化工学报 ›› 2019, Vol. 70 ›› Issue (9): 3248-3255.DOI: 10.11949/0438-1157.20190354
收稿日期:2019-04-04
修回日期:2019-06-24
出版日期:2019-09-05
发布日期:2019-09-05
通讯作者:
杨昭
作者简介:陈裕博(1995—),男,硕士研究生,基金资助:
Yubo CHEN( ),Zhao YANG(
),Zhao YANG( ),Rui ZHAI,Biao FENG,Zijian LYU,Wenzhong ZHAO,Yingying GE
),Rui ZHAI,Biao FENG,Zijian LYU,Wenzhong ZHAO,Yingying GE
Received:2019-04-04
Revised:2019-06-24
Online:2019-09-05
Published:2019-09-05
Contact:
Zhao YANG
摘要:
制冷剂与冷冻机油的互溶性直接影响制冷系统的使用寿命和循环性能。为了解决新型制冷剂与冷冻机油的匹配问题,搭建了一套制冷剂与冷冻机油互溶性测试系统,在温度范围223.15~303.15 K内,开展了R290/R1234yf和矿物油的互溶性实验研究。结果表明:在含油率为10%±0.5%的情况下,当R290占混合制冷剂的质量分数在25%~35%变动时,R290/R1234yf和矿物油的临界互溶温度随着R290含量的增加而下降。在测试含油率范围内,基于常见的制冷工况,当R290在溶液中的质量分数高于30%时,溶液将呈现均一透明的状态;R290在溶液中的质量分数低于20%时,溶液将出现絮状物或分层现象。通过元素贡献评价方法,提供了理论临界互溶温度预测方程,并将结果以三元图的方式呈现,对新型环保制冷剂的发展具有现实指导意义。
中图分类号:
陈裕博, 杨昭, 翟瑞, 冯彪, 吕子建, 赵文仲, 葛滢滢. R290/R1234yf与矿物油的互溶性测试及评价方法[J]. 化工学报, 2019, 70(9): 3248-3255.
Yubo CHEN, Zhao YANG, Rui ZHAI, Biao FENG, Zijian LYU, Wenzhong ZHAO, Yingying GE. Miscibility measurement and evaluation method of R290/R1234yfwith mineral oil[J]. CIESC Journal, 2019, 70(9): 3248-3255.
| Refrigerants and oil | Chemical identifier | Half structural formula | Mass fraction purity/% | Boiling point/℃ | Saturated liquid density (15℃)/(kg·m-3) | Viscosity(40℃)/(mm2·s-1) | Global warming potential (GWP) |
|---|---|---|---|---|---|---|---|
| R1234yf | 2,3,3,3-tetra-fluoropropene | CH2=CFCF3 | ≥99.9 | -29.8 | 1127.2 | 0.125 | <1 |
| R290 | propane | CH3CH2CH3 | ≥99.96 | -42.2 | 507.5 | 0.177 | 3.3 |
| mineral oil | SUNISO 3GS | — | — | — | 909.0 | 29.5 | — |
表1 制冷剂与冷冻机油参数
Table 1 Properties of refrigerant and lubricant
| Refrigerants and oil | Chemical identifier | Half structural formula | Mass fraction purity/% | Boiling point/℃ | Saturated liquid density (15℃)/(kg·m-3) | Viscosity(40℃)/(mm2·s-1) | Global warming potential (GWP) |
|---|---|---|---|---|---|---|---|
| R1234yf | 2,3,3,3-tetra-fluoropropene | CH2=CFCF3 | ≥99.9 | -29.8 | 1127.2 | 0.125 | <1 |
| R290 | propane | CH3CH2CH3 | ≥99.96 | -42.2 | 507.5 | 0.177 | 3.3 |
| mineral oil | SUNISO 3GS | — | — | — | 909.0 | 29.5 | — |
| R290 mass fraction in R290/R1234yf refrigerant/% | Oil rate/% | Critical miscibility temperature/K |
|---|---|---|
| 24.68 | 9.75 | 305.15 |
| 25.37 | 9.67 | 294.15 |
| 25.96 | 9.60 | 285.65 |
| 26.73 | 9.85 | 276.65 |
| 27.03 | 10.27 | 273.15 |
| 28.41 | 10.07 | 259.65 |
| 29.28 | 9.51 | 250.65 |
| 30.90 | 10.13 | 243.15 |
| 33.28 | 9.81 | 233.15 |
| 34.95 | 10.25 | 226.65 |
表2 R290/R1234yf与矿物油互溶性测试结果
Table 2 Miscibility result of R290/R1234yf with
