化工学报 ›› 2021, Vol. 72 ›› Issue (1): 578-588.DOI: 10.11949/0438-1157.20200955
收稿日期:2020-07-16
修回日期:2020-10-19
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
孙世鹏
作者简介:刘宁(1994—),男,硕士研究生,基金资助:
LIU Ning( ),CHU Changhui(
),CHU Changhui( ),WANG Qian,SUN Shipeng(
),WANG Qian,SUN Shipeng( )
)
Received:2020-07-16
Revised:2020-10-19
Online:2021-01-05
Published:2021-01-05
Contact:
SUN Shipeng
摘要:
针对有机颜料废水中单价相似离子(例如CH3COO-和Cl-)分离难的问题,以表面活化能与脱水现象协同作用的分离机制为指导,在界面聚合中加入3,5-二氨基苯甲酸(DMA)来调控孔径、电性等性质,制备对醋酸根和氯离子具有高选择性的复合纳滤膜。XPS结果表明DMA参与界面聚合反应,形成疏松选择层;Zeta电位表明膜表面负电性增强。通过pH、操作压力等条件优化,得出0.6%(质量) DMA-TFC膜性能最佳,水通量较未改性复合膜提高44%,对于醋酸钠与硫酸钠的分离比达到15.0。本工作为相似离子分离纳滤膜的设计与制备提供了理论和实践基础,在颜料废水等水处理、物料分离等领域展现了良好的应用前景。
中图分类号:
刘宁, 褚昌辉, 王乾, 孙世鹏. 用于混合一价盐分离的纳滤膜的制备及性能研究[J]. 化工学报, 2021, 72(1): 578-588.
LIU Ning, CHU Changhui, WANG Qian, SUN Shipeng. Preparation of nanofiltration membrane for separation of mixed monovalent salts[J]. CIESC Journal, 2021, 72(1): 578-588.
| 铸膜液成分/g | 膜厚/μm | 孔隙率/% | 孔径分布/nm | 平均孔径/nm |
|---|---|---|---|---|
| NMP∶PEG∶PI=64∶16∶20 | 80±5 | 61.79±3.24 | 19.36~125.62 | 56.39 |
表1 铸膜液组成及超滤膜相关参数
Table 1 The composition of dope and the related parameters of the ultrafiltration membrane
| 铸膜液成分/g | 膜厚/μm | 孔隙率/% | 孔径分布/nm | 平均孔径/nm |
|---|---|---|---|---|
| NMP∶PEG∶PI=64∶16∶20 | 80±5 | 61.79±3.24 | 19.36~125.62 | 56.39 |
| Compound | Chemical structure | Molecular weight |
|---|---|---|
| PIP |  | 86.14 |
| DMA |  | 152.15 |
| TMC |  | 265.48 |
表2 PIP、DMA和TMC分子结构、分子量
Table 2 Structure and molecular weight of PIP, DMA, and TMC
| Compound | Chemical structure | Molecular weight |
|---|---|---|
| PIP |  | 86.14 |
| DMA |  | 152.15 |
| TMC |  | 265.48 |
| Membrane | N in different chemical states /% | 原子组成/% | N/O | |||
|---|---|---|---|---|---|---|
| —NH— | —NCO— | C 1s | N 1s | O 1s | ||
| 0 DMA-TFC | 16.88 | 83.12 | 82.49 | 9.42 | 8.09 | 1.16 |
| 0.2% DMA-TFC | 21.06 | 78.94 | 84.05 | 8.85 | 7.10 | 1.25 |
| 0.6% DMA-TFC | 26.43 | 73.57 | 83.27 | 10.22 | 6.51 | 1.57 |
| 1.0% DMA-TFC | 28.43 | 71.57 | 84.51 | 9.68 | 5.81 | 1.67 |
表3 DMA-TFC膜的X射线光电子能谱原子组成和不同化学态N的相对含量
Table 3 The X-ray photoelectron spectral atomic composition of the DMA-TFC membrane and the relative composition of N in different chemical states
| Membrane | N in different chemical states /% | 原子组成/% | N/O | |||
|---|---|---|---|---|---|---|
| —NH— | —NCO— | C 1s | N 1s | O 1s | ||
| 0 DMA-TFC | 16.88 | 83.12 | 82.49 | 9.42 | 8.09 | 1.16 |
