化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6218-6235.DOI: 10.11949/0438-1157.20250511
童逸凡1,2( ), 张宁霜1,2, 蔡星鹏1,2, 李成煜1,2, 李世友1,2(
), 张宁霜1,2, 蔡星鹏1,2, 李成煜1,2, 李世友1,2( )
)
收稿日期:2025-05-09
修回日期:2025-05-30
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
李世友
作者简介:童逸凡(1999—),男,硕士研究生,tyftjzy@126.com
基金资助:
Yifan TONG1,2( ), Ningshuang ZHANG1,2, Xingpeng CAI1,2, Chengyu LI1,2, Shiyou LI1,2(
), Ningshuang ZHANG1,2, Xingpeng CAI1,2, Chengyu LI1,2, Shiyou LI1,2( )
)
Received:2025-05-09
Revised:2025-05-30
Online:2025-12-31
Published:2026-01-23
Contact:
Shiyou LI
摘要:
近年来,钠离子电池(SIB)因其成本优势和对锂离子电池的替代作用而受到了越来越多的关注。层状氧化物正极作为最有潜力的钠电正极材料,具有理论比容量高、合成工艺简单等优势,但也存在着不可逆相变、结构退化以及电压衰减等缺陷,这些因素阻碍了SIB的商业化发展与大规模应用。高熵策略是一种结合了多元素掺杂、体相结构设计等多种手段的综合改性策略,可以有效提高电极材料的能量密度、长循环稳定性以及离子传输动力学等性能指标。总结了近年来高熵策略在钠离子电池层状氧化物材料改性研究领域的前沿成果,探讨了高熵效应与层状氧化物材料电化学性能之间的内在联系与作用机理,并展望了高熵策略未来的发展方向,为未来高性能钠离子电池层状正极材料的设计与合成提供新的见解。
中图分类号:
童逸凡, 张宁霜, 蔡星鹏, 李成煜, 李世友. 高熵策略驱动下的钠离子电池层状氧化物正极材料改性研究:进展、机理与展望[J]. 化工学报, 2025, 76(12): 6218-6235.
Yifan TONG, Ningshuang ZHANG, Xingpeng CAI, Chengyu LI, Shiyou LI. Research on modification of layered oxide cathode materials for sodium-ion battery driven by high-entropy strategy: progress, mechanism, and future[J]. CIESC Journal, 2025, 76(12): 6218-6235.
| 材料 | 优势 | 劣势 |
|---|---|---|
| 聚阴离子型材料[ | 结构稳定性强、工作电压高 | 电子电导率低,能量密度低,合成成本高昂 |
| 普鲁士蓝材料[ | 合成工艺简单、快速充放电能力强 | 循环寿命较短、工作温度范围较窄 |
| 过渡金属氧化物材料[ | 合成工艺简单、可逆比容量高、储钠能力强 | 充放电过程中易发生不可逆相变、空气稳定性差 |
表1 主流钠离子电池正极材料优缺点总结
Table 1 Summary of advantages and disadvantages of mainstream SIB cathode materials
| 材料 | 优势 | 劣势 |
|---|---|---|
| 聚阴离子型材料[ | 结构稳定性强、工作电压高 | 电子电导率低,能量密度低,合成成本高昂 |
| 普鲁士蓝材料[ | 合成工艺简单、快速充放电能力强 | 循环寿命较短、工作温度范围较窄 |
| 过渡金属氧化物材料[ | 合成工艺简单、可逆比容量高、储钠能力强 | 充放电过程中易发生不可逆相变、空气稳定性差 |
| 正极材料 | 放电比容量/(mAh·g-1) | 电压范围/V | 循环稳定性 | 倍率性能/(mAh·g-1) | 文献 |
|---|---|---|---|---|---|
| Na0.83Li0.1Ni0.25Co0.2Mn0.15Ti0.15Sn0.15O2-δ | 109.4 | 2.0~4.2 | 87.2%/200次循环/2.0C | 83.3 /10C | [ |
| NaCu0.1Ni0.25Co0.15Mn0.35Li0.05Ti0.05Sn0.05O2 | 144.5 | 2.2~4.4 | 90.1%/100次循环/1.0C | 76.7/5.0C | [ |
| NaNi0.3Cu0.1Fe0.2Mn0.3Ti0.1O2 | 141.5 | 2.0~4.0 | 85%/500次循环/1.0C | 120/5.0C | [ |
| NaNi0.2Fe0.2Mn0.35Cu0.05Zn0.1Sn0.1O2 | 128 | 2.0~4.0 | 87%/500次循环/3.0C | 64.3/2.0C | [ |
| Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 | 171.2 | 2.0~4.5 | 90%/30次/0.3C | 78.2/10C | [ |
| [Na0.67Zn0.05]Ni0.22Cu0.06Mn0.66Ti0.01O2 | 146.1 | 2.0~4.3 | 92.7%/100次循环/1.0C | 91.54/10C | [ |
| Na0.67Mn0.6Cu0.08Ni0.09Fe0.18Ti0.05O2 | 150.3 | 2.0~4.5 | 100%/500次循环/10C | 62.5/10C | [ |
| Na[FeCoNiTi]1/6Mn1/4Zn1/12O2 | 127.3 | 2.0~4.1 | 88%/1000次循环/1.0C | 30.7/10C | [ |
| Na0.9Ni0.2Fe0.2Co0.2Mn0.2Ti0.15Cu0.05O2 | 117.8 | 2.2~4.1 | 70.7%/1000次循环/1.0C | 98.6/10C | [ |
| Na(Fe0.2Co0.15Cu0.05Ni0.2Mn0.2Ti0.2)B0.02O2 | 120.5 | 2.0~4.1 | 95%/100次循环/1.0C | 103.3/2.0C | [ |
| Na0.95Li0.06Ni0.25Cu0.05Fe0.15Mn0.49O2 | 141.2 | 2.0~4.2 | 83.2/500次循环/8.0C | 83.5/20C | [ |
| Na0.89Li0.05Cu0.11Ni0.11Fe0.3Mn0.43O1.97F0.03 | 145 | 1.5~4.0 | 80%/300次循环/1.0C | 109/10C | [ |
| Na0.85Li0.05Ni0.25Cu0.025Mg0.025Fe0.05Al0.05Mn0.5Ti0.05O2 | 122 | 2.0~4.3 | 89%/1000次循环/10C | 81.8/10C | [ |
| Na0.85Li0.05Ni0.3Fe0.1Mn0.5Ti0.05O2 | 182.2 | 1.5~4.3 | 94.3/10次循环/10C | 68.4/10C | [ |
表2 钠离子电池中的高熵层状正极材料总结
Table 2 Summary of layered cathodes with high-entropy configurations in SIBs
| 正极材料 | 放电比容量/(mAh·g-1) | 电压范围/V | 循环稳定性 | 倍率性能/(mAh·g-1) | 文献 |
|---|---|---|---|---|---|
| Na0.83Li0.1Ni0.25Co0.2Mn0.15Ti0.15Sn0.15O2-δ | 109.4 | 2.0~4.2 | 87.2%/200次循环/2.0C | 83.3 /10C | [ |
| NaCu0.1Ni0.25Co0.15Mn0.35Li0.05Ti0.05Sn0.05O2 | 144.5 | 2.2~4.4 | 90.1%/100次循环/1.0C | 76.7/5.0C | [ |
| NaNi0.3Cu0.1Fe0.2Mn0.3Ti0.1O2 | 141.5 | 2.0~4.0 | 85%/500次循环/1.0C | 120/5.0C | [ |
| NaNi0.2Fe0.2Mn0.35Cu0.05Zn0.1Sn0.1O2 | 128 | 2.0~4.0 | 87%/500次循环/3.0C | 64.3/2.0C | [ |
| Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 | 171.2 | 2.0~4.5 | 90%/30次/0.3C | 78.2/10C | [ |
| [Na0.67Zn0.05]Ni0.22Cu0.06Mn0.66Ti0.01O2 | 146.1 | 2.0~4.3 | 92.7%/100次循环/1.0C | 91.54/10C | [ |
| Na0.67Mn0.6Cu0.08Ni0.09Fe0.18Ti0.05O2 | 150.3 | 2.0~4.5 | 100%/500次循环/10C | 62.5/10C | [ |
| Na[FeCoNiTi]1/6Mn1/4Zn1/12O2 | 127.3 | 2.0~4.1 | 88%/1000次循环/1.0C | 30.7/10C | [ |
| Na0.9Ni0.2Fe0.2Co0.2Mn0.2Ti0.15Cu0.05O2 | 117.8 | 2.2~4.1 | 70.7%/1000次循环/1.0C | 98.6/10C | [ |
| Na(Fe0.2Co0.15Cu0.05Ni0.2Mn0.2Ti0.2)B0.02O2 | 120.5 | 2.0~4.1 | 95%/100次循环/1.0C | 103.3/2.0C | [ |
| Na0.95Li0.06Ni0.25Cu0.05Fe0.15Mn0.49O2 | 141.2 | 2.0~4.2 | 83.2/500次循环/8.0C | 83.5/20C | [ |
| Na0.89Li0.05Cu0.11Ni0.11Fe0.3Mn0.43O1.97F0.03 | 145 | 1.5~4.0 | 80%/300次循环/1.0C | 109/10C | [ |
| Na0.85Li0.05Ni0.25Cu0.025Mg0.025Fe0.05Al0.05Mn0.5Ti0.05O2 | 122 | 2.0~4.3 | 89%/1000次循环/10C | 81.8/10C | [ |
| Na0.85Li0.05Ni0.3Fe0.1Mn0.5Ti0.05O2 | 182.2 | 1.5~4.3 | 94.3/10次循环/10C | 68.4/10C | [ |
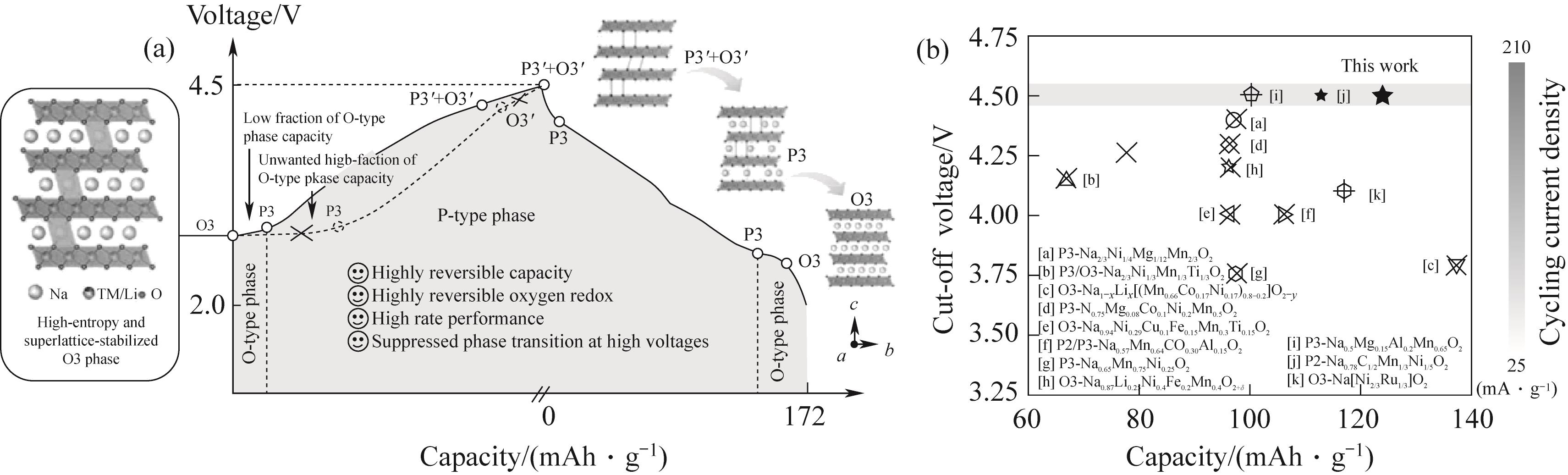
图4 (a)本研究提出的高熵和超晶格稳定的O3型正极的充放电行为示意图,其中低电压区的O3-P3相变被促进,高电压区的P3-O3相变被抑制;(b)NaLFCNM与之前报道的O3/P3型正极之间的电化学性能对比,其中所有正极都已经历超过50次充放电循环[39]
Fig.4 (a) Schematic illustration of charge/discharge behaviors for high-entropy and superlattice-stabilized O3-type cathodes proposed in this work,where the O3-P3 phase transition at the low-voltage region is facilitated and the P3-O3 phase transition at the high-voltage region is suppressed; (b) Electrochemical performance comparison between NaLFCNM and previously reported O3/P3-type cathodes, in which all cathodes havebeen cycled for more than 50 cycles[39]
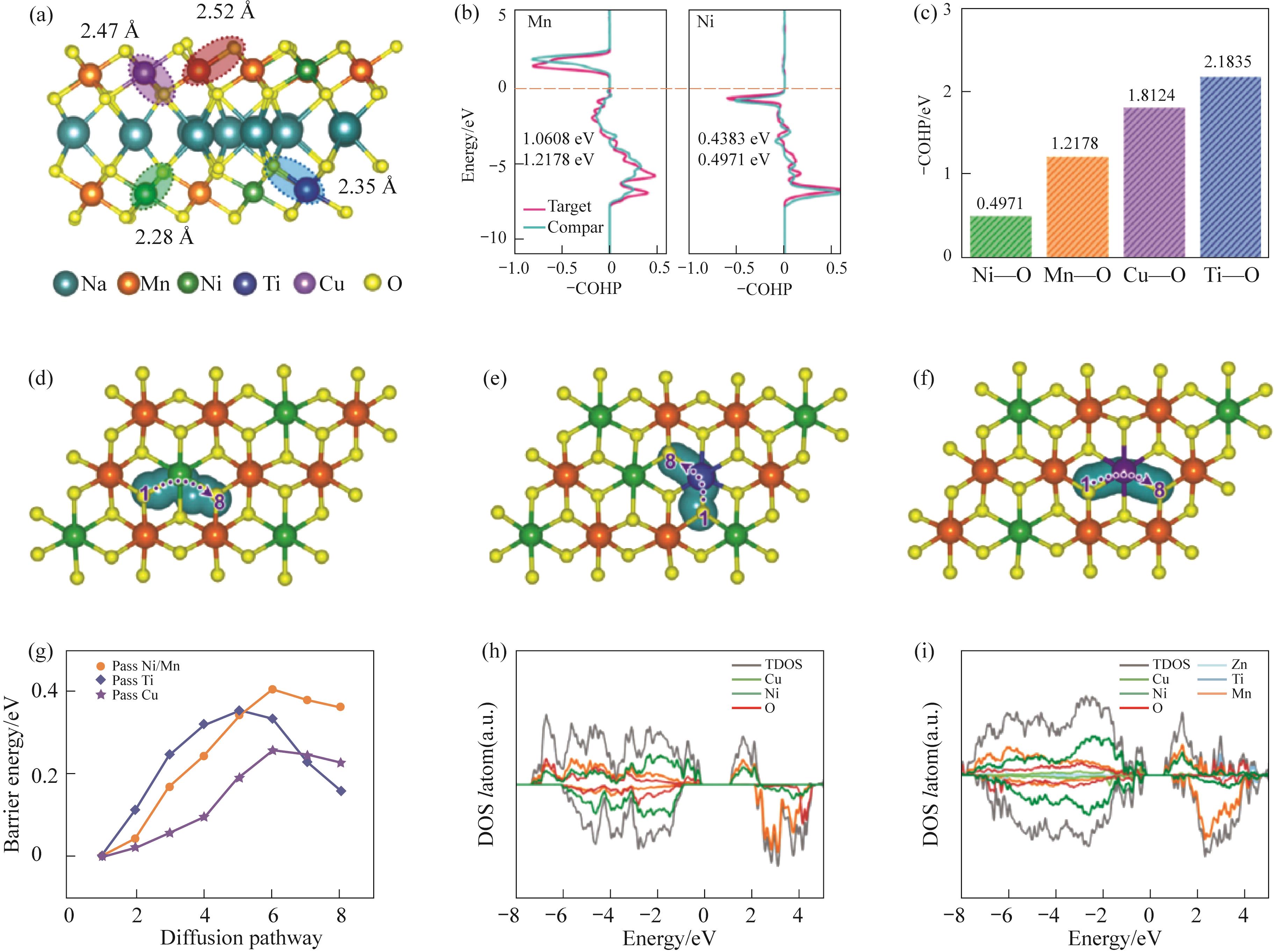