| R290 mass fraction in R290/R1234yf refrigerant/% | Oil rate/% | Critical miscibility temperature/K |
|---|---|---|
| 24.68 | 9.75 | 305.15 |
| 25.37 | 9.67 | 294.15 |
| 25.96 | 9.60 | 285.65 |
| 26.73 | 9.85 | 276.65 |
| 27.03 | 10.27 | 273.15 |
| 28.41 | 10.07 | 259.65 |
| 29.28 | 9.51 | 250.65 |
| 30.90 | 10.13 | 243.15 |
| 33.28 | 9.81 | 233.15 |
| 34.95 | 10.25 | 226.65 |
| w 1 | w 2 | w 3 | Z | T CMT/K | T Cal/K | ((T CMT-T Cal)/T CMT)/% |
|---|---|---|---|---|---|---|
| 0.0975 | 0.2227 | 0.6798 | 0.6457 | 301.15 | 299.14 | 0.67 |
| 0.0985 | 0.2250 | 0.6765 | 0.6431 | 296.15 | 296.62 | -0.16 |
| 0.0967 | 0.2292 | 0.6741 | 0.6391 | 294.15 | 291.08 | 1.04 |
| 0.1472 | 0.2532 | 0.5996 | 0.5984 | 286.65 | 272.43 | 4.96 |
| 0.0960 | 0.2346 | 0.6693 | 0.6336 | 285.65 | 284.69 | 0.34 |
| 0.1020 | 0.2329 | 0.6651 | 0.6338 | 285.15 | 288.10 | -1.04 |
| 0.1059 | 0.2417 | 0.6524 | 0.6234 | 274.15 | 279.26 | -1.87 |
| 0.1495 | 0.2571 | 0.5934 | 0.5934 | 273.15 | 268.74 | 1.62 |
| 0.0951 | 0.2419 | 0.6630 | 0.6262 | 272.65 | 276.56 | -1.44 |
| 0.1400 | 0.2669 | 0.5931 | 0.5858 | 270.15 | 259.15 | 4.07 |
| 0.1688 | 0.2484 | 0.5828 | 0.5966 | 269.15 | 277.77 | -3.20 |
| 0.2073 | 0.2674 | 0.5252 | 0.5607 | 264.65 | 258.27 | 2.41 |
| 0.1107 | 0.2527 | 0.6367 | 0.6104 | 262.65 | 268.99 | -2.42 |
| 0.1535 | 0.2641 | 0.5824 | 0.5843 | 261.65 | 262.39 | -0.28 |
| 0.1386 | 0.2740 | 0.5873 | 0.5786 | 256.15 | 252.89 | 1.27 |
| 0.1581 | 0.2720 | 0.5698 | 0.5740 | 252.65 | 255.51 | -1.13 |
| 0.2199 | 0.2739 | 0.5062 | 0.5481 | 251.15 | 251.83 | -0.27 |
| 0.2042 | 0.2784 | 0.5174 | 0.5492 | 250.15 | 249.16 | 0.39 |
| 0.1638 | 0.2818 | 0.5543 | 0.5610 | 248.15 | 247.47 | 0.27 |
| 0.1830 | 0.2693 | 0.5477 | 0.5679 | 247.15 | 257.75 | -4.29 |
| 0.1922 | 0.2864 | 0.5215 | 0.5449 | 247.15 | 243.45 | 1.50 |
| 0.1192 | 0.2721 | 0.6087 | 0.5871 | 246.15 | 252.55 | -2.60 |
| 0.2174 | 0.2822 | 0.5004 | 0.5393 | 244.65 | 245.24 | -0.24 |
| 0.2023 | 0.2852 | 0.5125 | 0.5421 | 242.65 | 243.85 | -0.49 |
| 0.1895 | 0.2963 | 0.5142 | 0.5347 | 239.65 | 236.13 | 1.47 |
| 0.1707 | 0.2937 | 0.5356 | 0.5452 | 239.15 | 238.37 | 0.33 |
| 0.1346 | 0.2952 | 0.5702 | 0.5573 | 237.65 | 236.01 | 0.69 |
| 0.1997 | 0.2943 | 0.5060 | 0.5328 | 235.15 | 237.16 | -0.85 |
| 0.1863 | 0.3081 | 0.5056 | 0.5228 | 233.15 | 228.01 | 2.21 |
| 0.2122 | 0.2994 | 0.4884 | 0.5214 | 231.15 | 232.74 | -0.69 |
| 0.1988 | 0.2926 | 0.5086 | 0.5351 | 230.65 | 238.46 | -3.38 |
| 0.1783 | 0.3066 | 0.5152 | 0.5278 | 230.65 | 229.17 | 0.64 |
| 0.1984 | 0.2989 | 0.5026 | 0.5280 | 230.15 | 233.86 | -1.61 |
