化工学报 ›› 2019, Vol. 70 ›› Issue (1): 179-188.DOI: 10.11949/j.issn.0438-1157.20180784
陈天华1,2( ),张若思1,2,姜国珍1,2,姚明东1,2,刘宏1,2,王颖1,2(
),张若思1,2,姜国珍1,2,姚明东1,2,刘宏1,2,王颖1,2( ),肖文海1,2,元英进1,2
),肖文海1,2,元英进1,2
收稿日期:2018-07-11
修回日期:2018-09-09
出版日期:2019-01-05
发布日期:2019-01-05
通讯作者:
王颖
作者简介:陈天华(1993—),女,硕士研究生,<email>13102217875@163.com</email>|王颖(1983—),女,副研究员,<email>ying.wang@tju.edu.cn</email>
基金资助:
Tianhua CHEN1,2( ),Ruosi ZHANG1,2,Guozhen JIANG1,2,Mingdong YAO1,2,Hong LIU1,2,Ying WANG1,2(
),Ruosi ZHANG1,2,Guozhen JIANG1,2,Mingdong YAO1,2,Hong LIU1,2,Ying WANG1,2( ),Wenhai XIAO1,2,Yingjin YUAN1,2
),Wenhai XIAO1,2,Yingjin YUAN1,2
Received:2018-07-11
Revised:2018-09-09
Online:2019-01-05
Published:2019-01-05
Contact:
Ying WANG
摘要:
蒎烯可衍生为高能量密度燃料,但在酿酒酵母中的全生物合成却未见报道。酿酒酵母由于拥有强大的蛋白表达和翻译后修饰系统以及完整的内膜系统,相比于大肠杆菌等原核生物更适于P450等蛋白的表达,因此将酿酒酵母作为宿主细胞,对于蒎烯或者其他物质实现如“疯狂碳环”的高能量化是至关重要的。本研究在酿酒酵母底盘中表达内源焦磷酸香叶酯合成酶(ERG20)的突变体ERG20ww和火炬松来源的蒎烯合酶(PtPS)构建了蒎烯的合成路径。通过截短PtPS N端2~51位氨基酸残基(tPtPS),蒎烯产量较初始产量(0.329 mg·L-1)提高了2.23倍。在过表达异戊二烯焦磷酸异构酶(IDI1)和RNA聚合酶Ш负调控因子(MAF1)的基础上,表达ERG20ww和tPtPS的融合蛋白,蒎烯产量进一步提高了5.16倍。通过将内源基因ERG20启动子原位替换为弱启动子HXT1,下调ERG20的转录,蒎烯的产量提高了26.0%。最终通过调节发酵过程中的培养基pH使蒎烯产量达11.7 mg·L-1,较初始产量提高了34.5倍。本研究在酿酒酵母中实现蒎烯的从头合成,并获得已知蒎烯摇瓶水平的最高产量。
中图分类号:
陈天华, 张若思, 姜国珍, 姚明东, 刘宏, 王颖, 肖文海, 元英进. 产蒎烯人工酵母细胞的构建[J]. 化工学报, 2019, 70(1): 179-188.
Tianhua CHEN, Ruosi ZHANG, Guozhen JIANG, Mingdong YAO, Hong LIU, Ying WANG, Wenhai XIAO, Yingjin YUAN. Metabolic engineering of Saccharomyces cerevisiae for pinene production[J]. CIESC Journal, 2019, 70(1): 179-188.
| Fuels | Density/(g·ml-1) | Heating value/(MJ·L-1) |
|---|---|---|
| pinene dimers | 0.938 | 39.5 |
| JP-10 | 0.94 | 39.6 |
| RJ-5 | 1.08 | 44.9 |
表1 先进生物燃料的能量密度
Table 1 Energy density of advanced biofuels
| Fuels | Density/(g·ml-1) | Heating value/(MJ·L-1) |
|---|---|---|
| pinene dimers | 0.938 | 39.5 |
| JP-10 | 0.94 | 39.6 |
| RJ-5 | 1.08 | 44.9 |
| Plasmids/strains | Description | Source |
|---|---|---|
| plasmids | ||
| pRS425K | shuttle plasmid for E. coli and S. cerevisiae, Kanr, LEU2 | this laboratory |
| pRS424 | shuttle plasmid for E. coli and S. cerevisiae, Amp, TRP3 | this laboratory |
| PRS425K_LDJ04 | pRS425K harboring cassette GPM1t_GAL7p_GPDt | this laboratory |
| PRS425K_LDJ09 | pRS425K harboring cassette GPDt_GAL1p_FBA1t | this laboratory |
| PRS425K_LDJ10 | pRS425K harboring cassette FBA1t_GAL7p_PGK1t | this laboratory |
| PRS425K_LDJ14 | pRS425K harboring cassette PGK1t_GAL3p_CYC1t | this laboratory |
| PRS424_LDJ04 | pRS424 harboring cassette GPM1t_GAL7p_GPDt | this study |
| ZRS1 | pRS424 harboring cassette GPM1t_GAL7p_PtPS_GPDt | this study |
| ZRS2 | pRS425K harboring cassette GPDt_GAL1p_ERG20ww _FBA1t | this study |
| ZRS3 | pRS424 harboring cassette GPM1t_GAL7p_tPtPS_GPDt | this study |
| ZRS4 | pRS424 harboring cassette GPM1t_GAL7p_ERG20ww -tPtPS_GPDt | this study |
| ZRS5 | pRS425K harboring cassette FBA1t_GAL7p_IDI1_PGK1t | this study |
| ZRS6 | pRS425K harboring cassette PGK1t_GAL3p_MAF1_CYC1t | this study |
| ZRS7 | pRS425K harboring cassette GPDt_GAL1p_ERG20ww _FBA1t_GAL7p _IDI1_PGK1t_GAL3p_MAF1_CYC1t | this study |
| S. cerevisiae strains | ||
| YJGZ1 | MAT a; URA3-52, TRP1-289, LEU2-3,112, HIS3Δ1, MAL2-8C, SUC2, GAL80 :: IDI1_GAL1,10p_tHMGR | this laboratory |
| YJGZ1_HXT1 | YJGZ1, ERG20p_ERG20 :: HXT1p_ERG20 | this study |
| SyBE_Sc02090001 | YJGZ1, ZRS1, ZRS2 | this study |
| SyBE_Sc02090002 | YJGZ1, ZRS3, ZRS2 | this study |
| SyBE_Sc02090003 | YJGZ1, ZRS4, ZRS2 | this study |
| SyBE_Sc02090004 | YJGZ1, ZRS3, ZRS7 | this study |
| SyBE_Sc02090005 | YJGZ1, ZRS4, ZRS7 | this study |
| SyBE_Sc02090006 | YJGZ1_HXT1, ZRS4, ZRS7 | this study |
表2 实验中涉及的质粒及酵母菌株
Table 2 Plasmids and yeast strains involved in this study
| Plasmids/strains | Description | Source |
|---|---|---|
| plasmids | ||
| pRS425K | shuttle plasmid for E. coli and S. cerevisiae, Kanr, LEU2 | this laboratory |
| pRS424 | shuttle plasmid for E. coli and S. cerevisiae, Amp, TRP3 | this laboratory |
| PRS425K_LDJ04 | pRS425K harboring cassette GPM1t_GAL7p_GPDt | this laboratory |
| PRS425K_LDJ09 | pRS425K harboring cassette GPDt_GAL1p_FBA1t | this laboratory |
| PRS425K_LDJ10 | pRS425K harboring cassette FBA1t_GAL7p_PGK1t | this laboratory |
| PRS425K_LDJ14 | pRS425K harboring cassette PGK1t_GAL3p_CYC1t | this laboratory |
| PRS424_LDJ04 | pRS424 harboring cassette GPM1t_GAL7p_GPDt | this study |
| ZRS1 | pRS424 harboring cassette GPM1t_GAL7p_PtPS_GPDt | this study |
| ZRS2 | pRS425K harboring cassette GPDt_GAL1p_ERG20ww _FBA1t | this study |
| ZRS3 | pRS424 harboring cassette GPM1t_GAL7p_tPtPS_GPDt | this study |
| ZRS4 | pRS424 harboring cassette GPM1t_GAL7p_ERG20ww -tPtPS_GPDt | this study |
| ZRS5 | pRS425K harboring cassette FBA1t_GAL7p_IDI1_PGK1t | this study |
| ZRS6 | pRS425K harboring cassette PGK1t_GAL3p_MAF1_CYC1t | this study |
| ZRS7 | pRS425K harboring cassette GPDt_GAL1p_ERG20ww _FBA1t_GAL7p _IDI1_PGK1t_GAL3p_MAF1_CYC1t | this study |
| S. cerevisiae strains | ||
| YJGZ1 | MAT a; URA3-52, TRP1-289, LEU2-3,112, HIS3Δ1, MAL2-8C, SUC2, GAL80 :: IDI1_GAL1,10p_tHMGR | this laboratory |
| YJGZ1_HXT1 | YJGZ1, ERG20p_ERG20 :: HXT1p_ERG20 | this study |
| SyBE_Sc02090001 | YJGZ1, ZRS1, ZRS2 | this study |
| SyBE_Sc02090002 | YJGZ1, ZRS3, ZRS2 | this study |
| SyBE_Sc02090003 | YJGZ1, ZRS4, ZRS2 | this study |
| SyBE_Sc02090004 | YJGZ1, ZRS3, ZRS7 | this study |
| SyBE_Sc02090005 | YJGZ1, ZRS4, ZRS7 | this study |
| SyBE_Sc02090006 | YJGZ1_HXT1, ZRS4, ZRS7 | this study |
| Primer name | Sequence(5'-3') |
|---|---|
| MAF1-F | GGTCTCCAATGAAAGTATGTTATCACTCTAAAACTG |
| MAF1-R | GGTCTCCTTTACTGTAGGGATTCTTCTTGATCTG |
| IDI1-BsaI-F | GGTCTCCAATGACTGCCGACAACAATAG |
| IDI1-BsaI-R | GGTCTCCTTTATAGCATTCTATGAATTTGCCTG |
| tPtPS-BsaI-F | GGTCTCCAATGAGAGGTGGTAAATCTATTGCACC |
| tPtPS-BsaI-R | GGTCTCCTTTATAATGGAACAGTTTCAACAACTGTTCTAG |
| ERG20ww-GS-F | GGTCTCCATAAGAATGCGGCCGCGGTCTCCAATGGCTTCAGAAAAAGAAATTAGG |
| GS-ERG20ww-R | GCTACCGCTACCGCTGCCGCTACCTTTGCTTCTCTTGTAAACTTTGTTCAAG |
| GS-tPtPS-F | GGTAGCGGCAGCGGTAGCGGTAGCATGGCTAGAAGAATTGCTGGTCATCATTC |
| tPtPS-GS-R | GGTCTCCATAAGAATGCGGCCGCGGTCTCCTTTATAATGGAACAGTTTCAACAACTGTTC |
| PHXT1-F | GTGATATCAGATCCACTAGTGGCCTATGCGTGCAGGTCTCATCTGGAATATAATTCC |
| PHXT1-R | CTCTCTCCTAATTTCTTTTTCTGAAGCCATGATTTTACGTATATCAACTAGTTGAC |
| URA3-Cre-loxp-F | ATTCTTTTTTCAATAGTCGAAGTCAGCTTCAGCTGAAGCTTCGTACGCTGCAGGTC |
| URA-Cre-loxp-R | AGATGAGACCTGCAGCATAGGCCACTAGTGGATCTGATATCACCTAATAACTTCG |
| Actin-F | GCCTTGGACTTCGAACAAGA |
| Actin-R | CCAAACCCAAAACAGAAGGA |
| ERG20-F | GCTATACCACGAATATGAAG |
| ERG20-R | GAACGCAGTTAAGACATC |
表3 本文所用引物
| Primer name | Sequence(5'-3') |
|---|---|
| MAF1-F | GGTCTCCAATGAAAGTATGTTATCACTCTAAAACTG |
| MAF1-R | GGTCTCCTTTACTGTAGGGATTCTTCTTGATCTG |
| IDI1-BsaI-F | GGTCTCCAATGACTGCCGACAACAATAG |
| IDI1-BsaI-R | GGTCTCCTTTATAGCATTCTATGAATTTGCCTG |
| tPtPS-BsaI-F | GGTCTCCAATGAGAGGTGGTAAATCTATTGCACC |
| tPtPS-BsaI-R | GGTCTCCTTTATAATGGAACAGTTTCAACAACTGTTCTAG |
| ERG20ww-GS-F | GGTCTCCATAAGAATGCGGCCGCGGTCTCCAATGGCTTCAGAAAAAGAAATTAGG |
| GS-ERG20ww-R | GCTACCGCTACCGCTGCCGCTACCTTTGCTTCTCTTGTAAACTTTGTTCAAG |
| GS-tPtPS-F | GGTAGCGGCAGCGGTAGCGGTAGCATGGCTAGAAGAATTGCTGGTCATCATTC |
| tPtPS-GS-R | GGTCTCCATAAGAATGCGGCCGCGGTCTCCTTTATAATGGAACAGTTTCAACAACTGTTC |
| PHXT1-F | GTGATATCAGATCCACTAGTGGCCTATGCGTGCAGGTCTCATCTGGAATATAATTCC |
| PHXT1-R | CTCTCTCCTAATTTCTTTTTCTGAAGCCATGATTTTACGTATATCAACTAGTTGAC |
| URA3-Cre-loxp-F | ATTCTTTTTTCAATAGTCGAAGTCAGCTTCAGCTGAAGCTTCGTACGCTGCAGGTC |
| URA-Cre-loxp-R | AGATGAGACCTGCAGCATAGGCCACTAGTGGATCTGATATCACCTAATAACTTCG |
| Actin-F | GCCTTGGACTTCGAACAAGA |
| Actin-R | CCAAACCCAAAACAGAAGGA |
| ERG20-F | GCTATACCACGAATATGAAG |
| ERG20-R | GAACGCAGTTAAGACATC |
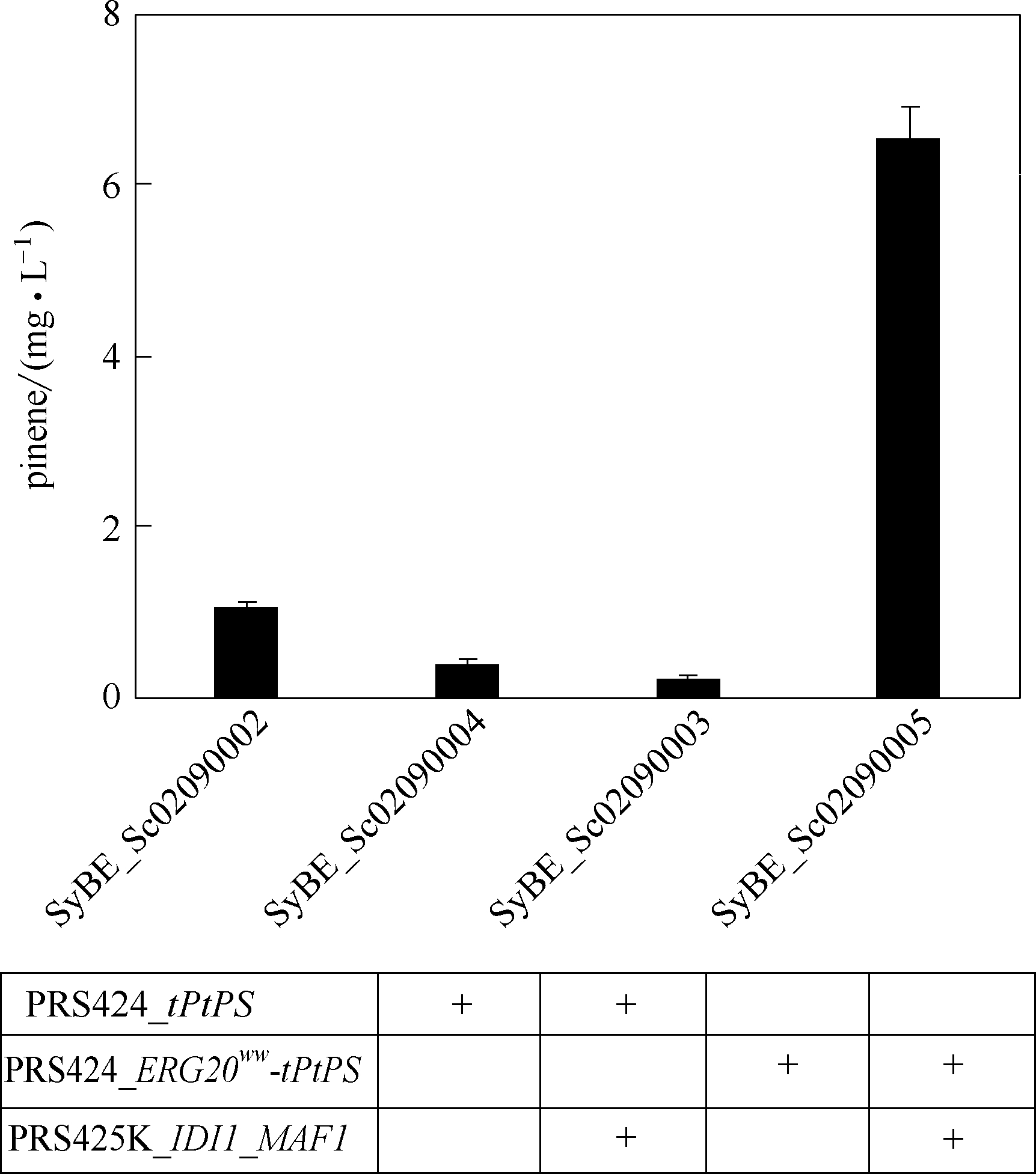
图5 过表达前体基因IDI1和MAF1以及融合表达ERG20ww/tPtPs对蒎烯产量的影响
Fig.5 Effects of overexpression of precursor genes IDI1 and MAF1 as well as expression fusion of ERG20ww/tPtPs on pinene produciton
| 1 | Harvey B G , Wright M E , Quintana R L . High-density renewable fuels based on the selective dimerization of pinenes [J]. Energy & Fuels, 2010, 24(1): 267-273. |
| 2 | 邹吉军, 张香文, 王莅, 等 . 高密度液体碳氢燃料合成及应用进展 [J]. 含能材料, 2007, (4): 411-415. |
| Zou J J , Zhang X W , Wang L , et al . Progress on the synthesis and application of high-density liquid hydrocarbon fuels [J]. Chinese Journal of Energetic Materials, 2007, (4): 411-415. | |
| 3 | 冯红茹, 杨建明, 秦利, 等 . β-蒎烯合成酶(QH6)在大肠杆菌中的表达及其产β-蒎烯的研究[J]. 生物加工过程, 2015, 13(1): 28-34. |
| Feng H R , Yang J M , Qin L , et al . Expression of β-pinene synthase (QH6) in Escherichia coli for the biosynthesis of β-pinene [J]. Chinese Journal of Bioprocess Engineering, 2015, 13(1): 28-34. | |
| 4 | Clomburg J M , Gonzalez R . Biofuel production in Escherichia coli: the role of metabolic engineering and synthetic biology [J]. Appl. Microbiol. Biotechnol., 2010, 86(2): 419-434. |
| 5 | Leonard E , Lim K H , Saw P N , et al . Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli [J]. Appl. Environ. Microbiol., 2007, 73(12): 3877-3886. |
| 6 | Schmidt-Dannert C , Umeno D , Arnold F H . Molecular breeding of carotenoid biosynthetic pathways [J]. Nat. Biotechnol., 2000, 18(7): 750-753. |
| 7 | Zhang L , Xiao W H , Wang Y , et al . Chassis and key enzymes engineering for monoterpenes production [J]. Biotechnol. Adv., 2017, 35(8): 1022-1031. |
| 8 | Niu F , Lu Q , Bu Y , et al . Metabolic engineering for the microbial production of isoprenoids: carotenoids and isoprenoid-based biofuels [J]. Synthetic and Systems Biotechnology, 2017, 2(3): 167-175. |
| 9 | Kang M , Eom J , Kim Y , et al . Biosynthesis of pinene from glucose using metabolically-engineered [J]. Biotechnology Letters, 2014, 36(10): 2069-2077. |
| 10 | Sarria S , Wong B , García Martín H , et al . Microbial synthesis of pinene [J]. ACS Synthetic Biology, 2014, 3(7): 466-475. |
| 11 | Yang J , Nie Q , Ren M , et al . Metabolic engineering of Escherichia coli for the biosynthesis of alpha-pinene [J]. Biotechnol. Biofuels, 2013, 6(1): 60. |
| 12 | Zhang X , Mcvay R C , Huang D D , et al . Formation and evolution of molecular products in alpha-pinene secondary organic aerosol [J]. Proc. Natl. Acad. Sci. USA, 2015, 112(46): 14168-14173. |
| 13 | 邓云祥, 王玉平, 王力昌, 等 . α-蒎烯选择性二聚合及低聚反应研究(Ⅱ):二聚异构体研究 [J]. 高分子学报, 1995, (2): 170-175. |
| Deng Y X , Wang Y P , Wang L C , et al . Studies on selective dimerization and oligomerization of α-pinene(Ⅱ): Studies of the dimeric isomers [J]. Acta Polymerica Sinica, 1995, (2): 170-175. | |
| 14 | Chen K , Huang X , Kan S , et al . Enzymatic construction of highly strained carbocycles [J]. Science, 2018, 360(6384): 71-75. |
| 15 | Kan S , Huang X , Gumulya Y , et al . Genetically programmed chiral organoborane synthesis [J]. Nature, 2017, 552(7683): 132-136. |
| 16 | Ajikumar P K , Xiao W H , Tyo K E , et al . Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli [J]. Science, 2010, 330(6000): 70-74. |
| 17 | Jiang G , Yao M , Wang Y , et al . Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2017, 41: 57-66. |
| 18 | 李博, 梁楠, 刘夺, 等 . 合成8-二甲基异戊烯基柚皮素的人工酿酒酵母菌株构建 [J]. 中国生物工程杂志, 2017, (9): 71-81. |
| Li B , Liang N , Liu D , et al . Metabolic engineering of Saccharomyces cerevisiae for production of 8-dimenthylally naringenin [J]. China Biotechnology, 2017, (9): 71-81. | |
| 19 | Gietz R D . Yeast transformation by the LiAc/SS carrier DNA/PEG method [J]. Methods Mol. Biol., 2014, 1205: 1-12. |
| 20 | Mathiasen D P , Lisby M . Cell cycle regulation of homologous recombination in Saccharomyces cerevisiae [J]. FEMS Microbiol. Rev., 2014, 38(2): 172-184. |
| 21 | Peck R F , Dassarma S , Krebs M P . Homologous gene knockout in the archaeon Halobacterium salinarum with ura3 as a counterselectable marker [J]. Mol. Microbiol., 2000, 35(3): 667-676. |
| 22 | Livak K J , Schmittgen T D . Analysis of relative gene expression data using real-time quantitative PCR and the 2 - Δ Δ C T method [J]. Methods, 2001, 25(4): 402-408. |
| 23 | Szkopinska A , Plochocka D . Farnesyl diphosphate synthase; regulation of product specificity [J]. Acta Biochim. Pol., 2005, 52(1): 45-55. |
| 24 | Grabinska K , Palamarczyk G . Dolichol biosynthesis in the yeast Saccharomyces cerevisiae: an insight into the regulatory role of farnesyl diphosphate synthase [J]. FEMS Yeast Res., 2002, 2(3): 259-265. |
| 25 | Ignea C , Pontini M , Maffei M E , et al . Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase [J]. ACS Synth. Biol., 2014, 3(5): 298-306. |
| 26 | Turner G , Gershenzon J , Nielson E E , et al . Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells [J]. Plant Physiol., 1999, 120(3): 879-886. |
| 27 | Bohlmann J , Meyer-Gauen G , Croteau R . Plant terpenoid synthases: molecular biology and phylogenetic analysis [J]. Proc. Natl. Acad. Sci. USA, 1998, 95(8): 4126-4133. |
| 28 | Köllner T G , Schnee C , Gershenzon J , et al . The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes [J]. The Plant Cell, 2004, 16(5): 1115-1131. |
| 29 | Cao Y , Zhang H , Liu H , et al . Biosynthesis and production of sabinene: current state and perspectives [J]. Applied Microbiology and Biotechnology, 2018, 102(4): 1535-1544. |
| 30 | Liu J , Zhang W , Du G , et al . Overproduction of geraniol by enhanced precursor supply in Saccharomyces cerevisiae [J]. Journal of Biotechnology, 2013, 168(4): 446-451. |
| 31 | Pluta K , Lefebvre O , Martin N C , et al . Maf1p, a negative effector of RNA polymerase III in Saccharomyces cerevisiae [J]. Molecular and Cellular Biology, 2001, 21(15): 5031-5040. |
| 32 | Clastre M , Bantignies B , Feron G , et al . Purification and characterization of geranyl diphosphate synthase from Vitis vinifera L. cv Muscat de Frontignan cell cultures [J]. Plant Physiol., 1993, 102(1): 205-211. |
| 33 | Dunlop M J , Dossani Z Y , Szmidt H L , et al . Engineering microbial biofuel tolerance and export using efflux pumps [J]. Molecular Systems Biology, 2011, 7(1): 487. |
| 34 | Albertsen L , Chen Y , Bach L S , et al . Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes [J]. Appl. Environ. Microbiol., 2011, 77(3): 1033-1040. |