图5 (a)经过高熵掺杂改性的NZNCMTO材料结构;(b)NZNCMTO和NNMO中Ni—O和Mn—O键的COHP值的对比;(b)熵调谐的NZNCMTO的COHP结果;(d)~(g)Na+在NNMO和熵调谐的NZNCMTO中通过Ni/Mn、Ti和Cu的迁移路径和迁移能垒示意图;(h)NNMO和(i)初始状态的NZNCMTO的总态密度(DOS)[40]
Fig.5 (a) The optimized structures of entropy-tuned NZNCMTO; (b) Comparison of COHP values of Ni—O and Mn—O bonds in NZNCMTO and NNMO; (c) COHP results of entropy-tuned NZNCMTO; (d)—(g) Schematic diagram of the migration paths and migration energy barrier of sodium ions through Ni/Mn, Ti, and Cu in NNMO and entropy-tuned NZNCMTO; (h) Total density of states (DOS) of NNMO and (i) entropy-tuned NZNCMTO of the initial state[40]
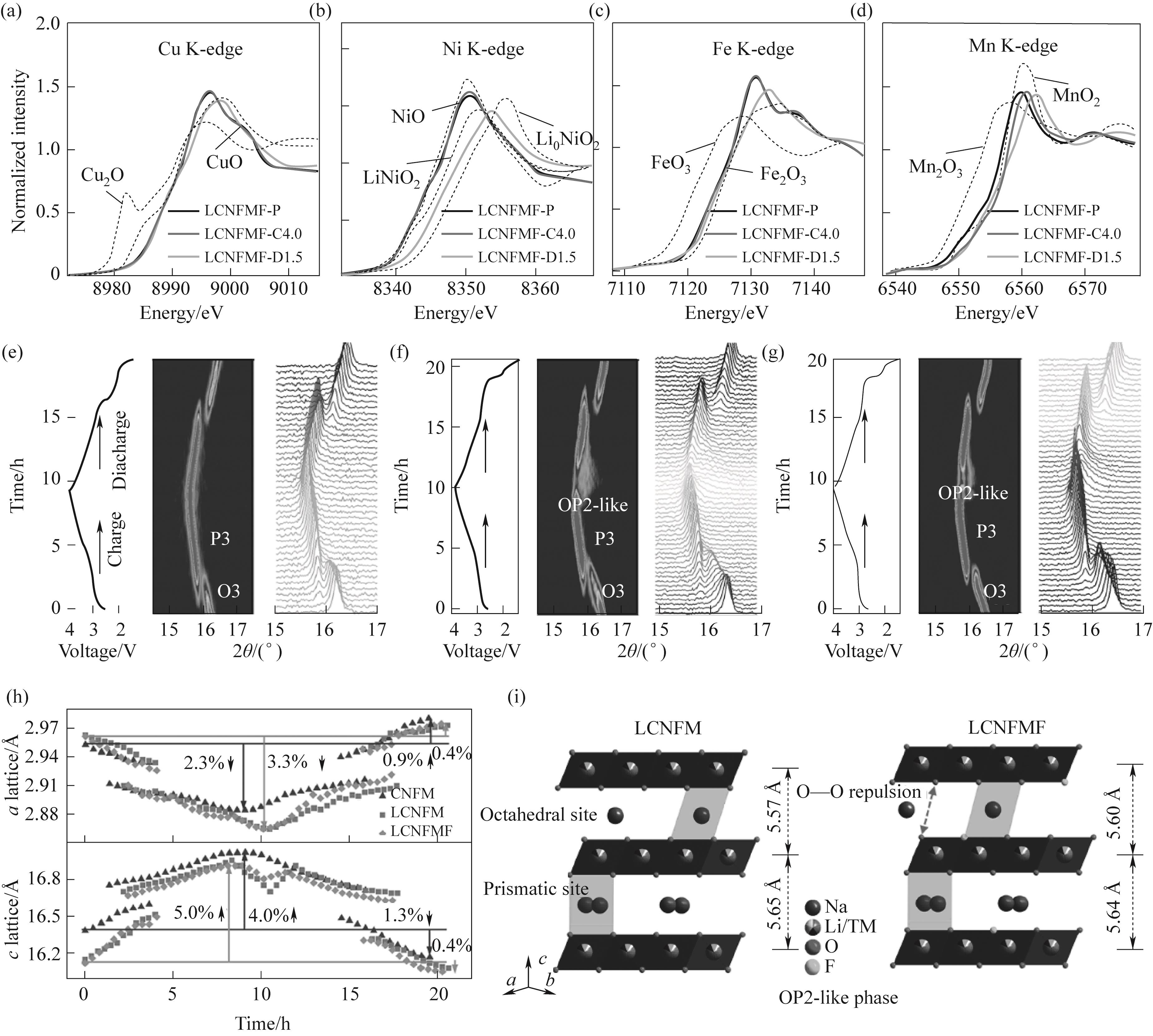
图10 氧化还原机理和晶体结构的演化过程:(a)~(d)不同充放电状态下的Cu、Ni、Fe和Mn的归一化K边XANES谱;(e)~(g)CNFM、LCNFM和LCNFMF的电压曲线和对应的原位XRD演化过程;(h)通过拟合原位XRD数据得到的3个样品的a/c晶格参数变化过程;(i)LCNFM和LCNFMF在充电过程结束时的晶体结构演化示意图[46]
Fig.10 Redox mechanism and crystal structural evolution: (a)—(d) Normalized Cu, Ni, Fe, and Mn K-edge XANES spectra at different charge- discharge states; (e)—(g) Voltage profile and corresponding in situ XRD evolution of CNFM, LCNFM, and LCNFMF; (h) The a/c-lattice parameters change in the three samples obtained by fitting the in situ XRD data; (i) Schematic illustration of the crystal structural evolution at the end of charging of LCNFM and LCNFMF[46]
| [1] | Guo S H, Yi J, Sun Y, et al. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries[J]. Energy & Environmental Science, 2016, 9(10): 2978-3006. |
| [2] | Delmas C. Sodium and sodium-ion batteries: 50 years of research[J]. Advanced Energy Materials, 2018, 8(17): 1703137. |
| [3] | Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| [4] | Liu Y C, Liu X B, Wang T S, et al. Research and application progress on key materials for sodium-ion batteries[J]. Sustainable Energy & Fuels, 2017, 1(5): 986-1006. |
| [5] | Deng J Q, Luo W B, Chou S L, et al. Sodium-ion batteries: from academic research to practical commercialization[J]. Advanced Energy Materials, 2018, 8(4): 1701428. |
| [6] | Bommier C, Ji X L. Electrolytes, SEI formation, and binders: a review of nonelectrode factors for sodium-ion battery anodes[J]. Small, 2018, 14(16): e1703576. |
| [7] | Chen M Z, Liu Q N, Wang S W, et al. High-abundance and low-cost metal-based cathode materials for sodium-ion batteries: problems, progress, and key technologies[J]. Advanced Energy Materials, 2019, 9(14): 1803609. |
| [8] | Xiang X D, Zhang K, Chen J. Recent advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials, 2015, 27(36): 5343-5364. |
| [9] | Hao Z Q, Shi X Y, Yang Z, et al. The distance between phosphate-based polyanionic compounds and their practical application for sodium-ion batteries[J]. Advanced Materials, 2024, 36(7): 2305135. |