| 0.1840 | 0.3166 | 0.4994 | 0.5142 | 227.15 | 222.52 | 2.04 |
| 0.1971 | 0.3035 | 0.4993 | 0.5233 | 225.15 | 230.70 | -2.46 |
| 0.2095 | 0.3083 | 0.4822 | 0.5124 | 224.65 | 226.84 | -0.98 |
表 3 变含油率下R290/R1234yf与矿物油互溶性测试结果
Table 3 Miscibility result of R290/R1234yf with mineral oil of various oil ratios
| w 1 | w 2 | w 3 | Z | T CMT/K | T Cal/K | ((T CMT-T Cal)/T CMT)/% |
|---|---|---|---|---|---|---|
| 0.0975 | 0.2227 | 0.6798 | 0.6457 | 301.15 | 299.14 | 0.67 |
| 0.0985 | 0.2250 | 0.6765 | 0.6431 | 296.15 | 296.62 | -0.16 |
| 0.0967 | 0.2292 | 0.6741 | 0.6391 | 294.15 | 291.08 | 1.04 |
| 0.1472 | 0.2532 | 0.5996 | 0.5984 | 286.65 | 272.43 | 4.96 |
| 0.0960 | 0.2346 | 0.6693 | 0.6336 | 285.65 | 284.69 | 0.34 |
| 0.1020 | 0.2329 | 0.6651 | 0.6338 | 285.15 | 288.10 | -1.04 |
| 0.1059 | 0.2417 | 0.6524 | 0.6234 | 274.15 | 279.26 | -1.87 |
| 0.1495 | 0.2571 | 0.5934 | 0.5934 | 273.15 | 268.74 | 1.62 |
| 0.0951 | 0.2419 | 0.6630 | 0.6262 | 272.65 | 276.56 | -1.44 |
| 0.1400 | 0.2669 | 0.5931 | 0.5858 | 270.15 | 259.15 | 4.07 |
| 0.1688 | 0.2484 | 0.5828 | 0.5966 | 269.15 | 277.77 | -3.20 |
| 0.2073 | 0.2674 | 0.5252 | 0.5607 | 264.65 | 258.27 | 2.41 |
| 0.1107 | 0.2527 | 0.6367 | 0.6104 | 262.65 | 268.99 | -2.42 |
| 0.1535 | 0.2641 | 0.5824 | 0.5843 | 261.65 | 262.39 | -0.28 |
| 0.1386 | 0.2740 | 0.5873 | 0.5786 | 256.15 | 252.89 | 1.27 |
| 0.1581 | 0.2720 | 0.5698 | 0.5740 | 252.65 | 255.51 | -1.13 |
| 0.2199 | 0.2739 | 0.5062 | 0.5481 | 251.15 | 251.83 | -0.27 |
| 0.2042 | 0.2784 | 0.5174 | 0.5492 | 250.15 | 249.16 | 0.39 |
| 0.1638 | 0.2818 | 0.5543 | 0.5610 | 248.15 | 247.47 | 0.27 |
| 0.1830 | 0.2693 | 0.5477 | 0.5679 | 247.15 | 257.75 | -4.29 |
| 0.1922 | 0.2864 | 0.5215 | 0.5449 | 247.15 | 243.45 | 1.50 |
| 0.1192 | 0.2721 | 0.6087 | 0.5871 | 246.15 | 252.55 | -2.60 |
| 0.2174 | 0.2822 | 0.5004 | 0.5393 | 244.65 | 245.24 | -0.24 |
| 0.2023 | 0.2852 | 0.5125 | 0.5421 | 242.65 | 243.85 | -0.49 |
| 0.1895 | 0.2963 | 0.5142 | 0.5347 | 239.65 | 236.13 | 1.47 |
| 0.1707 | 0.2937 | 0.5356 | 0.5452 | 239.15 | 238.37 | 0.33 |
| 0.1346 | 0.2952 | 0.5702 | 0.5573 | 237.65 | 236.01 | 0.69 |
| 0.1997 | 0.2943 | 0.5060 | 0.5328 | 235.15 | 237.16 | -0.85 |
| 0.1863 | 0.3081 | 0.5056 | 0.5228 | 233.15 | 228.01 | 2.21 |
| 0.2122 | 0.2994 | 0.4884 | 0.5214 | 231.15 | 232.74 | -0.69 |
| 0.1988 | 0.2926 | 0.5086 | 0.5351 | 230.65 | 238.46 | -3.38 |
| 0.1783 | 0.3066 | 0.5152 | 0.5278 | 230.65 | 229.17 | 0.64 |
| 0.1984 | 0.2989 | 0.5026 | 0.5280 | 230.15 | 233.86 | -1.61 |
| 0.1840 | 0.3166 | 0.4994 | 0.5142 | 227.15 | 222.52 | 2.04 |
