 | -0.076 |
| 150 | -0.515 | -0.236 | -0.074 | -0.086 |
表2 两种涂层在不同温度下的腐蚀速率
Table 2 Corrosion rate constants of the two coatings at different temperatures
| 温度/℃ | 腐蚀速率KL/(mg/(cm2·h)) | |||
|---|---|---|---|---|
| HCl | NaOH | |||
| EP-SiAlCa | EP-SiTiMg | EP-SiAlCa | EP-SiTiMg | |
| 30 | -0.053 | -0.043 | -0.034 | -0.048 |
| 60 | -0.233 | -0.109 | -0.034 | -0.066 |
| 90 | -0.253 | -0.146 | -0.042 | -0.065 |
| 120 | -0.348 | -0.198 | -0.071 | -0.076 |
| 150 | -0.515 | -0.236 | -0.074 | -0.086 |
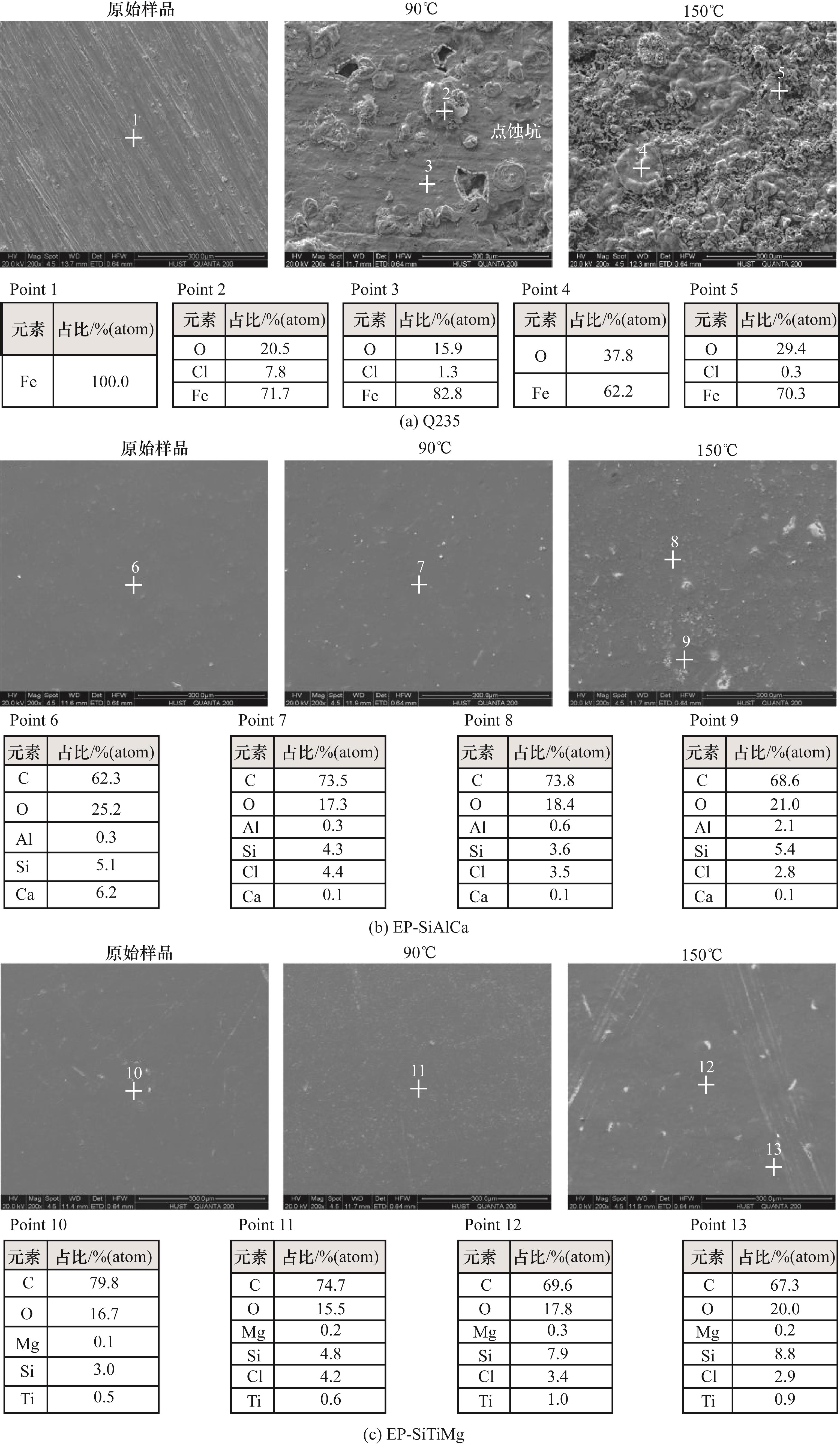
图7 酸性环境下Q235、EP-SiAlCa和EP-SiTiMg原始样品,90、150℃腐蚀后微观形貌
Fig.7 Microstructure of the original sample and after corrosion at 90℃ and 150℃ of Q235, Ep-SiAlCa and EP-SiTiMg under acidic environment
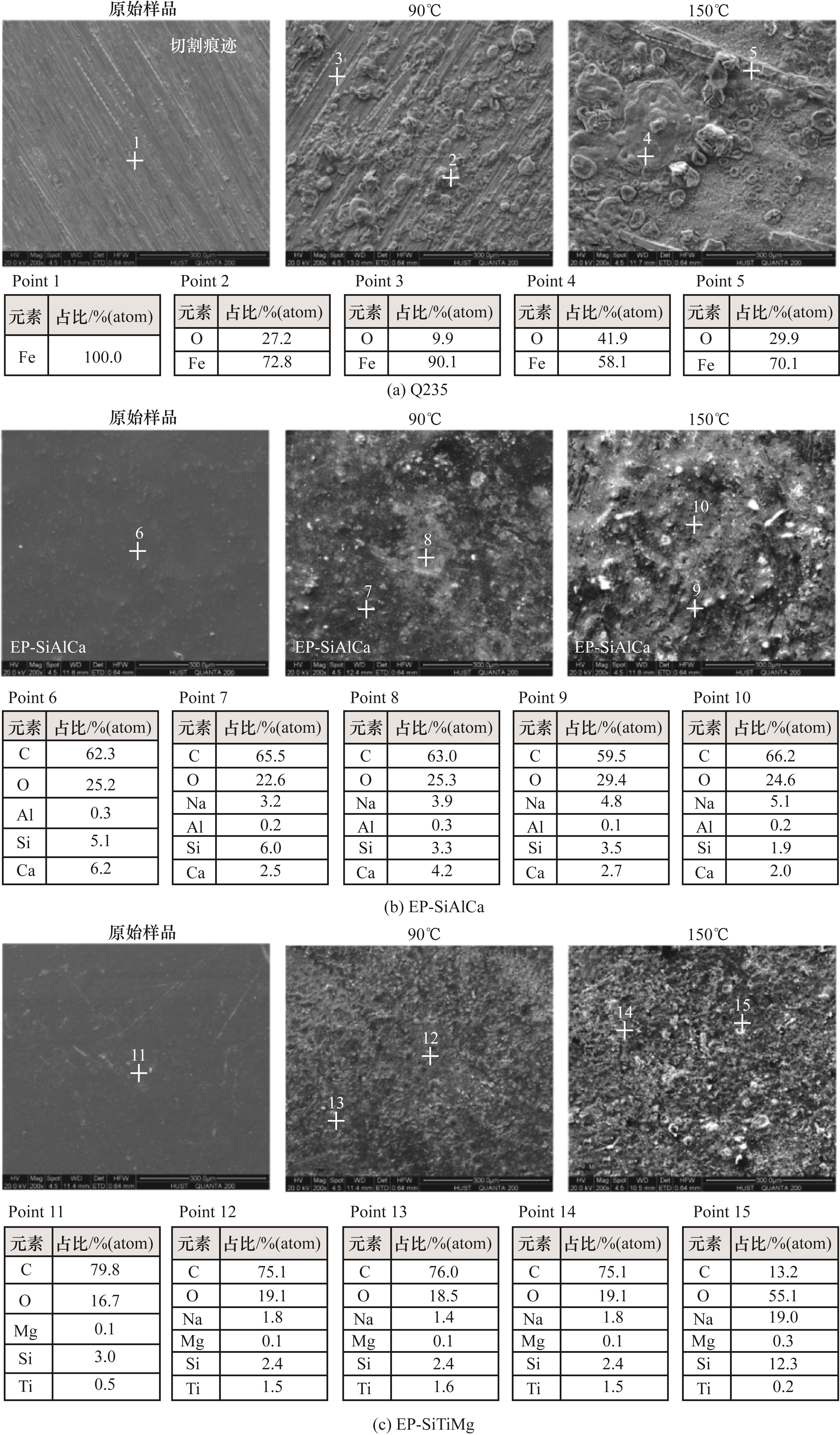
图8 碱性环境下Q235、EP-SiAlCa和EP-SiTiMg原始样品,90、150℃腐蚀后微观形貌
Fig.8 Microstructure of original sample and after corrosion at 90℃ and 150℃ of Q235, EP-SiAlCa and EP-SiTiMg under alkaline environment

图10 酸性环境中Q235[(a)~(c)]、EP-SiAlCa[(d)~(f)]和EP-SiTiMg[(g)~(i)]的电化学阻抗谱Nyquist和Bode图
Fig.10 Nyquist and Bode diagrams of electrochemical impedance spectra of Q235[(a)~(c)], EP-SiAlCa[(d)~(f)] and EP-SiTiMg[(g)~(i)] in acidic environment
| 样品 | Rc/(Ω·cm2) | CPEc/(F/cm2) | n1 | |
|---|---|---|---|---|
| Q235 | 原始 | 1000.40 | 9.69×10-4 | 0.77 |
| 90℃ | 447.29 | 1.42×10-2 | 0.69 | |
| 150℃ | 399.68 | 7.85×10-3 | 0.64 | |
| EP-SiAlCa | 原始 | 6.38×109 | 1.10×10-9 | 0.96 |
| 90℃ | 1.63×109 | 1.07×10-9 | 0.96 | |
| 150℃ | 3.58×105 | 1.15×10-9 | 0.96 | |
| EP-SiTiMg | 原始 | 6.94×109 | 1.09×10-9 | 0.96 |