| [10] | Zhao L N, Bi S Y, Li J Y, et al. Prussian blue analogues for advanced non-aqueous sodium ion batteries: redox mechanisms, key challenges and modification strategies[J]. Energy Storage Materials, 2025, 78: 104256. |
| [11] | Gao Y, Zhang H, Liu X H, et al. Low-cost polyanion-type sulfate cathode for sodium-ion battery[J]. Advanced Energy Materials, 2021, 11(42): 2101751. |
| [12] | Li J J, Li H J, Huang Q, et al. Study on the mechanism of the influence of doping on the properties of cathode materials of sodium ion batteries[J]. Prog Chem, 2022, 34(4): 857-869. |
| [13] | Delmas C, Fouassier C, Hagenmuller P. Structural classification and properties of the layered oxides[J]. Physica B+C, 1980, 99(1/2/3/4): 81-85. |
| [14] | Wei F L, Zhang Q P, Zhang P, et al. Review: research progress on layered transition metal oxide cathode materials for sodium ion batteries[J]. Journal of the Electrochemical Society, 2021, 168(5): 050524. |
| [15] | Zuo W H, Qiu J M, Liu X S, et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres[J]. Nature Communications, 2020, 11(1): 3544. |
| [16] | Li X, Wang Y, Wu D, et al. Jahn-Teller assisted Na diffusion for high performance Na ion batteries[J]. Chemistry of Materials, 2016, 28(18): 6575-6583. |
| [17] | Zhang L, Wang C C, Liu Y C, et al. Suppressing interlayer-gliding and Jahn-Teller effect in P2-type layered manganese oxide cathode via Mo doping for sodium-ion batteries[J]. Chemical Engineering Journal, 2021, 426: 130813. |
| [18] | Zhang R W, Liang J N, Zeng C, et al. Air degradation and rehealing of high-voltage Na0.7Ni0.35Sn0.65O2 cathode for sodium ion batteries[J]. Science China Materials, 2023, 66(1): 88-96. |
| [19] | Gao X, Liu H Q, Chen H Y, et al. Cationic-potential tuned biphasic layered cathodes for stable desodiation/sodiation[J]. Science Bulletin, 2022, 67(15): 1589-1602. |
| [20] | Brugnetti G, Triolo C, Massaro A, et al. Structural evolution of air-exposed layered oxide cathodes for sodium-ion batteries: an example of Ni-doped Na x MnO2 [J]. Chemistry of Materials, 2023, 35(20): 8440-8454. |
| [21] | Wang Y Y, Cao Z T, Du Z Y, et al. Research progress of iron-based polyanionic cathode materials for sodium-ion batteries[J]. Acta Physico-Chimica Sinica, 2025, 41(4): 100035. |
| [22] | Yao H, Gao Y, Lin X H, et al. Prussian blue analogues for aqueous sodium-ion batteries: progress and commercialization assessment[J]. Advanced Energy Materials, 2024, 14(32): 2401984. |
| [23] | Zhang J L, Yu D Y W. Stabilizing Na0.7MnO2 cathode for Na-ion battery via a single-step surface coating and doping process[J]. Journal of Power Sources, 2018, 391: 106-112. |
| [24] | Li S Y, Fan X Q, Wang S M, et al. Probing the account of phase transition upon electrochemical cycling of the P2-Na0.67Ni0.15Fe0.2Mn0.65O2 layered oxide cathodes for sodium-ion batteries[J]. Materials Research Express, 2024, 11(3): 035504. |
| [25] | Kong W J, Wang H B, Sun L M, et al. Understanding the synergic roles of MgO coating on the cycling and rate performance of Na0.67Mn0.5Fe0.5O2 cathode[J]. Applied Surface Science, 2019, 497: 143814. |
| [26] | Fan Z W, Song W D, Yang N, et al. Insights into the phase purity and storage mechanism of nonstoichiometric Na3.4Fe2.4(PO4)1.4P2O7 cathode for high-mass-loading and high-power-density sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(8): e202316957. |
| [27] | Chen C, Huang W Y, Li Y W, et al. P2/O3 biphasic Fe/Mn-based layered oxide cathode with ultrahigh capacity and great cyclability for sodium ion batteries[J]. Nano Energy, 2021, 90: 106504. |
| [28] | Cai C C, Li X Y, Li J T, et al. Transition metal vacancy and position engineering enables reversible anionic redox reaction for sodium storage[J]. Nature Communications, 2025, 16(1): 100. |
| [29] | Wu L R, Zhang Y H, Wu Z, et al. Stabilized O3-type layered sodium oxides with enhanced rate performance and cycling stability by dual-site Ti4+/K+ substitution[J]. Advanced Science, 2023, 10(32): 2304067. |
| [30] | Feng S, Zheng C J, Song Z Y, et al. Boosting fast ionic transport and stability of O3-NaNi1/3Fe1/3Mn1/3O2 cathode via Al/Cu synergistically modulating microstructure for high-rate sodium-ion batteries[J]. Chemical Engineering Journal, 2023, 475: 146090. |
| [31] | Ahmad N, Yu L, Muzaffar M U, et al. Dual-pillar effect in P2-type Na0.67Ni0.33Mn0.67O2 through Na site substitution achieve superior electrochemical and air/water dual-stability as cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2025, 15(20): 2404093. |
| [32] | Zhou B, Wong D, Fu Z H, et al. K-doping suppresses oxygen redox in P2-Na0.67Ni0.11Cu0.22Mn0.67O2 cathode materials for sodium-ion batteries[J]. Small, 2024, 20(43): 2402991. |
| [33] | Xiao B, Wu G, Wang T D, et al. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries[J]. Nano Energy, 2022, 95: 106962. |
| [34] | Huang L P, Zhu J T, Liu J X, et al. Emerging high-entropy strategy: a booster to the development of cathode materials for power batteries[J]. Journal of Advanced Ceramics, 2024, 13(8): 1093-1118. |
| [35] | Wang H J, Gao X, Zhang S, et al. High-entropy Na-deficient layered oxides for sodium-ion batteries[J]. ACS Nano, 2023, 17(13): 12530-12543. |
| [36] | Wang H J, Gao J Q, Mei Y, et al. Halting oxygen evolution to achieve long cycle life in sodium layered cathodes[J]. Angewandte Chemie International Edition, 2025, 64(6): e202418605. |
| [37] | Ding F X, Ji P X, Han Z, et al. Tailoring planar strain for robust structural stability in high-entropy layered sodium oxide cathode materials[J]. Nature Energy, 2024, 9: 1529-1539. |
| [38] | Wang B, Ma J, Wang K J, et al. High-entropy phase stabilization engineering enables high-performance layered cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2024, 14(23): 2401090. |
| [39] | Yao L B, Zou P C, Wang C Y, et al. High-entropy and superstructure-stabilized layered oxide cathodes for sodium-ion batteries[J]. Advanced Energy Materials, 2022, 12(41): 2201989. |
| [40] | Liu J, Huang W Y, Liu R B, et al. Entropy tuning stabilizing P2-type layered cathodes for sodium-ion batteries[J]. Advanced Functional Materials, 2024, 34(24): 2315437. |
| [41] | Liu Z G, Liu R X, Xu D S, et al. Achieving a deeply desodiated stabilized cathode material by the high entropy strategy for sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(29): e202405620. |
| [42] | Zeng Z Y, Abulikemu A, Zhang J K, et al. High-entropy O3-type cathode enabling low-temperature performance for sodium-ion batteries[J]. Nano Energy, 2024, 128: 109813. |
| [43] | Wang X Z, Zuo Y T, Qin Y B, et al. Fast Na+ kinetics and suppressed voltage hysteresis enabled by a high-entropy strategy for sodium oxide cathodes[J]. Advanced Materials, 2024, 36(24): 2312300. |
| [44] | Dang Y Z, Xu Z, Wu Y R, et al. Boron-doped high-entropy oxide toward high-rate and long-cycle layered cathodes for wide-temperature sodium-ion batteries[J]. Journal of Energy Chemistry, 2024, 95: 577-587. |
| [45] | Cai T X, Cai M Z, Mu J X, et al. High-entropy layered oxide cathode enabling high-rate for solid-state sodium-ion batteries[J]. Nano-Micro Letters, 2023, 16(1): 10. |
| [46] | Ding F X, Wang H B, Zhang Q H, et al. Tailoring electronic structure to achieve maximum utilization of transition metal redox for high-entropy Na layered oxide cathodes[J]. Journal of the American Chemical Society, 2023, 145(25): 13592-13602. |
| [47] | Mu J X, Cai T X, Dong W J, et al. Biphasic high-entropy layered oxide as a stable and high-rate cathode for sodium-ion batteries[J]. Chemical Engineering Journal, 2023, 471: 144403. |
| [48] | Hao D B, Zhang G Y, Ning D, et al. Design of high-entropy P2/O3 hybrid layered oxide cathode material for high-capacity and high-rate sodium-ion batteries[J]. Nano Energy, 2024, 125: 109562. |
| [49] | Hu J, Guo T Q, Zhong X Y, et al. In situ reconstruction of high-entropy heterostructure catalysts for stable oxygen evolution electrocatalysis under industrial conditions[J]. Advanced Materials, 2024, 36(14): 2310918. |
| [50] | Gao X D, Zhang X Y, Liu X Y, et al. Recent advances for high-entropy based layered cathodes for sodium ion batteries[J]. Small Methods, 2023, 7(9): 2300152. |
| [51] | Yeh J W, Chen S K, Lin S J, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes[J]. Advanced Engineering Materials, 2004, 6(5): 299-303. |
| [52] | Rost C M, Sachet E, Borman T, et al. Entropy-stabilized oxides[J]. Nature Communications, 2015, 6: 8485. |
| [53] | Aamlid S S, Oudah M, Rottler J, et al. Understanding the role of entropy in high entropy oxides[J]. Journal of the American Chemical Society, 2023, 145(11): 5991-6006. |
| [54] | George E P, Raabe D, Ritchie R O. High-entropy alloys[J]. Nature Reviews Materials, 2019, 4(8): 515-534. |
| [55] | Zhang W T, Wang X Q, Zhang F Q, et al. Frontiers in high entropy alloys and high entropy functional materials[J]. Rare Metals, 2024, 43(10): 4639-4776. |
| [56] | Zhang R Z, Reece M J. Review of high entropy ceramics: design, synthesis, structure and properties[J]. Journal of Materials Chemistry A, 2019, 7(39): 22148-22162. |
| [57] | Xu T Y, Feng H W, Liu W, et al. Opportunities and challenges of high-entropy materials in lithium-ion batteries[J]. Rare Metals, 2024, 43(10): 4884-4902. |
| [58] | Wang L, Sunariwal N, He Y F, et al. Elemental stability rules for high entropy disordered rocksalt type Li-ion battery positive electrodes[J]. Advanced Energy Materials, 2025, 15(22): 2404982. |
| [59] | Fracchia M, Ghigna P, Pozzi T, et al. Stabilization by configurational entropy of the Cu(Ⅱ) active site during CO oxidation on Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O[J]. The Journal of Physical Chemistry Letters, 2020, 11(9): 3589-3593. |
| [60] | Zheng W, Liang G M, Liu Q, et al. The promise of high-entropy materials for high-performance rechargeable Li-ion and Na-ion batteries[J]. Joule, 2023, 7(12): 2732-2748. |
| [61] | Kong X K, Gu R, Jin Z Z, et al. Maximizing interface stability in all-solid-state lithium batteries through entropy stabilization and fast kinetics[J]. Nature Communications, 2024, 15(1): 7247. |
| [62] | Li M, Zhuo H X, Lei J W, et al. Unravelling the structure-stability interplay of O3-type layered sodium cathode materials via precision spacing engineering[J]. Nature Communications, 2025, 16(1): 2010. |
| [63] | Wang J H, Xu F T, Fan X M, et al. Study on the impact of cutoff voltage on structural and electrochemical stability of sodium-ion layered cathodes[J]. Chemical Engineering Journal, 2024, 500: 157032. |
| [64] | Huang Q, Wang M Y, Zhang L, et al. Shear-resistant interface of layered oxide cathodes for sodium ion batteries[J]. Energy Storage Materials, 2022, 45: 389-398. |
| [65] | Liu S Q, Liu F Z, Zhao S, et al. A high-entropy engineering on sustainable anionic redox Mn-based cathode with retardant stress for high-rate sodium-ion batteries[J]. Angewandte Chemie International Edition, 2025, 64(10): e202421089. |
| [66] | Tian K H, Dang Y Z, Xu Z, et al. A three-in-one strategy of high-entropy, single-crystal, and biphasic approaches to design O3- type layered cathodes for sodium-ion batteries[J]. Energy Storage Materials, 2024, 73: 103841. |
| [67] | Sekine S, Hosaka T, Maejima H, et al. Na[Mn0.36Ni0.44Ti0.15Fe0.05]O2 predicted via machine learning for high energy Na-ion batteries[J]. Journal of Materials Chemistry A, 2024, 12(45): 31103-31107. |
| [68] | Guo R N, Yang Y, Zhao C C, et al. The role of high-entropy materials in lithium-based rechargeable batteries[J]. Advanced Functional Materials, 2024, 34(18): 2313168. |
| [69] | Quinn A, Moutinho H, Usseglio-Viretta F, et al. Electron backscatter diffraction for investigating lithium-ion electrode particle architectures[J]. Cell Reports Physical Science, 2020, 1(8): 100137. |
| [70] | Chen D, Indris S, Schulz M, et al. In situ scanning electron microscopy on lithium-ion battery electrodes using an ionic liquid[J]. Journal of Power Sources, 2011, 196(15): 6382-6387. |
| [71] | He K, Zhang S, Li J, et al. Visualizing non-equilibrium lithiation of spinel oxide via in situ transmission electron microscopy[J]. Nature Communications, 2016, 7: 11441. |
| [72] | Wu K, Ran P L, Yin W, et al. Dynamic evolution of antisite defect and coupling anionic redox in high-voltage ultrahigh-Ni cathode[J]. Angewandte Chemie International Edition, 2024, 63(42): e202410326. |
| [1] | 张圣美, 李明, 张莹, 易茜, 杨依婷, 刘雅莉. 乳化剂和温度对相变微胶囊性能的影响分析[J]. 化工学报, 2025, 76(S1): 444-452. |
| [2] | 郭松源, 周晓庆, 缪五兵, 汪彬, 耑锐, 曹庆泰, 陈成成, 杨光, 吴静怡. 火箭上升段含多孔板液氧贮箱增压输运数值研究[J]. 化工学报, 2025, 76(S1): 62-74. |
| [3] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [4] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [5] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [6] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [7] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [8] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [9] | 吴林凯, 林志敏, 王良璧. 基于热质传递效应的准稳态结霜模型改进及数值验证[J]. 化工学报, 2025, 76(8): 4004-4016. |
| [10] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [11] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [12] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [13] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| [14] | 夏天炜, 王谙词, 句子涵, 孙晓霞, 胡定华. 基于三周期极小曲面结构的高密度储热器蓄放热特性研究[J]. 化工学报, 2025, 76(7): 3605-3614. |
| [15] | 陈培强, 郑群, 姜玉廷, 熊春华, 陈今茂, 王旭东, 黄龙, 阮曼, 徐万里. 电液流量及电流密度对海水激活电池输出特性的影响[J]. 化工学报, 2025, 76(7): 3235-3245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号