| 0.1971 | 0.3035 | 0.4993 | 0.5233 | 225.15 | 230.70 | -2.46 |
| 0.2095 | 0.3083 | 0.4822 | 0.5124 | 224.65 | 226.84 | -0.98 |
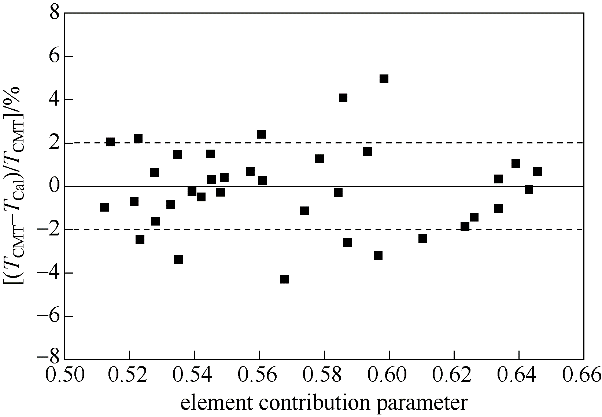
图5 R290/R1234yf矿物油溶液的临界互溶温度与理论计算值的偏差
Fig.5 Deviation between critical miscibility temperature and theoretical calculation value of R290/R1234yf mineral oil solution
| 1 | 秦大河, Stocker Thomas . IPCC第五次评估报告第一工作组报告的亮点结论[J]. 气候变化研究进展, 2014, (1): 1-6. |
| Qin D H , Thomas S . Highlights of the report of working group I of the fifth assessment report of the IPCC[J]. Progressus Inquisitiones DE Mutatione Climatis, 2014, (1): 1-6. | |
| 2 | Mclinden M O , Kazakov A F , Steven Brown J , et al . A thermodynamic analysis of refrigerants: possibilities and tradeoffs for low-GWP refrigerants[J]. International Journal of Refrigeration, 2014, 38: 80-92. |
| 3 | Del Col D , Torresin D , Cavallini A . Heat transfer and pressure drop during condensation of the low GWP refrigerant R1234yf[J]. International Journal of Refrigeration, 2010, 33(7): 1307-1318. |
| 4 | Saitoh S , Dang C , Nakamura Y , et al . Boiling heat transfer of HFO-1234yf flowing in a smooth small-diameter horizontal tube[J]. International Journal of Refrigeration, 2011, 34(8): 1846-1853. |
| 5 | Wang C . An overview for the heat transfer performance of HFO-1234yf[J]. Renewable and Sustainable Energy Reviews, 2013, 19: 444-453. |
| 6 | Cho H , Lee H , Park C . Performance characteristics of an automobile air conditioning system with internal heat exchanger using refrigerant R1234yf[J]. Applied Thermal Engineering, 2013, 61(2): 563-569. |
| 7 | Zilio C , Brown J S , Schiochet G , et al . The refrigerant R1234yf in air conditioning systems[J]. Energy, 2011, 36(10): 6110-6120. |
| 8 | Jarall S . Study of refrigeration system with HFO-1234yf as a working fluid[J]. International Journal of Refrigeration, 2012, 35(6): 1668-1677. |
| 9 | Boumaraf L , Haberschill P , Lallemand A . Investigation of a novel ejector expansion refrigeration system using the working fluid R134a and its potential substitute R1234yf[J]. International Journal of Refrigeration, 2014, 45: 148-159. |
| 10 | Qin Y , Zhang H , Wu Y , et al . Thermodynamic modeling of VLE and VLLE for the ternary system of 2, 3, 3, 3-tetrafluoroprop-1-ene(R1234yf)+propane (R290)+1,1,1,2-tetrafluoroethane(R134a) at 253.15 K—313.15 K[J]. Chemical Engineering Science, 2018, 187: 134-147. |
| 11 | Tanaka K , Higashi Y . Thermodynamic properties of HFO-1234yf (2, 3, 3, 3-tetrafluoropropene)[J]. International Journal of Refrigeration, 2010, 33(3): 474-479. |
