化工学报 ›› 2020, Vol. 71 ›› Issue (8): 3575-3584.DOI: 10.11949/0438-1157.20200200
收稿日期:2020-02-28
修回日期:2020-05-19
出版日期:2020-08-05
发布日期:2020-08-05
通讯作者:
李季
作者简介:吴林(1993—),男,硕士研究生,基金资助:
Lin WU( ),Ji LI(
),Ji LI( ),Jiahua ZHU,Yuan GONG,Jing GE
),Jiahua ZHU,Yuan GONG,Jing GE
Received:2020-02-28
Revised:2020-05-19
Online:2020-08-05
Published:2020-08-05
Contact:
Ji LI
摘要:
磷石膏-氨-水固碳反应体系中石膏颗粒的溶解,是三相流化矿化反应系统的控制性步骤。在氨水溶液中石膏颗粒能与氨分子形成N—H…O氢键,导致石膏溶解速率受氨浓度影响。基于已知粒度分布的石膏颗粒群在不同氨浓度下的溶解实验数据,采用种群平衡模型与物料衡算相结合的方法,探究了氨浓度对石膏颗粒群溶解特性的影响规律。结果表明:氨浓度的增加会降低石膏溶解速率,随着溶解的进行,氨对溶解的抑制作用会减弱。此外,随着氨浓度的增大,增加单位氨浓度石膏溶解速率的降幅将变小。结合实验数据拟合关联溶解动力学参数,构建了氨浓度影响下石膏的溶解速率模型,预测了三相流化矿化反应系统中两个串联回路石膏料浆所需的停留时间,为磷石膏-氨-水固碳反应体系矿化烟气CO2的放大设计提供了理论依据。
中图分类号:
吴林, 李季, 朱家骅, 宫源, 葛敬. 磷石膏-氨-水固碳反应体系氨浓度对石膏颗粒溶解速率的影响[J]. 化工学报, 2020, 71(8): 3575-3584.
Lin WU, Ji LI, Jiahua ZHU, Yuan GONG, Jing GE. Effect of ammonia concentration on dissolution rate of gypsum particles in phosphogypsum-ammonia-water reaction system for carbon sequestration[J]. CIESC Journal, 2020, 71(8): 3575-3584.
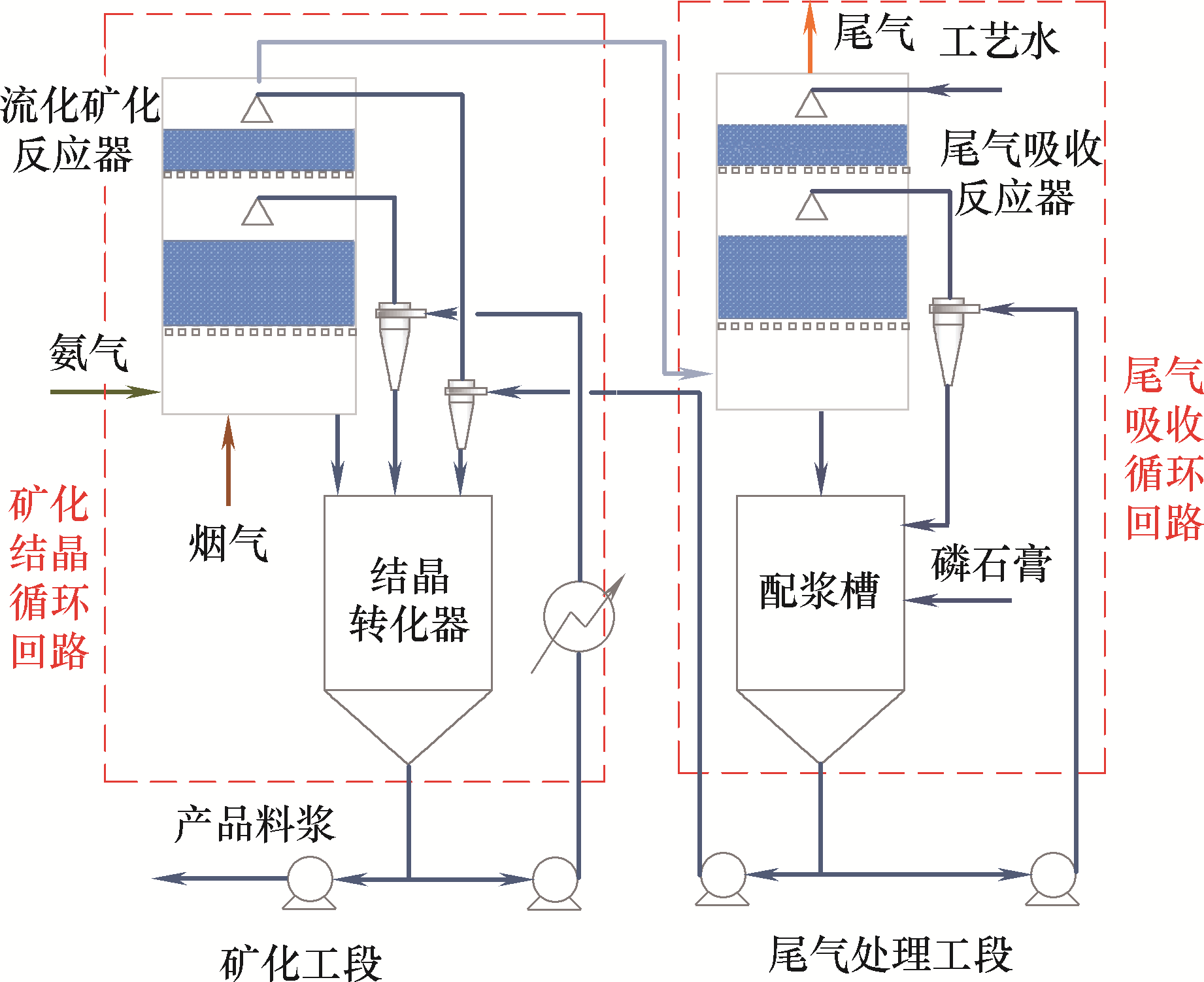
图1 磷石膏-氨-水固碳体系三相流化矿化反应系统[6]
Fig.1 The three-phase fluidized mineralization reaction system in the phosphogypsum-ammonia-water system for carbon sequestration
| ω/% | 时间/s | (mol·L-1) | (mol·L-1) | k/ (mol·m-2·s-1) | n | |
|---|---|---|---|---|---|---|
| 0 | 120 | 0.010 | 0.0101 | 9.0 | 1.73×10-4 | 1.10 |
| 240 | 0.0129 | 0.0126 | 5.4 | |||
| 360 | 0.0141 | 0.0142 | 2.8 | |||
| 480 | 0.0146 | 0.0146 | 2.1 | |||
| 600 | 0.0148 | 0.0145 | 0.7 | |||
| 2 | 120 | 0.0075 | 0.0077 | 2.6 | 1.01×10-4 | 1.12 |
| 240 | 0.0110 | 0.0109 | 0.9 | |||
| 360 | 0.0128 | 0.0126 | 1.6 | |||
| 480 | 0.0137 | 0.0136 | 0.7 | |||
| 600 | 0.0144 | 0.0143 | 0.7 | |||
| 4 | 120 | 0.0059 | 0.0067 | 11.9 | 0.70×10-4 | 1.13 |
| 240 | 0.0091 | 0.0096 | 5.2 | |||
| 360 | 0.0111 | 0.0107 | 3.7 | |||
| 480 | 0.0124 | 0.0120 | 3.3 | |||
| 600 | 0.0133 | 0.0127 | 4.7 | |||
| 6 | 120 | 0.0046 | 0.0051 | 9.8 | 0.46×10-4 | 1.12 |