| 90℃ | 4.89×109 | 1.09×10-9 | 0.96 | |
| 150℃ | 4.26×109 | 1.10×10-9 | 0.96 | |
表3 酸性环境中电化学阻抗谱拟合结果
Table 3 Fitting results of electrochemical impedance spectra in acidic environment
| 样品 | Rc/(Ω·cm2) | CPEc/(F/cm2) | n1 | |
|---|---|---|---|---|
| Q235 | 原始 | 1000.40 | 9.69×10-4 | 0.77 |
| 90℃ | 447.29 | 1.42×10-2 | 0.69 | |
| 150℃ | 399.68 | 7.85×10-3 | 0.64 | |
| EP-SiAlCa | 原始 | 6.38×109 | 1.10×10-9 | 0.96 |
| 90℃ | 1.63×109 | 1.07×10-9 | 0.96 | |
| 150℃ | 3.58×105 | 1.15×10-9 | 0.96 | |
| EP-SiTiMg | 原始 | 6.94×109 | 1.09×10-9 | 0.96 |
| 90℃ | 4.89×109 | 1.09×10-9 | 0.96 | |
| 150℃ | 4.26×109 | 1.10×10-9 | 0.96 | |
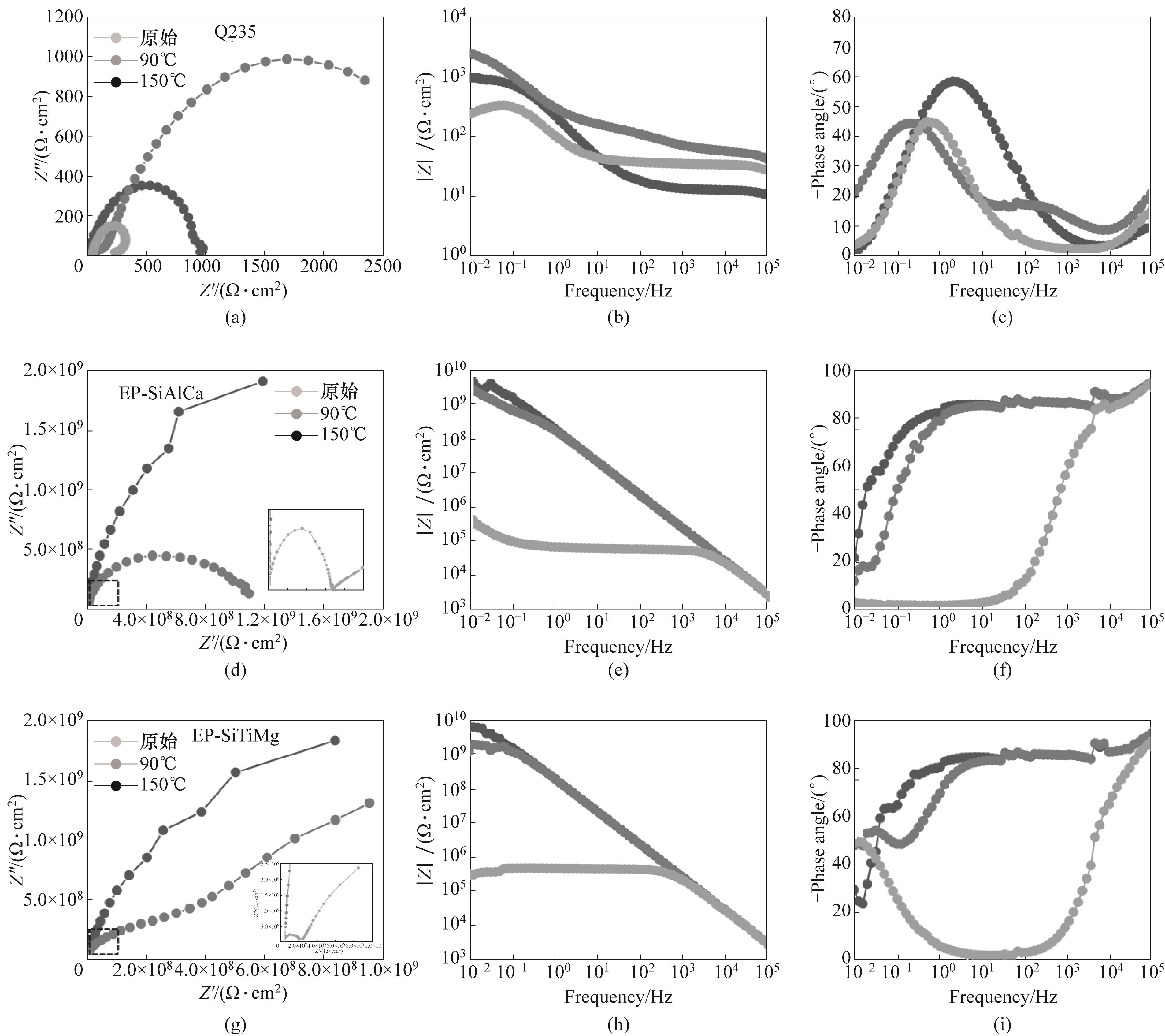
图12 碱性环境中Q235[(a)~(c)]、EP-SiAlCa[(d)~(f)]和EP-SiTiMg[(g)~(i)]电化学阻抗谱Nyquist和Bode图
Fig.12 Nyquist and Bode diagrams of electrochemical impedance spectra of Q235[(a)~(c)], EP-SiAlCa[(d)~(f)] and EP-SiTiMg[(g)~(i)] in alkaline environment
| 样品 | Rc/(Ω·cm2) | CPEc/(F/cm2) | n1 | Rct/(Ω·cm2) | CPEdl/(F/cm2) | n2 | |
|---|---|---|---|---|---|---|---|
| Q235 | 原始 | 1000.40 | 9.69×10-4 | 0.77 | — | — | — |
| 90℃ | 110.24 | 3.15×10-4 | 0.53 | 3075.71 | 12374×10-3 | 0.73 | |
| 150℃ | 306.97 | 2.27×10-3 | 0.84 | — | — | — | |
| EP-SiAlCa | 原始 | 6.38×109 | 1.10×10-9 | 0.96 | |||
| 90℃ | 1.02×109 | 1.08×10-9 | 0.96 | — | — | — | |
| 150℃ | 38978 | 1.02×10-9 | 0.97 | ||||
| EP-SiTiMg | 原始 | 6.94×109 | 1.09×10-9 | 0.96 | — | — | — |
| 90℃ | 6.40×108 | 1.13×10-9 | 0.96 | 8.75×109 | 2.65×10-9 | 0.73 | |
| 150℃ | 53977.00 | 1.07×10-9 | 0.97 | 1.01×106 | 3.11×10-5 | 0.82 | |
表4 碱性环境中电化学阻抗谱拟合结果