| 12 | Dang Y , Kamiaka T , Dang C , et al . Liquid viscosity of low-GWP refrigerant mixtures (R32+R1234yf) and (R125+R1234yf)[J]. The Journal of Chemical Thermodynamics, 2015, 89: 183-188. |
| 13 | Qiu G , Meng X , Wu J . Density measurements for 2, 3, 3, 3-tetrafluoroprop-1-ene (R1234yf) and trans-1, 3, 3, 3-tetrafluoropropene (R1234ze(E))[J]. The Journal of Chemical Thermodynamics, 2013, 60: 150-158. |
| 14 | 孙维栋, 张鹏, 张昌 . R1234yf/R134a二元混合工质的热物理性质[J]. 流体机械, 2017, (8): 78-83. |
| Sun W D , Zhang P , Zhang C . The thermophysical properties of binary mixed refrigerant R1234yf/R134a[J]. Fluid Machinery, 2017, (8): 78-83. | |
| 15 | 田田, 杨昭, 吴曦, 等 . RE170、RE170/R227ea与矿物油的互溶性评价[J]. 化工学报, 2015, 66(6): 2005-2010. |
| Tian T , Yang Z , Wu X , et al . Miscibility evaluation of RE170 and RE170/R227ea with a mineral oil[J]. CIESC Journal, 2015, 66(6): 2005-2010. | |
| 16 | 熊爱凌, 韩厚德, 曹红奋 . R290及其冷冻油混合物互溶特性研究[J]. 上海海事大学学报, 2004, (3): 66-70. |
| Xiong A L , Han H D , Cao H F . A research on R290 and solubility of propane and refrigerating oils[J]. Journal of Shanghai Maritime University, 2015, 66, (6): 2005-2010. | |
| 17 | Choudhari C S , Sapali S N . Performance investigation of natural refrigerant R290 as a substitute to R22 in refrigeration systems[J]. Energy Procedia, 2017, 109: 346-352. |
| 18 | 杨瑞杰, 杨忠学, 孙蓉, 等 . 自然制冷剂R290与冷冻机油的相溶性研究[J]. 制冷学报, 2013, (5): 23-27. |
| Yang R J , Yang Z X , Sun R , et al . Investigation on the miscibility of lubricants with natural refrigerant R290[J]. Journal of Refrigeration, 2013, (5): 23-27. | |
| 19 | Pate M B , Zoz S , Berkenbosch L . Miscibility of lubricants with refrigerants [C]//International Refrigeration and Air Conditioning Conference. 1992: 210. |
| 20 | Yang Z , Tian T , Wu X , et al . Miscibility measurement and evaluation for the binary refrigerant mixture isobutane (R600a) + 1, 1, 1, 2, 3, 3, 3-heptafluoropropane (R227ea) with a mineral oil[J]. Journal of Chemical & Engineering Data, 2015, 60(6): 1781-1786. |
| 21 | 毕胜山, 韦俊, 刘志刚, 等 . DME、DME/R125与润滑油互溶性实验研究[J]. 工程热物理学报, 2012, 33(11): 1836-1838. |
| Bi S S , Wei J , Liu Z G , et al . The miscibility of DME and DME/R125 and lubricant oil[J]. Journal of Engineering Thermophysics, 2012, 33(11): 1836-1838. | |
| 22 | 金梧凤, 于斌, 高攀, 等 . R32与新型PVE油的互溶性及其对空调性能的影响[J]. 化工学报, 2018, 69(4): 1631-1637. |
| Jin W F , Yu B , Gao P , et al . Effect of solubility between R32 and new PVE oil on performance of air conditioning system[J]. CIESC Journal, 2018, 69(4): 1631-1637. | |
| 23 | Bobbo S , Zilio C , Scattolini M , et al . R1234yf as a substitute of R134a in automotive air conditioning. Solubility measurements in two commercial PAG oils[J]. International Journal of Refrigeration, 2014, 40: 302-308. |
| 24 | Sun Y , Wang X , Gong N , et al . Solubility of trans-1, 3, 3, 3-tetrafluoroprop-1-ene (R1234ze(E)) in pentaerythritol tetrapentanoate (PEC5) in the temperature range from 283.15 to 353.15 K[J]. International Journal of Refrigeration, 2014, 48: 114-120. |
| 25 | Sun Y , Wang X , Wang D , et al . Measurement and correlation for phase equilibrium of HFO1234yf with three pentaerythritol esters from 293.15 K to 348.15 K[J]. The Journal of Chemical Thermodynamics, 2017, 112: 122-128. |
| 26 | 中国石油天然气股份有限公司兰州炼化分公司 . 冷冻机油与制冷剂相溶性试验法: SH/T 0699—2000[S]. 北京: 中国标准出版社, 2000. |
| China Petroleum & Chemical Corporation Lanzhou Petrochemical Company . Text method for miscibility of refrigerator oils with refrigerants: SH/T 0699—2000[S]. Beijing: Standards Press of China, 2000. | |
| 27 | Technical Committee on Chemical Products . Text method for miscibility of refrigerator oils with refrigerants: JIS K2211—2009[S]. 2009. |
| 28 | Ashour I . Liquid-liquid equilibrium of MTBE+ ethanol+ water and MTBE + 1-hexanol + water over the temperature range of 288.15 to 308.15 K[J]. Journal of Chemical and Engineering Data, 2005, 50(1): 113-118. |
| 29 | Hildebrand J H , Scott R L . The Solubility of Nonelectrolyte[M]. New York: Reinhold Publ. Corp., 1950. |
| 30 | 王如竹 . 制冷原理与技术[M]. 北京: 科学出版社, 2003: 39-40. |
| Wang R Z . Refrigeration Principle and Technology[M]. Beijing: Science Press, 2003: 39-40. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [4] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [5] | 闫琳琦, 王振雷. 基于STA-BiLSTM-LightGBM组合模型的多步预测软测量建模[J]. 化工学报, 2023, 74(8): 3407-3418. |
| [6] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [7] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [8] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [9] | 罗来明, 张劲, 郭志斌, 王海宁, 卢善富, 相艳. 1~5 kW高温聚合物电解质膜燃料电池堆的理论模拟与组装测试[J]. 化工学报, 2023, 74(4): 1724-1734. |
| [10] | 许文烜, 江锦波, 彭新, 门日秀, 刘畅, 彭旭东. 宽速域三种典型型槽油气密封泄漏与成膜特性对比研究[J]. 化工学报, 2023, 74(4): 1660-1679. |
| [11] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [12] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [13] | 唐茹意, 潘罕骞, 郑侠俊, 张广欣, 汪星平, 崔希利, 邢华斌. Z型全氟聚醚的结构表征[J]. 化工学报, 2023, 74(1): 479-486. |
| [14] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [15] | 杨克, 王辰升, 纪虹, 郑凯, 邢志祥, 毕海普, 蒋军成. 聚多巴胺包覆混合粉体抑制甲烷爆炸的实验研究[J]. 化工学报, 2022, 73(9): 4245-4254. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号