| 0.2% DMA-TFC | 21.06 | 78.94 | 84.05 | 8.85 | 7.10 | 1.25 |
| 0.6% DMA-TFC | 26.43 | 73.57 | 83.27 | 10.22 | 6.51 | 1.57 |
| 1.0% DMA-TFC | 28.43 | 71.57 | 84.51 | 9.68 | 5.81 | 1.67 |
| Membranes | MWCO | rp/nm |
|---|---|---|
| 0 DMA-TFC | 212 | 0.14 |
| 0.2% DMA-TFC | 344 | 0.18 |
| 0.6% DMA-TFC | 397 | 0.21 |
| 1.0% DMA-TFC | 457 | 0.22 |
表4 不同浓度DMA-TFC膜截留分子量及平均孔径
Table 4 MWCO and rp for TFC membranes with different DMA contents
| Membranes | MWCO | rp/nm |
|---|---|---|
| 0 DMA-TFC | 212 | 0.14 |
| 0.2% DMA-TFC | 344 | 0.18 |
| 0.6% DMA-TFC | 397 | 0.21 |
| 1.0% DMA-TFC | 457 | 0.22 |

图6 不同浓度的DMA-TFC膜混合单价盐截留与通量(压力1 MPa, 温度25℃)
Fig.6 Nanofiltration performance of DMA-TFC membranes with different DMA contents (under the pressure of 1 MPa and the temperature of 25℃)
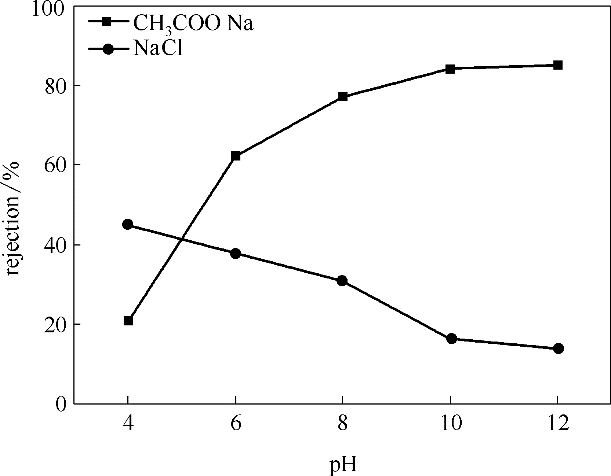
图7 pH对于0.6%(质量) DMA-TFC膜分离混合盐性能的影响(压力0.6 MPa, 温度25℃)
Fig.7 Effects of pH on performance of 0.6%(mass) DMA-TFC membrane (under the pressure of 0.6 MPa and the temperature of 25℃)
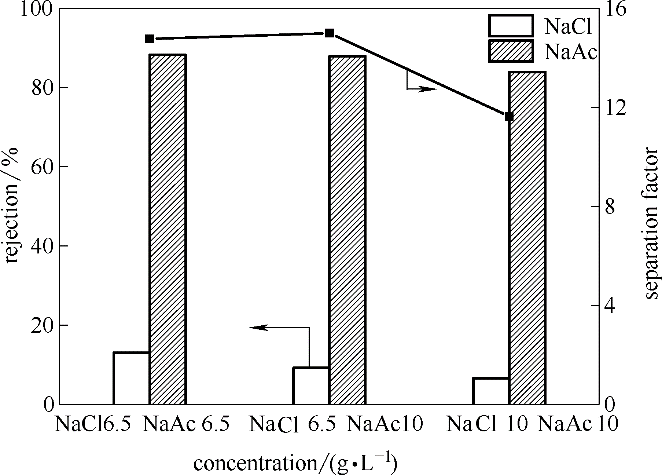
图9 0.6%(质量) DMA-TFC膜对于不同浓度比的混合单价盐的分离(压力0.6 MPa,温度25℃)
Fig.9 Rejection of 0.6%(mass) DMA-TFC membrane for mixed monovalent salts with different concentration ratios (under the pressure of 0.6 MPa and the temperature of 25℃)
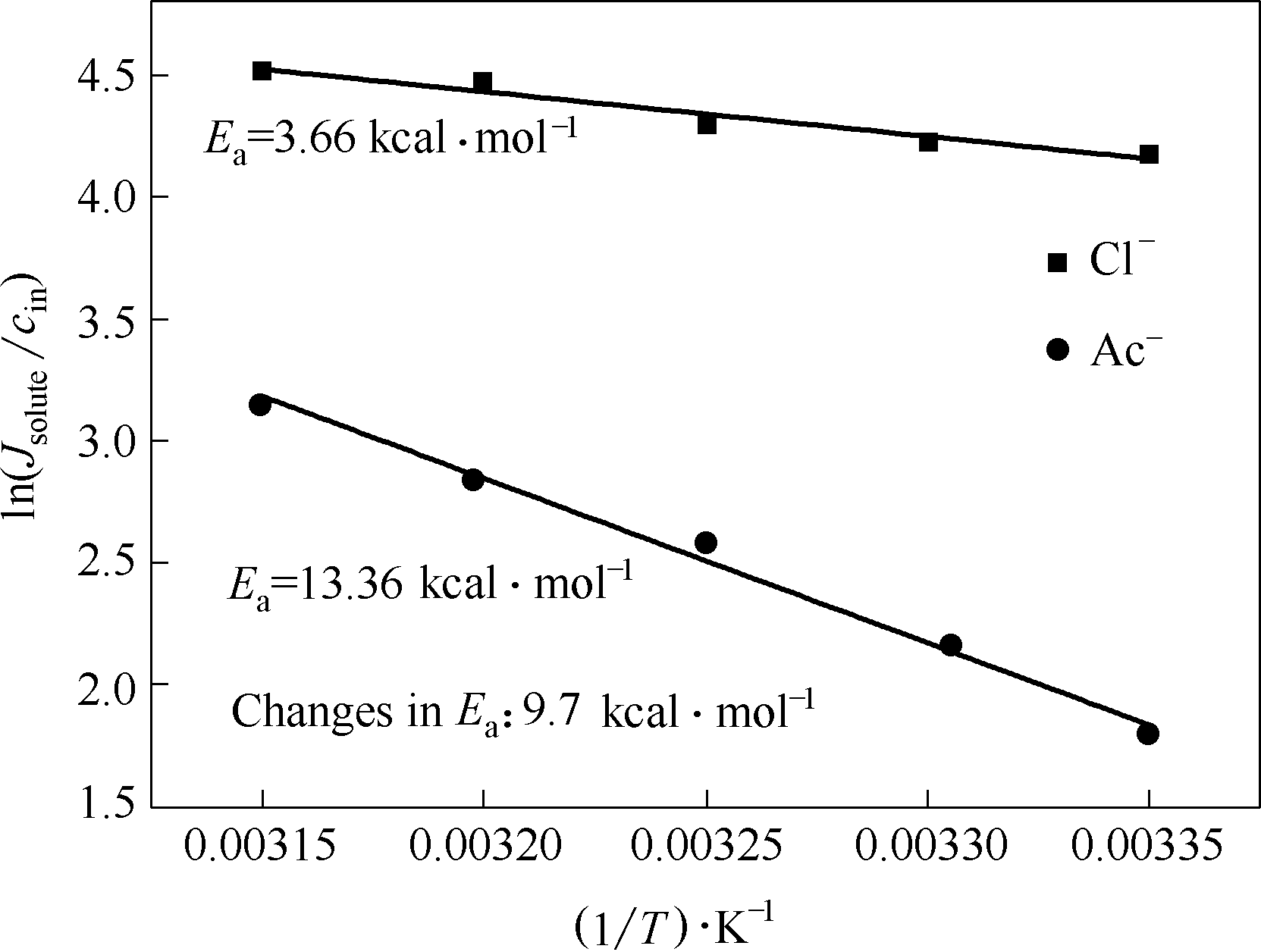
图10 在pH=10时,氯离子和醋酸根阴离子通过膜孔所需的活化能(1 kcal=4186 J)
Fig.10 Activation energy required for chloride and acetate anions to pass through the membrane at pH=10
| Ion | r/nm① | Δr/nm② | N③ | ΔhydGcalc*/ (kJ·mol-1)④ |
|---|---|---|---|---|
| CH3COO- | 0.162 | 0.055 | 2.2 | -300 |
| Cl- | 0.181 | 0.043 | 2.0 | -270 |
表5 CH3COO-和Cl-的物理特性[42]
Table 5 Physical properties of CH3COO- and Cl- ions[42]
| Ion | r/nm① | Δr/nm② | N③ | ΔhydGcalc*/ (kJ·mol-1)④ |
|---|---|---|---|---|
| CH3COO- | 0.162 | 0.055 | 2.2 | -300 |
| Cl- | 0.181 | 0.043 | 2.0 | -270 |
| 1 | 陈荣圻. 有机颜料的生态环保问题探讨(一)[J]. 印染, 2003, (2): 36-41. |
| Chen R Q. Discussion on ecological and environmental protection problems of organic colors (I)[J]. Dyeing & Finishing, 2003, (2): 36-41. | |
| 2 | 陈荣圻. 有机颜料的生态环保问题探讨(二)[J]. 印染, 2003, (3): 35-41. |
| Chen R Q. Discussion on ecological and environmental protection problems of organic colors (Ⅱ)[J]. Dyeing & Finishing, 2003, (3): 35-41. | |
| 3 | Abbasian M, Jaymand M, Niroomand P, et al. Grafting of aniline derivatives onto chitosan and their applications for removal of reactive dyes from industrial effluents[J]. International Journal Biological Macromolecules, 2017, 95: 393-403. |
| 4 | Huang X, Gao B, Yue Q, et al. Compound bioflocculant used as a coagulation aid in synthetic dye wastewater treatment: the effect of solution pH[J]. Separation and Purification Technology, 2015, 154: 108-114. |
| 5 | Khan M A N, Siddique M, Wahid F, et al. Removal of reactive blue 19 dye by sono, photo and sonophotocatalytic oxidation using visible light[J]. Ultrason Sonochem, 2015, 26: 370-377. |
| 6 | Sarkar A K, Saha A, Tarafder A, et al. Efficient removal of toxic dyes via simultaneous adsorption and solar light driven photodegradation using recyclable functionalized amylopectin–TiO2–Au nanocomposite[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1679-1688. |
| 7 | Cervantes F J, Dos Santos A B. Reduction of azo dyes by anaerobic bacteria: microbiological and biochemical aspects[J]. Reviews in Environmental Science and Bio/Technology, 2011, 10(2): 125-137. |
| 8 | Singh R L, Singh P K, Singh R P. Enzymatic decolorization and degradation of azo dyes: a review[J]. International Biodeterioration & Biodegradation, 2015, 104: 21-31. |
| 9 | Pennemann H, Forster S, Kinkel J, et al. Improvement of dye properties of the azo pigment yellow 12 using a micromixer-based process[J]. Organic Process Research & Development, 2005, 9: 188-192. |
| 10 | 蔡李鹏, 祁晓婷, 王娟, 等. 有机颜料黄的合成研究现状[J]. 化学与生物工程, 2013, 30(7): 10-12. |
| Cai L P, Qi X T, Wang J, et al. Synthetic research status of organic pigment yellow[J]. Chemistry & Bioengineering, 2013, 30(7): 10-12. | |
| 11 | 王仲旭. 颜料废水治理工程实例[J]. 工业水处理, 2018, 38(2): 95-98. |
| Wang Z X. Project example of pigment wastewater treatment[J]. Industrial Water Treatment, 2018, 38(2): 95-98. | |
| 12 | Wen Y H, Yuan J M, Ma X M, et al. Polymeric nanocomposite membranes for water treatment: a review[J]. Environmental Chemistry Letters, 2019, 17(4): 1539-1551. |
| 13 | 蔡媛媛,郭百涛,邢卫红, 等. 面向健康产业应用需求的膜技术与膜材料[J]. 化工学报, 2020, 71(7): 2921-2932. |
| Cai Y Y, Guo B T, Xing W H, et al. Progress research on development of membrane technology and materials for health industry[J]. CIESC Journal, 2020, 71(7): 2921-2932. | |
| 14 | Xiong S, Zhang D Y, Mei S, et al. Thin film composite membranes containing intrinsic CD cavities in the selective layer[J]. Journal of Membrane Science, 2018, 551: 294-304. |