Table 4 Fitting results of electrochemical impedance spectra in alkaline environment
| 样品 | Rc/(Ω·cm2) | CPEc/(F/cm2) | n1 | Rct/(Ω·cm2) | CPEdl/(F/cm2) | n2 | |
|---|---|---|---|---|---|---|---|
| Q235 | 原始 | 1000.40 | 9.69×10-4 | 0.77 | — | — | — |
| 90℃ | 110.24 | 3.15×10-4 | 0.53 | 3075.71 | 12374×10-3 | 0.73 | |
| 150℃ | 306.97 | 2.27×10-3 | 0.84 | — | — | — | |
| EP-SiAlCa | 原始 | 6.38×109 | 1.10×10-9 | 0.96 | |||
| 90℃ | 1.02×109 | 1.08×10-9 | 0.96 | — | — | — | |
| 150℃ | 38978 | 1.02×10-9 | 0.97 | ||||
| EP-SiTiMg | 原始 | 6.94×109 | 1.09×10-9 | 0.96 | — | — | — |
| 90℃ | 6.40×108 | 1.13×10-9 | 0.96 | 8.75×109 | 2.65×10-9 | 0.73 | |
| 150℃ | 53977.00 | 1.07×10-9 | 0.97 | 1.01×106 | 3.11×10-5 | 0.82 | |
| [1] | 侯保荣. 中国腐蚀成本[M]. 北京: 科学出版社, 2017. |
| Hou B R. The Cost of Corrosion in China[M]. Beijing: Science Press, 2017. | |
| [2] | 张俊平. 氯碱工业中的设备腐蚀与防护[J]. 中国氯碱, 2019(7): 23-26. |
| Zhang J P. Equipment corrosion and protection in chlor-alkali industry[J]. China Chlor-Alkali, 2019(7): 23-26. | |
| [3] | 梁诚. 新形势下氯碱行业高质量发展路径分析[J]. 氯碱工业, 2020, 56(12): 1-7. |
| Liang C. Analysis of high quality development path of chlor-alkali industry under the new situations[J]. Chlor-Alkali Industry, 2020, 56(12): 1-7. | |
| [4] | 张丽, 由钢, 乔霄峰, 等. 氯碱电解槽内压力波动的混沌分析及流型识别[J]. 化工学报, 2019, 70(S1): 35-44. |
| Zhang L, You G, Qiao X F, et al. Chaotic analysis of pressure fluctuation and identification of flow regime in chlor-alkali electrolyzer[J]. CIESC Journal, 2019, 70(S1): 35-44. | |
| [5] | 高云, 马万荣, 鲁斌. 氯碱生产中常见腐蚀及防护[J]. 氯碱工业, 2023, 59(2): 26-28. |
| Gao Y, Ma W R, Lu B. Common corrosion and protection in chlor-alkali production[J]. Chlor-Alkali Industry, 2023, 59(2): 26-28. | |
| [6] | 肖光桥. 氯碱电解槽阳极钛板腐蚀与防护研究[D]. 北京: 北京化工大学, 2020. |
| Xiao G Q. Study on corrosion and protection of anode titanium plate in chlor-alkali electrolyzer[D]. Beijing: Beijing University of Chemical Technology, 2020. | |
| [7] | 李书声. 38DD350型电解槽钛板及钢板腐蚀原因分析及密封条设计[D]. 北京: 北京化工大学, 2009. |
| Li S S. Corrosion analysis and sealing strip design of titanium plate and steel plate in 38DD350 electrolyzer[D]. Beijing: Beijing University of Chemical Technology, 2009. | |
| [8] | Mirseyed S F, Jafarzadeh K, Rostamian A, et al. A new insight on the mechanisms of corrosion deactivation of a typical Ti/IrO2 + RuO2 +TiO2 coating in the presence of Ta2O5 in chlor-alkali medium[J]. Corrosion Science, 2023, 214: 111005. |
| [9] | Tang J W, Shao Y W, Zhang T, et al. Corrosion behaviour of carbon steel in different concentrations of HCl solutions containing H2S at 90℃[J]. Corrosion Science, 2011, 53(5): 1715-1723. |
| [10] | Yao H C, Li R, Xiang H Y, et al. Experimental study on the dew point corrosion behavior in atmospheric tower system[J]. Materials Today Communications, 2023, 36: 106829. |
| [11] | 刘亚丽, 王守华, 傅俊丰, 等. 碱液设备及管道的腐蚀与防护[J]. 石油化工设备技术, 2014, 35(5): 40-41, 7. |
| Liu Y L, Wang S H, Fu J F, et al. Corrosion and protection of equipment and pipelines with alkali lye[J]. Petro-chemical Equipment Technology, 2014, 35(5): 40-41, 7. | |
| [12] | 赵天雷, 路伟, 吕海武, 等. 10号碳钢在NaOH溶液中的腐蚀电化学行为[J]. 腐蚀与防护, 2018, 39(11): 833-837, 842. |
| Zhao T L, Lu W, Lü H W, et al. Electrochemical corrosion behavior of 10# carbon steel in NaOH solution[J]. Corrosion & Protection, 2018, 39(11): 833-837, 842. | |
| [13] | 严明亮. 电石乙炔法制氯乙烯腐蚀分析及防护技术[J]. 中国氯碱, 2007(5): 40-42, 45. |
| Yan M L. Corrosion analysis of vinyl chloride with carbide acetylene method and preventive technology[J]. China Chlor-Alkali, 2007(5): 40-42, 45. | |
| [14] | 王尚, 时杰, 徐映. 氯碱化工的腐蚀与防护[J]. 中国氯碱, 2016(11): 28-30. |
| Wang S, Shi J, Xu Y. Brief analysis about corrosion and protection of chlor-alkali chemical industry[J]. China Chlor-Alkali, 2016(11): 28-30. | |
| [15] | 刘雪辉. 基于纳米容器自修复防腐涂层的制备及其性能研究[D]. 济南: 山东大学, 2019. |
| Liu X H. Preparation and properties of self-repairing anticorrosive coatings based on nanocontainers[D]. Jinan: Shandong University, 2019. | |
| [16] | 赵婧, 顾程文, 蹇锡高, 等. 厚朴酚基环氧树脂防腐涂层的制备及性能评价[J]. 化工学报, 2023, 74(7): 3010-3017. |
| Zhao J, Gu C W, Jian X G, et al. Preparation and performance evaluation of magnolol-based epoxy resin anti-corrosion coatings[J]. CIESC Journal, 2023, 74(7): 3010-3017. | |
| [17] | Weng W H, Chen H, Tsai S P, et al. Thermal property of epoxy/SiO2 hybrid material synthesized by the sol-gel process[J]. Journal of Applied Polymer Science, 2004, 91(1): 532-537. |
| [18] | Liu L S, Zhao M Y, Pei X Y, et al. Improving corrosion resistance of epoxy coating by optimizing the stress distribution and dispersion of SiO2 filler[J]. Progress in Organic Coatings, 2023, 179: 107522. |
| [19] | Yu M D, Fan C Q, Han S K, et al. Anticorrosion behavior of superhydrophobic particles reinforced epoxy coatings for long-time in the high salinity liquid[J]. Progress in Organic Coatings, 2020, 147: 105867. |
| [20] | Li H, Lai J, Yao M, et al. Development of high performance superhydrophobic coating with excellent corrosion resistance, durability, and self-cleaning properties using M-TiO2@EP composites[J]. Applied Surface Science, 2022, 601: 154109. |
| [21] | 付海波, 刘晓茹, 孙媛, 等. 环氧树脂/重结晶碳化硅复合材料的抗腐蚀性能[J]. 中国腐蚀与防护学报, 2020, 40(4): 373-380. |
| Fu H B, Liu X R, Sun Y, et al. Corrosion resistance of epoxy resin/recrystallized silicon carbide composite[J]. Journal of Chinese Society for Corrosion and Protection, 2020, 40(4): 373-380. | |
| [22] | 中国腐蚀与防护学会. 金属防腐蚀手册[M]. 上海: 科学技术出版社, 1989. |
| Chinese Society for Corrosion and Protection. Handbook of Metal Corrosion Prevention[M]. Shanghai: Scientific & Technical Publishers,1989. | |
| [23] | Liu Y, Dai H L, Chen S, et al. Study on stress corrosion behavior of 316L austenitic stainless steel in hot NaOH solution[J]. Journal of Materials Research and Technology, 2024, 30: 8894-8905. |
| [24] | Dos Santos R S G, Cabral T S, Rodrigues L A S, et al. Study of the corrosion resistance of high chromium white cast iron welds in the presence of NaOH (30% w/w)[J]. Journal of Materials Research and Technology, 2024, 30: 7563-7574. |
| [25] | Qian Z Z, Hu G J, Zhang S M, et al. Preparation and characterization of montmorillonite-silica nanocomposites: a sol-gel approach to modifying clay surfaces[J]. Physica B: Condensed Matter, 2008, 403(18): 3231-3238. |