| 240 | 0.0071 | 0.0073 | 2.7 | |||
| 360 | 0.0091 | 0.0095 | 4.2 | |||
| 480 | 0.0105 | 0.0104 | 1.0 | |||
| 600 | 0.0116 | 0.0110 | 5.5 |
表1 溶解动力学参数及Ca2+浓度实验值与模型计算值对比(T=25℃)
Table 1 Dissolution kinetics parameters and comparisons between experimental results and model calculations of Ca2+ concentrations (T=25℃)
| ω/% | 时间/s | (mol·L-1) | (mol·L-1) | k/ (mol·m-2·s-1) | n | |
|---|---|---|---|---|---|---|
| 0 | 120 | 0.010 | 0.0101 | 9.0 | 1.73×10-4 | 1.10 |
| 240 | 0.0129 | 0.0126 | 5.4 | |||
| 360 | 0.0141 | 0.0142 | 2.8 | |||
| 480 | 0.0146 | 0.0146 | 2.1 | |||
| 600 | 0.0148 | 0.0145 | 0.7 | |||
| 2 | 120 | 0.0075 | 0.0077 | 2.6 | 1.01×10-4 | 1.12 |
| 240 | 0.0110 | 0.0109 | 0.9 | |||
| 360 | 0.0128 | 0.0126 | 1.6 | |||
| 480 | 0.0137 | 0.0136 | 0.7 | |||
| 600 | 0.0144 | 0.0143 | 0.7 | |||
| 4 | 120 | 0.0059 | 0.0067 | 11.9 | 0.70×10-4 | 1.13 |
| 240 | 0.0091 | 0.0096 | 5.2 | |||
| 360 | 0.0111 | 0.0107 | 3.7 | |||
| 480 | 0.0124 | 0.0120 | 3.3 | |||
| 600 | 0.0133 | 0.0127 | 4.7 | |||
| 6 | 120 | 0.0046 | 0.0051 | 9.8 | 0.46×10-4 | 1.12 |
| 240 | 0.0071 | 0.0073 | 2.7 | |||
| 360 | 0.0091 | 0.0095 | 4.2 | |||
| 480 | 0.0105 | 0.0104 | 1.0 | |||
| 600 | 0.0116 | 0.0110 | 5.5 |
| 1 | 张含. 大气二氧化碳、全球变暖、海洋酸化与海洋碳循环相互作用的模拟研究[D]. 杭州: 浙江大学, 2018. |
| Zhang H. Simulation of interaction between atmospheric carbon dioxide, global warming, ocean acidification and ocean carbon cycle[D]. Hangzhou: Zhejiang University, 2018. | |
| 2 | Styring P, Jansen D, De Coninck H, et al. Carbon Capture and Utilisation in the Green Economy[M]. New York: Centre for Low Carbon Futures, 2011. |
| 3 | Zhang M, Guo Y. Rate based modeling of absorption and regeneration for CO2 capture by aqueous ammonia solution[J]. Applied Energy, 2013, 111: 142-152. |
| 4 | Mathias P M, Reddy S, connell J P O. Quantitative evaluation of the aqueous-ammonia process for CO2 capture using fundamental data and thermodynamic analysis[J]. Energy Procedia, 2009, 1(1): 1227-1234. |
| 5 | