化工学报 ›› 2022, Vol. 73 ›› Issue (3): 1403-1415.DOI: 10.11949/0438-1157.20211801
• 材料化学工程与纳米技术 • 上一篇
收稿日期:2021-12-22
修回日期:2022-01-27
出版日期:2022-03-15
发布日期:2022-03-14
通讯作者:
刘育红
作者简介:王建(1996—),男,硕士研究生,基金资助:
Jian WANG1( ),Zixuan LEI1,Jiayu YAO1,Jian LI2,Yuhong LIU1(
),Zixuan LEI1,Jiayu YAO1,Jian LI2,Yuhong LIU1( )
)
Received:2021-12-22
Revised:2022-01-27
Online:2022-03-15
Published:2022-03-14
Contact:
Yuhong LIU
摘要:
基于酚醛树脂原料中甲醛的危害性与不可再生性,使用安全、可再生的对苯二甲醛代替甲醛,合成了一种新型的酚醛树脂——对苯二甲醛酚醛树脂。采用核磁、红外、GPC和流变仪等分析手段对此类树脂的结构与性能进行了表征。为了进一步提高该树脂的热性能,使用二茂铁甲醛对其进行改性。采用Kissinger方程、等转换法及双参数自催化模型对改性前后树脂的固化动力学进行了研究,明确了二茂铁甲醛在树脂固化中的作用机理。最后通过MDSC和TG研究了改性前后树脂固化物的热性能,结果表明:在加入15%的二茂铁甲醛后,改性树脂呈现出优异的热性能,其玻璃化转变温度为319.3℃,起始分解温度为397.7℃,在800℃氮气气氛下质量保持率高达76.07%。
中图分类号:
王建, 雷子萱, 姚家钰, 李建, 刘育红. 对苯二甲醛酚醛树脂的制备及其固化动力学研究[J]. 化工学报, 2022, 73(3): 1403-1415.
Jian WANG, Zixuan LEI, Jiayu YAO, Jian LI, Yuhong LIU. Synthesis and curing kinetics of terephthalaldehyde phenolic resin[J]. CIESC Journal, 2022, 73(3): 1403-1415.
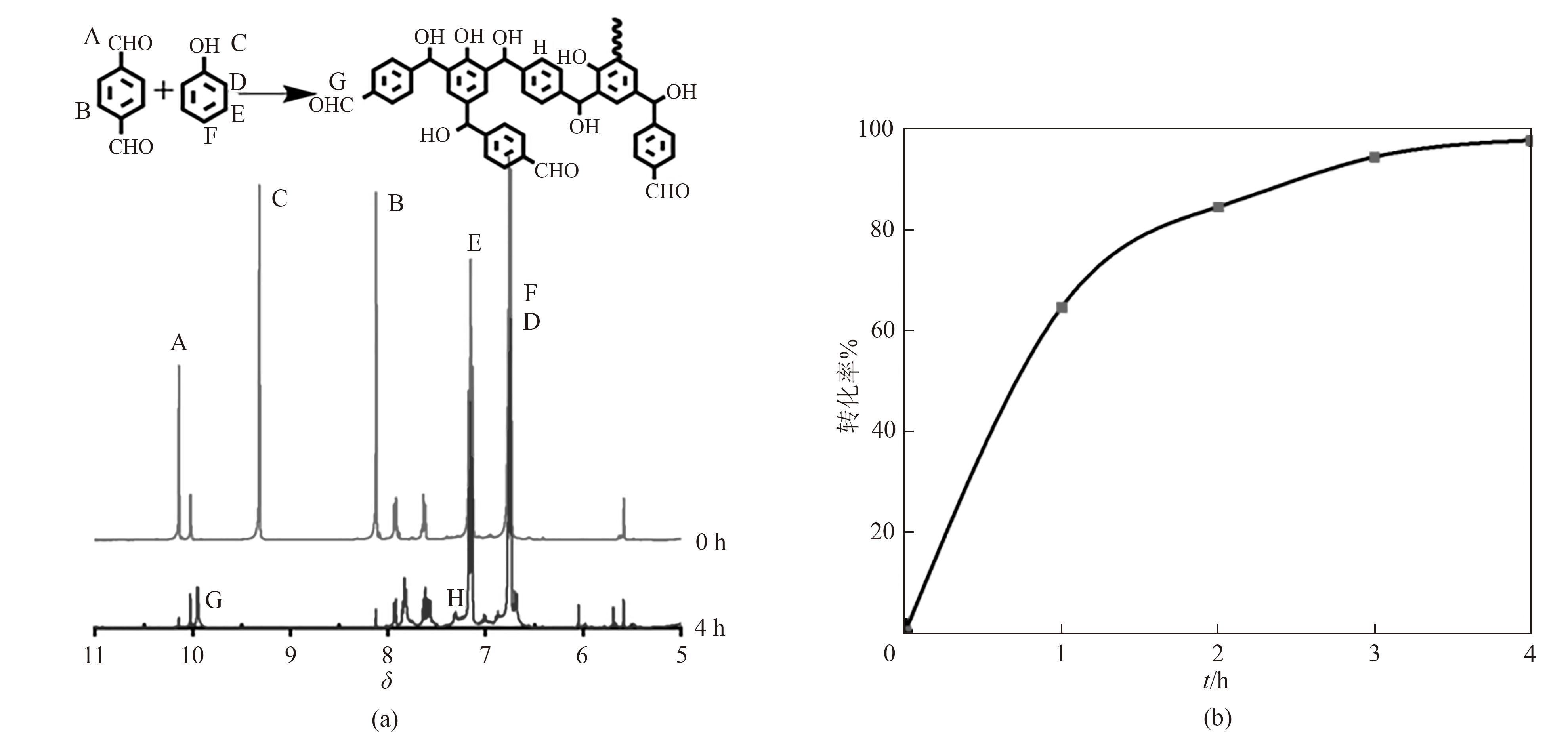
图1 对苯二甲醛酚醛树脂合成过程中0 h和4 h的核磁图以及可能的预聚物结构(a);反应过程中参与反应的对苯二甲醛的量(b)
Fig.1 NMR images at 0 h and 4 h during the synthesis of terephthalaldehyde phenolic resin and possible prepolymer structure(a); The amount of terephthalaldehyde involved in the reaction process(b)
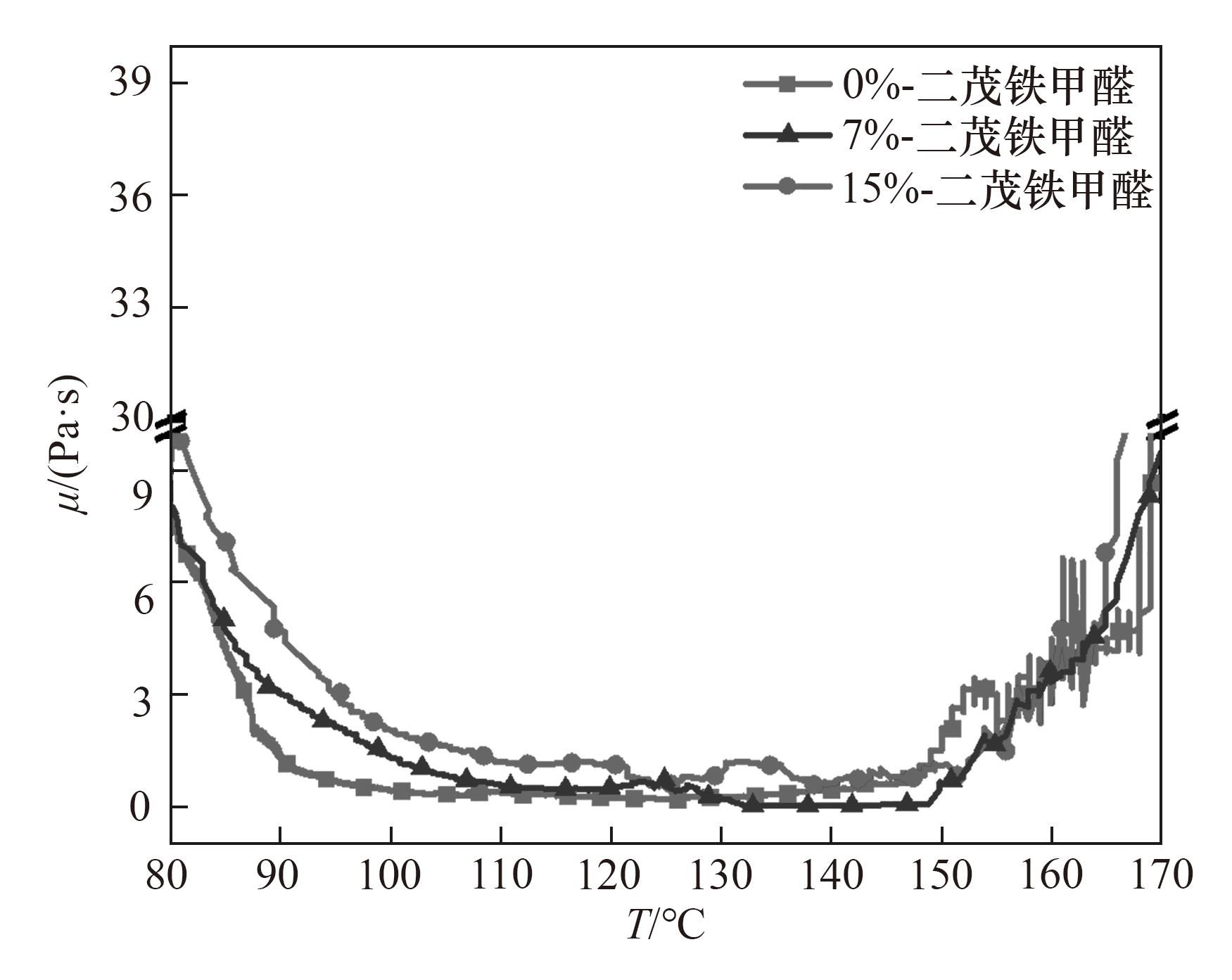
图4 不同二茂铁甲醛含量(0%、7%、15%)的对苯二甲醛酚醛树脂在升温过程中(80~170℃)的表观黏度曲线
Fig.4 Rheological viscosity diagrams of terephthalaldehyde phenolic resins with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%) during heating (80—170℃)
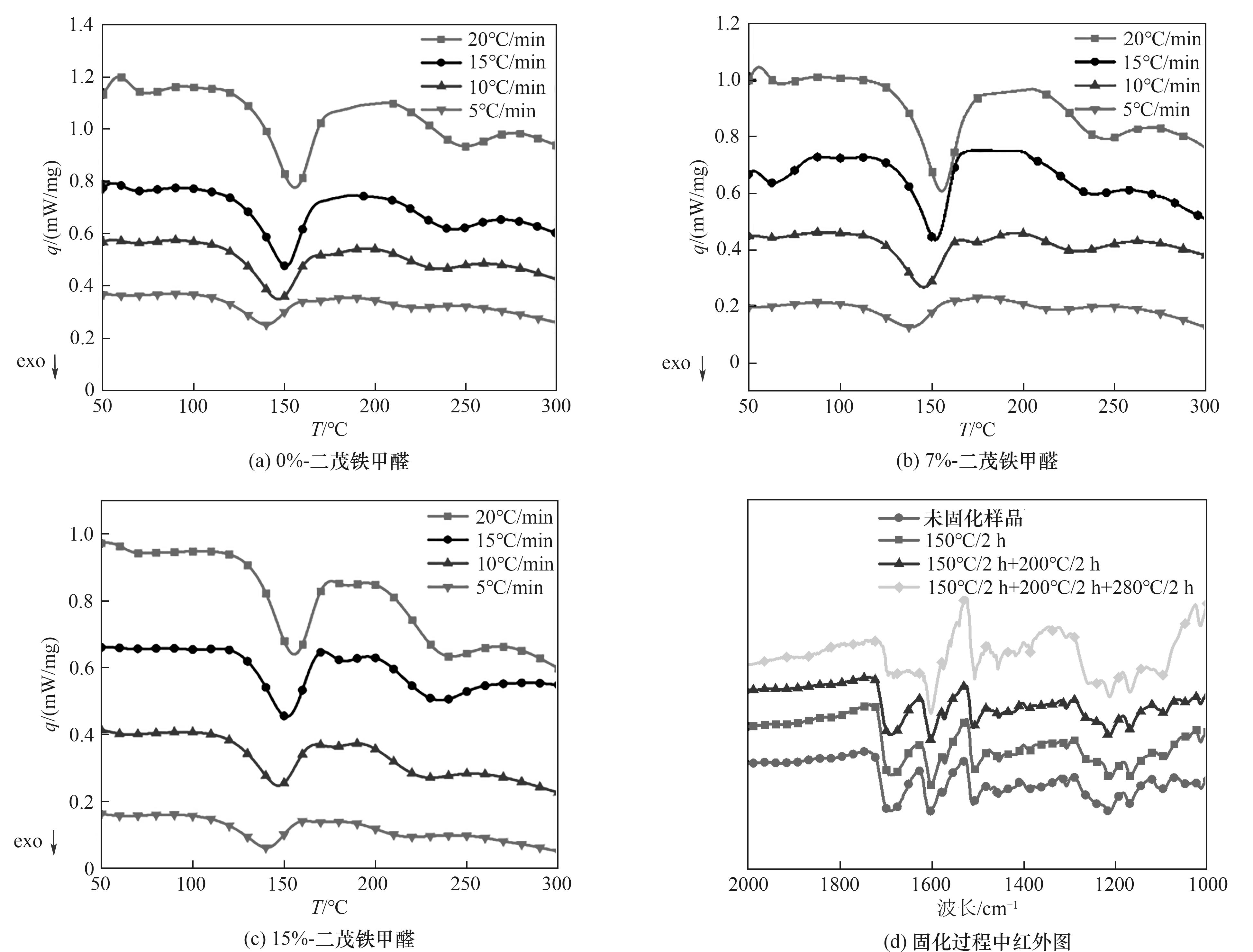
图5 0%-二茂铁甲醛(a)、7%-二茂铁甲醛(b)、15%-二茂铁甲醛(c)改性对苯二甲醛酚醛树脂的DSC图及树脂固化过程中的FTIR图(d)
Fig.5 DSC of 0%-ferrocenecarboxaldehyde (a), 7%-ferrocenecarboxaldehyde (b), 15%-ferrocenecarboxaldehyde (c) modified terephthalaldehyde phenolic resin and FTIR diagram during resin curing process(d)

图6 对苯二甲醛酚醛树脂(a)和二茂铁甲醛改性对苯二甲醛酚醛树脂(b)的固化机理图(R:H或其取代物)
Fig.6 Curing mechanism diagram of terephthalaldehyde phenolic resin (a) and ferrocenecarboxaldehyde modified terephthalaldehyde phenolic resin (b) (R:H or its substitute)
| β/(℃/min) | 0% | 7% | 15% | |||
|---|---|---|---|---|---|---|
| Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | |
| 5 | 142.1 | 222.8 | 141.3 | 218.8 | 142.8 | 218.4 |
| 10 | 148.6 | 235.7 | 147.5 | 230.6 | 148.7 | 228.8 |
| 15 | 152.5 | 242.7 | 152.0 | 238.8 | 153.2 | 236.0 |
| 20 | 156.5 | 250.4 | 155.9 | 246.1 | 156.6 | 240.6 |
表1 不同二茂铁甲醛含量(0%、7%、15%)的对苯二甲醛酚醛树脂两个固化峰的峰值温度
Table 1 Peak temperatures of two curing peaks of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| β/(℃/min) | 0% | 7% | 15% | |||
|---|---|---|---|---|---|---|
| Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | Tp1/℃ | Tp2/℃ | |
| 5 | 142.1 | 222.8 | 141.3 | 218.8 | 142.8 | 218.4 |
| 10 | 148.6 | 235.7 | 147.5 | 230.6 | 148.7 | 228.8 |
| 15 | 152.5 | 242.7 | 152.0 | 238.8 | 153.2 | 236.0 |
| 20 | 156.5 | 250.4 | 155.9 | 246.1 | 156.6 | 240.6 |

图7 0%-二茂铁甲醛[(a)、(b)]、7%-二茂铁甲醛[(c)、(d)]、15%-二茂铁甲醛[(e)、(f)]改性对苯二甲醛酚醛树脂的拟合曲线
Fig.7 Fitting curves of 0%- ferrocenecarboxaldehyde [(a),(b)], 7%- ferrocenecarboxaldehyde [(c),(d)], 15%-ferrocenecarboxaldehyde [(e),(f)] modified terephthalaldehyde phenolic resin
| Peak | Ea/(kJ/mol) | ||
|---|---|---|---|
| 0% | 7% | 15% | |
| peak 1 | 137.82 | 134.07 | 143.14 |
| peak 2 | 101.81 | 100.16 | 121.89 |
| total | 239.63 | 234.23 | 265.03 |
表2 不同二茂铁甲醛含量(0%、7%、15%)的对苯二甲醛酚醛树脂的活化能
Table 2 Activation energy of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| Peak | Ea/(kJ/mol) | ||
|---|---|---|---|
| 0% | 7% | 15% | |
| peak 1 | 137.82 | 134.07 | 143.14 |
| peak 2 | 101.81 | 100.16 | 121.89 |
| total | 239.63 | 234.23 | 265.03 |

图8 不同二茂铁甲醛含量(0%、7%、15%)的对苯二甲醛酚醛树脂峰1(a)和峰2(b)活化能
Fig.8 Activation energy of peak 1(a) and peak 2(b) of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)

图9 0%-二茂铁甲醛[(a)、(b)]、7%-二茂铁甲醛[(c)、(d)]、15%-二茂铁甲醛[(e)、(f)]改性对苯二甲醛酚醛树脂固化度与温度曲线
Fig.9 Curves of curing degree and temperature of 0%- ferrocenecarboxaldehyde [(a),(b)], 7%- ferrocenecarboxaldehyde [(c),(d)], 15%- ferrocenecarboxaldehyde [(e),(f)] modified terephthalaldehyde phenolic resin
| Sample | β/(℃/min) | lnA1 | m1 | n1 | m1/n1 | lnA2 | m2 | n2 | m2/n2 |
|---|---|---|---|---|---|---|---|---|---|
| 0% | 5 | 39.39 | 0.75 | 0.08 | 7.90 | 23.94 | 0.78 | 0.28 | 3.53 |
| 10 | 39.37 | 0.73 | 0.04 | 23.86 | 0.78 | 0.25 | |||
| 15 | 39.56 | 0.79 | 0.11 | 23.77 | 0.76 | 0.17 | |||
| 20 | 39.48 | 0.81 | 0.16 | 23.60 | 0.75 | 0.17 | |||
| 7% | 5 | 38.36 | 0.87 | 0.08 | 5.38 | 23.46 | 0.78 | 0.19 | 3.54 |
| 10 | 38.37 | 0.76 | 0.07 | 23.89 | 0.84 | 0.30 | |||
| 15 | 38.80 | 0.85 | 0.35 | 23.78 | 0.78 | 0.19 | |||
| 20 | 38.37 | 0.93 | 0.14 | 23.63 | 0.76 | 0.22 | |||
| 15% | 5 | 40.88 | 0.80 | 0.03 | 5.20 | 28.77 | 0.75 | 0.02 | 9.70 |
| 10 | 41.00 | 0.79 | 0.12 | 28.92 | 0.80 | 0.15 | |||
| 15 | 41.05 | 0.85 | 0.30 | 28.88 | 0.83 | 0.15 | |||
| 20 | 40.86 | 0.80 | 0.17 | 28.81 | 0.78 | 0.01 |
表3 不同含量的二茂铁甲醛 (0%、7%、15%)改性对苯二甲醛酚醛树脂的固化动力学参数
Table 3 Curing kinetic parameters of terephthalaldehyde phenolic resin with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%)
| Sample | β/(℃/min) | lnA1 | m1 | n1 | m1/n1 | lnA2 | m2 | n2 | m2/n2 |
|---|---|---|---|---|---|---|---|---|---|
| 0% | 5 | 39.39 | 0.75 | 0.08 | 7.90 | 23.94 | 0.78 | 0.28 | 3.53 |
| 10 | 39.37 | 0.73 | 0.04 | 23.86 | 0.78 | 0.25 | |||
| 15 | 39.56 | 0.79 | 0.11 | 23.77 | 0.76 | 0.17 | |||
| 20 | 39.48 | 0.81 | 0.16 | 23.60 | 0.75 | 0.17 | |||
| 7% | 5 | 38.36 | 0.87 | 0.08 | 5.38 | 23.46 | 0.78 | 0.19 | 3.54 |
| 10 | 38.37 | 0.76 | 0.07 | 23.89 | 0.84 | 0.30 | |||
| 15 | 38.80 | 0.85 | 0.35 | 23.78 | 0.78 | 0.19 | |||
| 20 | 38.37 | 0.93 | 0.14 | 23.63 | 0.76 | 0.22 | |||
| 15% | 5 | 40.88 | 0.80 | 0.03 | 5.20 | 28.77 | 0.75 | 0.02 | 9.70 |
| 10 | 41.00 | 0.79 | 0.12 | 28.92 | 0.80 | 0.15 | |||
| 15 | 41.05 | 0.85 | 0.30 | 28.88 | 0.83 | 0.15 | |||
| 20 | 40.86 | 0.80 | 0.17 | 28.81 | 0.78 | 0.01 |
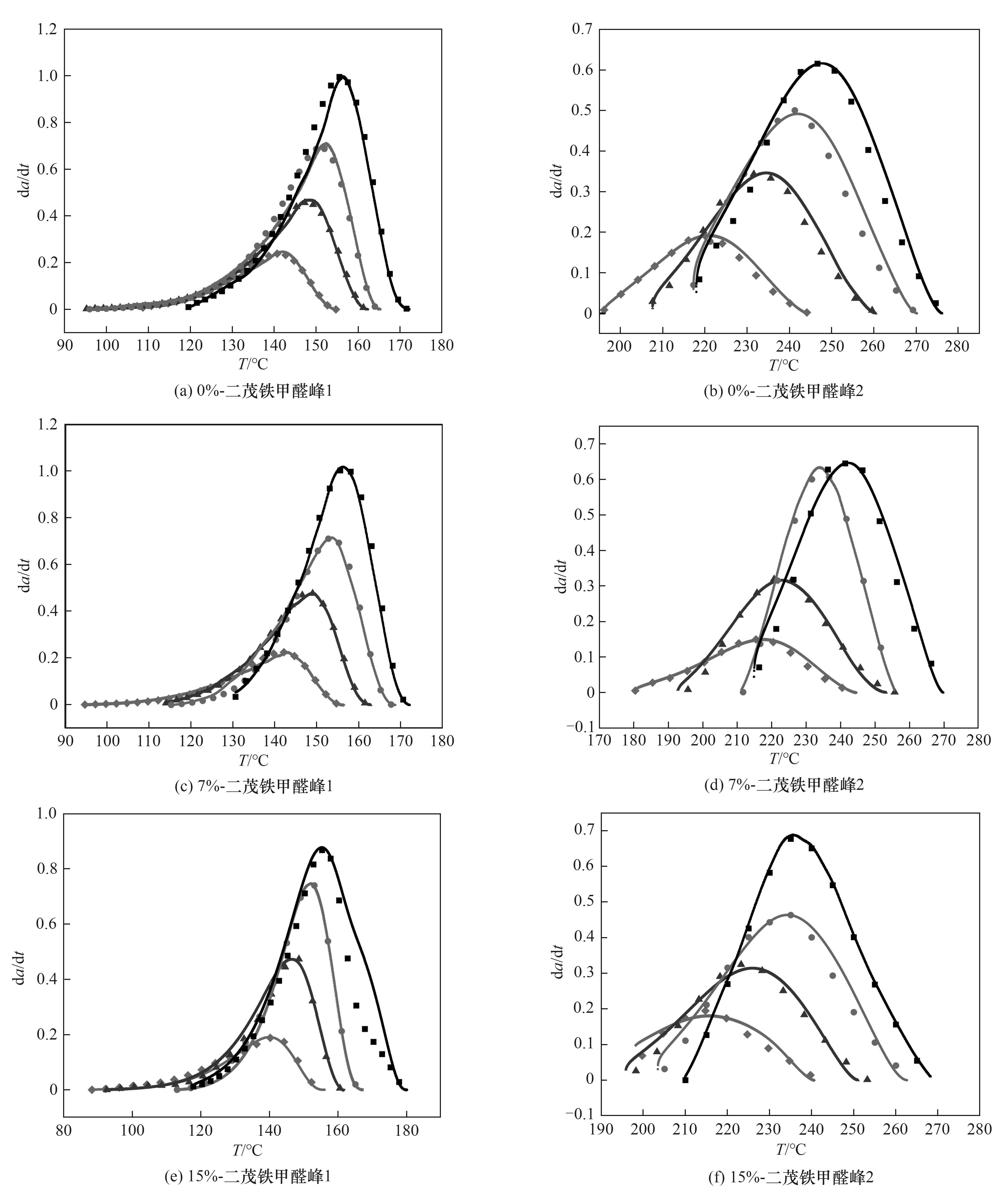
图10 0%-二茂铁甲醛[(a)、(b)]、7%-二茂铁甲醛[(c)、(d)]、15%-二茂铁甲醛[(e)、(f)]改性对苯二甲醛酚醛树脂的拟合曲线(实线为模拟数据所得,散点为实验数据所得)
Fig.10 Fitting curves of 0%- ferrocenecarboxaldehyde[(a),(b)], 7%- ferrocenecarboxaldehyde[(c),(d)], 15%- ferrocenecarboxaldehyde[ (e),(f) ] modified terephthalaldehyde phenolic resin (the solid line is the curve obtained from simulated data, and the scattered point is the curve obtained from experimental data)
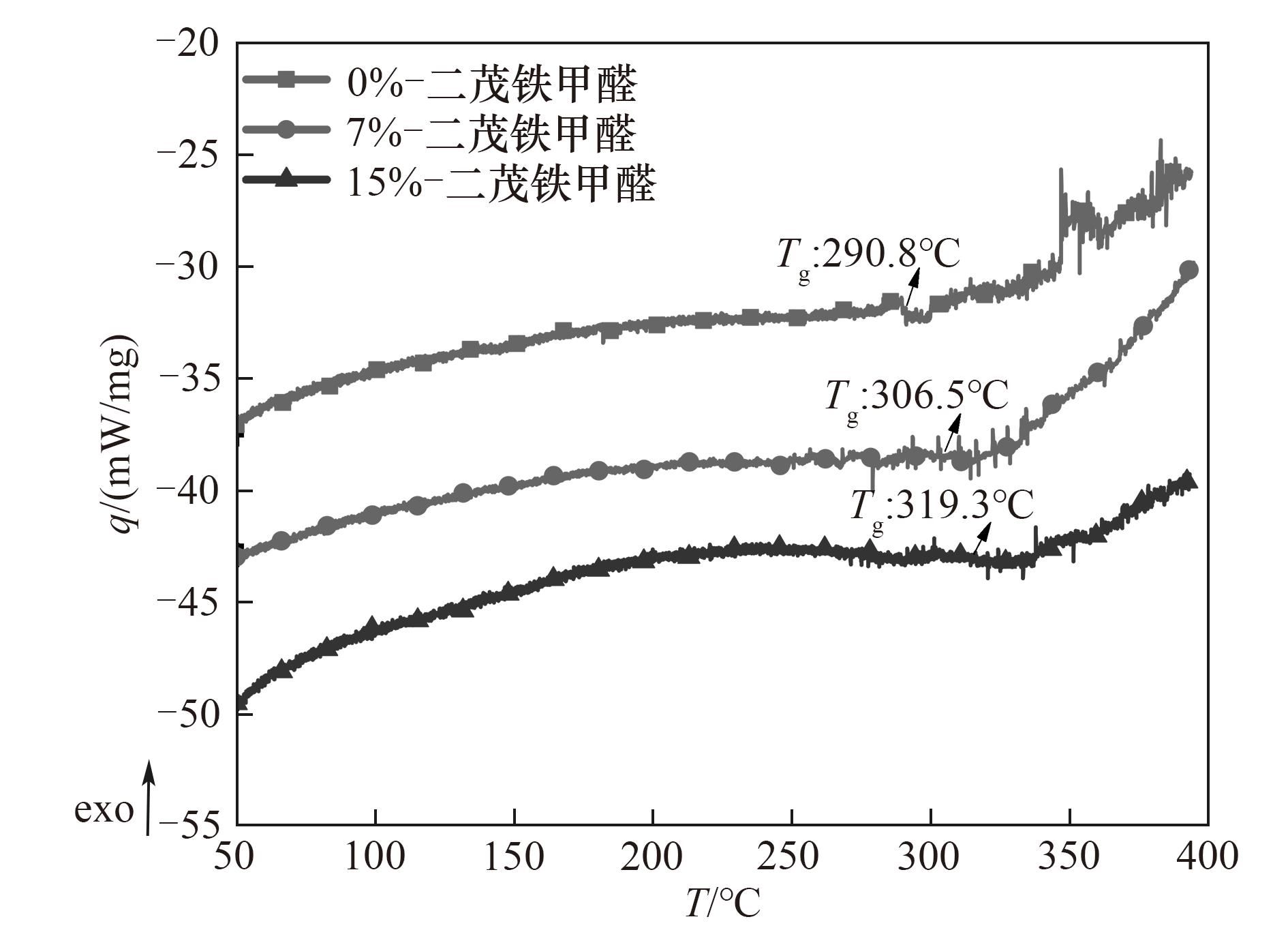
图11 不同含量的二茂铁甲醛(0%、7%、15%)改性对苯二甲醛酚醛树脂固化物的MDSC曲线
Fig.11 MDSC curves of cured terephthalaldehyde phenolic resin modified by different contents of ferrocenecarboxaldehyde(0%,7%,15%)

图12 不同含量的二茂铁甲醛 (0%、7%、15%)改性对苯二甲醛酚醛树脂的TG(a)和DTG(b)曲线(N2气氛)
Fig.12 TG(a) and DTG(b) curves of terephthalaldehyde phenolic resin modified with different contents of ferrocenecarboxaldehyde (0%, 7%, 15%) in N2 atmosphere
| 1 | Reghunadhan Nair C P, Bindu R L, Ninan K N. Thermal characteristics of addition-cure phenolic resins[J]. Polymer Degradation and Stability, 2001, 73(2): 251-257. |
| 2 | Yang W S, Jiao L, Wang X, et al. Formaldehyde-free self-polymerization of lignin-derived monomers for synthesis of renewable phenolic resin[J]. International Journal of Biological Macromolecules, 2021, 166: 1312-1319. |
| 3 | Sarika P R, Nancarrow P, Khansaheb A, et al. Bio-based alternatives to phenol and formaldehyde for the production of resins[J]. Polymers, 2020, 12(10): 2237. |
| 4 | Rivero G, Fasce L A, Ceré S M, et al. Furan resins as replacement of phenolic protective coatings: structural, mechanical and functional characterization[J]. Progress in Organic Coatings, 2014, 77(1): 247-256. |
| 5 | Younesi-Kordkheili H. Ionic liquid modified lignin-phenol-glyoxal resin: a green alternative resin for production of particleboards[J]. The Journal of Adhesion, 2019, 95(12): 1075-1087. |
| 6 | Foyer G, Chanfi B H, Virieux D, et al. Aromatic dialdehyde precursors from lignin derivatives for the synthesis of formaldehyde-free and high char yield phenolic resins[J]. European Polymer Journal, 2016, 77: 65-74. |
| 7 | 朱其仁, 李锦春, 王丽娟, 等. 苯酚-亚联苯型酚醛树脂的合成与表征[J]. 化工学报, 2009, 60(4): 1052-1056. |
| Zhu Q R, Li J C, Wang L J, et al. Synthesis and characterization of phenol-biphenylene resin[J]. CIESC Journal, 2009, 60(4): 1052-1056. | |
| 8 | Yun J, Chen L X, Zhang X F, et al. The effects of silicon and ferrocene on the char formation of modified novolac resin with high char yield[J]. Polymer Degradation and Stability, 2017, 139: 97-106. |
| 9 | Xu S H, Zhang F Y, Kang Q, et al. The effect of magnetic field on the catalytic graphitization of phenolic resin in the presence of Fe-Ni[J]. Carbon, 2009, 47(14): 3233-3237. |
| 10 | Zhang Y, Shen S H, Liu Y J. The effect of titanium incorporation on the thermal stability of phenol-formaldehyde resin and its carbonization microstructure[J]. Polymer Degradation and Stability, 2013, 98(2): 514-518. |
| 11 | Naderi A, Mazinani S, Javad Ahmadi S, et al. Modified thermo-physical properties of phenolic resin/carbon fiber composite with nano zirconium dioxide[J]. Journal of Thermal Analysis and Calorimetry, 2014, 117(1): 393-401. |
| 12 | Zhou J, Li X G, Du J Z, et al. Conversion of phenolic mixture to refractory resins: a resourcezation strategy for phenolic distillation residues[J]. Journal of Hazardous Materials, 2021, 414: 125357. |
| 13 | Granado L, Tavernier R, Foyer G, et al. Catalysis for highly thermostable phenol-terephthalaldehyde polymer networks[J]. Chemical Engineering Journal, 2020, 379: 122237. |
| 14 | Yi C, Rostron P, Vahdati N, et al. Curing kinetics and mechanical properties of epoxy based coatings: the influence of added solvent[J]. Progress in Organic Coatings, 2018, 124: 165-174. |
| 15 | Granado L, Tavernier R, Foyer G, et al. Comparative curing kinetics study of high char yield formaldehyde- and terephthalaldehyde-phenolic thermosets[J]. Thermochimica Acta, 2018, 667: 42-49. |
| 16 | 张西莹, 刘育红. 酚醛树脂/碳化硼/聚硼氮烷复合物的固化行为及其热解性能[J]. 化工学报, 2014, 65(8): 3268-3276. |
| Zhang X Y, Liu Y H. Curing and pyrolysis behavior of PF/B4C/PBZ composite[J]. CIESC Journal, 2014, 65(8): 3268-3276. | |
| 17 | 代洁, 李鹏程, 朱蓉琪, 等. 一种高残炭新型苯并𫫇嗪树脂的固化及热解动力学[J]. 功能高分子学报, 2018, 31(2): 114-120. |
| Dai J, Li P C, Zhu R Q, et al. Curing and pyrolysis kinetics of a new benzoxazine resin with high char yield[J]. Journal of Functional Polymers, 2018, 31(2): 114-120. | |
| 18 | Gao J G, Huo L, Du Y G. Nonisothermal cure kinetics and diffusion effect of liquid-crystalline epoxy sulfonyl bis(1, 4-phenylene)bis[4-(2, 3-epoxypropyloxy)benzoate]resin with aromatic diamine[J]. Journal of Applied Polymer Science, 2012, 125(5): 3329-3334. |
| 19 | Xu M Z, Luo Y S, Lei Y X, et al. Phthalonitrile-based resin for advanced composite materials: curing behavior studies[J]. Polymer Testing, 2016, 55: 38-43. |
| 20 | Li C, Ma Z Z, Zhang X W, et al. Silicone-modified phenolic resin: relationships between molecular structure and curing behavior[J]. Thermochimica Acta, 2016, 639: 53-65. |
| 21 | Lei Z X, Jiang X, Lv Y, et al. Time-temperature-transformation diagram of modified resol phenolic resin and the thermomechanical performance of resol phenolic resin/glass fabric composite[J]. Polymers for Advanced Technologies, 2018, 29(11): 2827-2837. |
| 22 | Asaro L, Manfredi L B, Pellice S, et al. Innovative ablative fire resistant composites based on phenolic resins modified with mesoporous silica particles[J]. Polymer Degradation and Stability, 2017, 144: 7-16. |
| 23 | Wang S J, Jia Q X, Liu Y H, et al. An investigation on the effect of phenylboronic acid on the processibilities and thermal properties of bis-benzoxazine resins[J]. Reactive and Functional Polymers, 2015, 93: 111-119. |
| 24 | Wang S J, Jing X L, Wang Y, et al. Synthesis and characterization of novel phenolic resins containing aryl-boron backbone and their utilization in polymeric composites with improved thermal and mechanical properties[J]. Polymers for Advanced Technologies, 2014, 25(2): 152-159. |
| 25 | Fox T G, Loshaek S. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers[J]. Journal of Polymer Science, 1955, 15(80): 371-390. |
| 26 | Trick K A, Saliba T E. Mechanisms of the pyrolysis of phenolic resin in a carbon/phenolic composite[J]. Carbon, 1995, 33(11): 1509-1515. |
| 27 | Xing X L, Zhang P, Zhao Y H, et al. Pyrolysis mechanism of phenylboronic acid modified phenolic resin[J]. Polymer Degradation and Stability, 2021, 191: 109672. |
| 28 | Wang S J, Wang Y, Bian C, et al. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: the effect of phenyl borates on the char formation[J]. Applied Surface Science, 2015, 331: 519-529. |
| 29 | Amer W A, Wang L, Amin A M, et al. Study on the electrochemical, thermal, and liquid crystalline properties of poly(diethyleneglycol 1, 1'-ferrocene dicarboxylate)[J]. Designed Monomers and Polymers, 2013, 16(2): 160-169. |
| 30 | Talabi S I, Luz A P, Pandolfelli V C, et al. Synthesis and graphitization of resole resins by ferrocene[J]. Progress in Natural Science: Materials International, 2019, 29(1): 71-80. |
| [1] | 杨天阳, 邹慧明, 周晖, 王春磊, 田长青. -30℃电动汽车补气式CO2热泵制热性能实验研究[J]. 化工学报, 2023, 74(S1): 272-279. |
| [2] | 徐文杰, 贾献峰, 王际童, 乔文明, 凌立成, 王任平, 余子舰, 张寅旭. 有机硅/酚醛杂化气凝胶的制备和性能研究[J]. 化工学报, 2023, 74(8): 3572-3583. |
| [3] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [4] | 郑杰元, 张先伟, 万金涛, 范宏. 丁香酚环氧有机硅树脂的制备及其固化动力学研究[J]. 化工学报, 2023, 74(2): 924-932. |
| [5] | 孟辉波, 蒙彤, 禹言芳, 王宗勇, 吴剑华. Ross LPD型静态混合器内湍流传热与混合强化特性[J]. 化工学报, 2022, 73(8): 3541-3552. |
| [6] | 王哲, 祖愿, 胡方圆, 蹇锡高. 含杂萘联苯结构的环氧树脂固化动力学分析[J]. 化工学报, 2022, 73(2): 681-688. |
| [7] | 郑哲楠, 高翔, 罗英武, 黄杰. 紫外光交联法制备全固态聚合物电解质[J]. 化工学报, 2022, 73(1): 441-450. |
| [8] | 罗介霖, 杨凯寅, 赵朕, 王勤, 陈光明. 低GWP混合工质回热热泵采暖性能[J]. 化工学报, 2021, 72(S1): 84-90. |
| [9] | 徐玲玲, 蒲亮. 基于热短路问题的仿生地埋管换热器模拟[J]. 化工学报, 2021, 72(S1): 134-139. |
| [10] | 赵岩, 李秀萍, 赵荣祥. 苯酚型低共熔溶剂中硫酸钛作为催化剂高效氧化脱硫[J]. 化工学报, 2021, 72(8): 4391-4400. |
| [11] | 张锐, 邵琦, 张华宇, 金泽龙, 张小亮. 硼掺杂二氧化硅杂化膜的制备及渗透汽化脱盐性能[J]. 化工学报, 2021, 72(4): 2317-2327. |
| [12] | 刘宁, 褚昌辉, 王乾, 孙世鹏. 用于混合一价盐分离的纳滤膜的制备及性能研究[J]. 化工学报, 2021, 72(1): 578-588. |
| [13] | 周一帆, 姚丛雪, 王靖文, 郭文文, 宋磊, 牧小卫, 胡源. 大豆的热分解特性及其动力学探究[J]. 化工学报, 2020, 71(S2): 187-194. |
| [14] | 邵索拉, 张欢, 由世俊, 郑万冬. 带有蓄热型直接冷凝式加热板的空气源热泵系统性能研究[J]. 化工学报, 2020, 71(8): 3480-3489. |
| [15] | 魏利平,江国栋,古玉宽,滕海鹏. 五彩湾煤和吐鲁番煤热解动力学模型评估与应用[J]. 化工学报, 2019, 70(S2): 275-286. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号