| [26] | Zhang C C, Li S M, Xiao J, et al. Enhanced heat and corrosion resistance of organic silicone coatings by dual shielding effects of Zn powder[J]. Electrochimica Acta, 2025, 509: 145329. |
| [27] | Shu T, Zhang Y L, Cao Y H, et al. Anticorrosive and anti-icing/deicing behavior of epoxy composite coatings reinforced with GO-PPy@SiO2 photothermal fillers[J]. Chemical Engineering Journal Advances, 2024, 18: 100592. |
| [1] | 张畅, 解强, 沙雨桐, 王炳杰, 梁鼎成, 刘金昌. 低灰低硅竹炭的制备及衍生硬炭的电化学性能[J]. 化工学报, 2025, 76(6): 3073-3083. |
| [2] | 郭明钢, 杨晓航, 代岩, 米盼盼, 马世鑫, 贺高红, 肖武, 崔福军. 贫氦管输天然气提氦多元化产品耦合工艺优化设计[J]. 化工学报, 2025, 76(5): 2251-2261. |
| [3] | 李坤, 黄锐, 丛君, 马海涛, 常龙娇, 罗绍华. NCM622正极材料结构形态和储锂特性的同步演变[J]. 化工学报, 2025, 76(4): 1831-1840. |
| [4] | 林纬, 杜建, 姚晨, 朱家豪, 汪威, 郑小涛, 徐建民, 喻九阳. 电化学水软化过程中离子输运与成核机理研究[J]. 化工学报, 2025, 76(4): 1788-1799. |
| [5] | 吴迪, 刘世朋, 王文伟, 姜久春, 杨晓光. 机械压力对锂金属电池性能影响的研究进展[J]. 化工学报, 2025, 76(4): 1422-1431. |
| [6] | 马钟琛, 魏子杰, 朱明涛, 叶恒棣, 郭学益, 谭磊. 一步氧化法制备锰酸锂正极材料用电池级四氧化三锰[J]. 化工学报, 2025, 76(3): 1363-1374. |
| [7] | 肖志华, 房浩楠, 郑方植, 孙冬, 陶丽达, 李永峰, 徐春明, 马新龙. NaCl辅助构筑高性能沥青基硬炭负极材料[J]. 化工学报, 2025, 76(2): 846-857. |
| [8] | 徐子易, 席阳, 宋泽文, 周海骏. 碳纳米材料在锌离子电池中的应用研究进展[J]. 化工学报, 2025, 76(1): 40-52. |
| [9] | 纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39. |
| [10] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [11] | 李彦, 郭红利, 苏国庆, 张建文. 加氢装置空冷器气液两相流动与冲刷腐蚀问题[J]. 化工学报, 2025, 76(1): 141-150. |
| [12] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [13] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [14] | 彭丹, 卢俊杰, 倪文静, 杨媛, 汪靖伦. 高电压钴酸锂电池电解液研究进展[J]. 化工学报, 2024, 75(9): 3028-3040. |
| [15] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号