| 15 | Paul M, Jons S D. Chemistry and fabrication of polymeric nanofiltration membranes: a review[J]. Polymer, 2016, 103: 417-456. |
| 16 | Zhang Z, Kang G D, Yu H J, et al. From reverse osmosis to nanofiltration: precise control of the pore size and charge of polyamide membranes via interfacial polymerization[J]. Desalination, 2019, 466: 16-23. |
| 17 | Xu Y Q, Wu M Y, Yu S Y, et al. Ultrathin and stable graphene oxide film via intercalation polymerization of polydopamine for preparation of digital inkjet printing dye[J]. Journal of Membrane Science, 2019, 586: 15-22. |
| 18 | Yang X B, Sun P, Zhang H R, et al. Polyphenol-sensitized atomic layer deposition for membrane interface hydrophilization[J]. Advanced Functional Materials, 2020, 30(15): 1910062-1910071. |
| 19 | 唐元晖, 扈阳, 燕至琴, 等. 高浓度含盐草甘膦溶液的纳滤分离实验研究[J]. 化工学报, 2019, 70(7): 2574-2583. |
| Tang Y H, Hu Y, Yan Z Q, et al. Experimental study on nanofiltration separation of high concentrated saline glyphosate solution[J]. CIESC Journal, 2019, 70(7): 2574-2583. | |
| 20 | 赵德伟. 纳滤膜分离技术在废水处理中的应用[J]. 节能与环保, 2020, (Z1): 70-71. |
| Zhao D W. Application of nanofiltration membrane separation technology in wastewater treatment[J]. Energy Conservation & Environmental Protection, 2020, (Z1): 70-71. | |
| 21 | 汪菊, 牛淑锋, 漆虹, 等. GO/Al2O3复合纳滤膜的制备及其稳定性能研究[J]. 化工学报, 2020, 71(6): 2795-2803. |
| Wang J, Niu S F, Qi H, et al. Fabrication and stability of GO/Al2O3 composite nanofiltration membranes[J]. CIESC Journal, 2020, 71(6): 2795-2803. | |
| 22 | Liang B, Li Q, Cao B, et al. Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes[J]. Desalination, 2018, 433: 132-140. |
| 23 | Wang Q, Wang Y, Chen B Z, et al. Designing high-performance nanofiltration membranes for high-salinity separation of sulfate and chloride in the chlor-alkali process[J]. Industrial & Engineering Chemistry Research, 2019, 58: 12280-12290. |
| 24 | 徐颜军, 徐泽海, 孟琴, 等. 新型还原氧化石墨烯/氮化碳复合纳滤膜制备及其性能[J]. 化工学报, 2019, 70(9): 3565-3572. |
| Xu Y J, Xu Z H, Meng Q, et al. Preparation and performance of novel rGO/uCN composite nanofiltration membrane[J]. CIESC Journal, 2019, 70(9): 3565-3572. | |
| 25 | Lin J Y, Tang C Y, Huang C M, et al. A comprehensive physico-chemical characterization of superhydrophilic loose nanofiltration membranes[J]. Journal of Membrane Science, 2016, 501: 1-14. |
| 26 | Epsztein R, Shaulsky E, Dizge N, et al. Role of ionic charge density in Donnan exclusion of monovalent anions by nanofiltration[J]. Environmental Science & Technology, 2018, 52: 4108-4116. |