| 35 | Ohto C , Muramatsu M , Obata S , et al . Production of geranylgeraniol on overexpression of a prenyl diphosphate synthase fusion gene in Saccharomyces cerevisiae [J]. Appl. Microbiol. Biotechnol., 2010, 87(4): 1327-1334. |
| 36 | Zhao J , Li C , Zhang Y , et al . Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2017, 16(1): 17. |
| 37 | Xie W , Ye L , Lv X , et al . Sequential control of biosynthetic pathways for balanced utilization of metabolic intermediates in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2015, 28: 8-18. |
| 38 | Wang Y , Lim L , Diguistini S , et al . A specialized ABC efflux transporter GcABC-G1 confers monoterpene resistance to Grosmannia clavigera, a bark beetle-associated fungal pathogen of pine trees [J]. New Phytol., 2013, 197(3): 886-898. |
| 39 | Piper P W . The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap [J]. FEMS Microbiol. Lett., 1995, 134(2/3): 121-127. |
| 40 | Jiang X , Zhang H , Yang J , et al . Induction of gene expression in bacteria at optimal growth temperatures [J]. Appl. Microbiol. Biotechnol., 2013, 97(12): 5423-5431. |
| [1] | 赵春雷, 郭亮, 高聪, 宋伟, 吴静, 刘佳, 刘立明, 陈修来. 代谢工程改造大肠杆菌生产软骨素[J]. 化工学报, 2023, 74(5): 2111-2122. |
| [2] | 刘昕, 戈钧, 李春. 光驱动微生物杂合系统提高生物制造水平[J]. 化工学报, 2023, 74(1): 330-341. |
| [3] | 刘雪, 张莉娟, 赵广荣. 大肠杆菌偏利共培养系统合成大豆苷元[J]. 化工学报, 2022, 73(9): 4015-4024. |
| [4] | 王靖楠, 庞建, 秦磊, 郭超, 吕波, 李春, 王超. 丁烯基多杀菌素高产菌株的选育和改造策略[J]. 化工学报, 2022, 73(2): 566-576. |
| [5] | 孙怡, 张腾, 吕波, 李春. 胞内生物传感器提高微生物细胞工厂的精细调控[J]. 化工学报, 2022, 73(2): 521-534. |
| [6] | 王欣慧, 王颖, 姚明东, 肖文海. 维生素A生物合成的研究进展[J]. 化工学报, 2022, 73(10): 4311-4323. |
| [7] | 周武林, 高惠芳, 吴玉玲, 张显, 徐美娟, 杨套伟, 邵明龙, 饶志明. 重组酿酒酵母生物合成菜油甾醇[J]. 化工学报, 2021, 72(8): 4314-4324. |
| [8] | 王欣, 赵鹏, 李清扬, 田平芳. 半导体合成生物学的研究进展[J]. 化工学报, 2021, 72(5): 2426-2435. |
| [9] | 毛金竹, 肖淑玲, 杨智淳, 王孝宇, 张诗, 陈俊宏, 谢佶晟, 陈福德, 黄子诺, 冯天宇, 张瑷珲, 方柏山. 合成生物学在农残检测领域的应用[J]. 化工学报, 2021, 72(5): 2413-2425. |
| [10] | 郑煜堃, 孙青, 陈振, 于慧敏. 微生物细胞工厂生产化学品的研究进展——以几种典型小分子和大分子化学品为例[J]. 化工学报, 2021, 72(12): 6109-6121. |
| [11] | 王炼, 吴迪, 周景文. 木脂素的生物合成及其微生物法生产的研究进展[J]. 化工学报, 2021, 72(1): 320-333. |
| [12] | 赵贞尧, 张保财, 李锋, 宋浩. 产电细胞的合成生物学设计构建[J]. 化工学报, 2021, 72(1): 468-482. |
| [13] | 王凯峰, 王金鹏, 韦萍, 纪晓俊. 代谢工程改造解脂耶氏酵母生产脂肪酸及其衍生物[J]. 化工学报, 2021, 72(1): 351-365. |
| [14] | 高虎涛, 申晓林, 孙新晓, 王佳, 袁其朋. 代谢工程调控策略在生物合成氨基酸及其衍生物中的应用[J]. 化工学报, 2020, 71(9): 4058-4070. |
| [15] | 徐静, 由紫暄, 张君奇, 陈正, 吴德光, 李锋, 宋浩. 合成生物学方法改造电活性生物膜研究进展[J]. 化工学报, 2020, 71(9): 3950-3962. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号