| 27 | Xue S, Wu C M, Wu Y H, et al. An optimized process for treating sodium acetate waste residue: coupling of diffusion dialysis or electrodialysis with bipolar membrane electrodialysis[J]. Chemical Engineering Research and Design, 2018, 129: 237-247. |
| 28 | Chu C H, Wang C, Xiao H F, et al. Separation of ions with equivalent and similar molecular weights by nanofiltration: sodium chloride and sodium acetate as an example[J]. Separation and Purification Technology, 2020, 250: 117199. |
| 29 | Xiao H F, Shao D D, Wu Z L, et al. Zero liquid discharge hybrid membrane process for separation and recovery of ions with equivalent and similar molecular weights[J]. Desalination, 2020, 482: 114387. |
| 30 | Chai G Y, Krantz W B. Formation and characterization of polyamide membranes via interfacial polymerization[J]. Journal of Membrane Science, 1994, 93: 175-192. |
| 31 | Kwon Y, Leckie J O. Hypochlorite degradation of crosslinked polyamide membranes (Ⅱ): Changes in hydrogen bonding behavior and performance[J]. Journal of Membrane Science, 2006, 282: 456-464. |
| 32 | Tang C Y, Kwon Y L, Leckie J O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes (Ⅰ): FTIR and XPS characterization of polyamide and coating layer chemistry[J]. Desalination, 2009, 242: 149-167. |
| 33 | Zhang Y, Jia L L, Cai T, et al. Sulfonated hyperbranched polyglycerol grafted membranes with antifouling properties for sustainable osmotic power generation using municipal wastewater[J]. Journal of Membrane Science, 2018, 563: 521-530. |
| 34 | Misdan N, Lau W J, Ismail A F, et al. Study on the thin film composite poly(piperazine-amide) nanofiltration membrane: impacts of physicochemical properties of substrate on interfacial polymerization formation[J]. Desalination, 2014, 344: 198-205. |
| 35 | Morao A, Alves A, Afonso M. Concentration of clavulanic acid broths: influence of the membrane surface charge density on NF operation[J]. Journal of Membrane Science, 2006, 281: 417-428. |
| 36 | Liu Y L, Wang X M, Yang H W, et al. Preparation of nanofiltration membranes for high rejection of organic micropollutants and low rejection of divalent cations[J]. Journal of Membrane Science, 2019, 572: 152-160. |
| 37 | Andrade L H, Aguiar A O, Pires W L, et al. Comprehensive bench- and pilot-scale investigation of NF for gold mining effluent treatment: membrane performance and fouling control strategies[J]. Separation and Purification Technology, 2017, 174: 44-56. |
| 38 | Fridman-Bishop N, Tankus K A, Freger V. Permeation mechanism and interplay between ions in nanofiltration[J]. Journal of Membrane Science, 2018, 548: 449-458. |
| 39 | Ge Q C, H G, Chung T S. Effective As(Ⅲ) removal by a multi-charged hydroacid complex draw solute facilitated forward osmosis-membrane distillation (FO-MD) processes[J]. Environmental Science & Technology, 2016, 50(5): 2363-2370. |
| 40 | Zhou F L, Wang C W, Wei J. Separation of acetic acid from monosaccharides by NF and RO membranes: performance comparison[J]. Journal of Membrane Science, 2013, 429: 243-251. |
| 41 | Epsztein R, Cheng W, Shaulsky E, et al. Elucidating the mechanisms underlying the difference between chloride and nitrate rejection in nanofiltration[J]. Journal of Membrane Science, 2018, 548: 694-701. |
| 42 | Marcus Y. Thermodynamics of solvation of ions[J]. Journal of the Chemical Society-Faraday Transactions, 1991, 87 (18): 2995-2999. |
| [1] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [2] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [3] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [4] | 张艳梅, 袁涛, 李江, 刘亚洁, 孙占学. 高效SRB混合菌群构建及其在酸胁迫条件下的性能研究[J]. 化工学报, 2023, 74(6): 2599-2610. |
| [5] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [6] | 闫新龙, 黄志刚, 胡清勋, 张新, 胡晓燕. Cu/Co掺杂多孔炭活化过硫酸盐降解水中硝基酚研究[J]. 化工学报, 2023, 74(3): 1102-1112. |
| [7] | 李承威, 骆华勇, 张铭轩, 廖鹏, 方茜, 荣宏伟, 王竞茵. 氢氧化镧交联壳聚糖微球的微流控制备及其除磷性能[J]. 化工学报, 2022, 73(9): 3929-3939. |
| [8] | 贾艳萍, 丁雪, 刚健, 佟泽为, 张海丰, 张兰河. Mn强化Fe/C微电解工艺条件优化及降解油墨废水机理[J]. 化工学报, 2022, 73(5): 2183-2193. |
| [9] | 赵希强, 张健, 孙爽, 王文龙, 毛岩鹏, 孙静, 刘景龙, 宋占龙. 生物质炭改性微球去除化工废水中无机磷的性能研究[J]. 化工学报, 2022, 73(5): 2158-2173. |
| [10] | 王建, 雷子萱, 姚家钰, 李建, 刘育红. 对苯二甲醛酚醛树脂的制备及其固化动力学研究[J]. 化工学报, 2022, 73(3): 1403-1415. |
| [11] | 王祺, 房阔, 贺聪慧, 王凯军. 流动电极电容去离子技术综述:研究进展与未来挑战[J]. 化工学报, 2022, 73(3): 975-989. |
| [12] | 毛恒, 王月, 王森, 刘伟民, 吕静, 陈甫雪, 赵之平. APTES改性ZIF-L/PEBA混合基质膜强化渗透汽化分离苯酚研究[J]. 化工学报, 2022, 73(3): 1389-1402. |
| [13] | 郑喜, 王涛, 任永胜, 赵珍珍, 王雪琪, 赵之平. 聚间苯二甲酰间苯二胺平板膜的制备及其性能研究[J]. 化工学报, 2022, 73(10): 4707-4721. |
| [14] | 张兰河, 汪露, 李梓萌, 唐宏, 郭静波, 贾艳萍, 张明爽. 电极超滤膜生物反应器处理阴离子表面活性剂废水[J]. 化工学报, 2022, 73(10): 4679-4691. |
| [15] | 付鹏波,田金乙,吕文杰,黄渊,刘毅,卢浩,杨强,修光利,汪华林. 物理法水处理技术[J]. 化工学报, 2022, 73(1): 59-72. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号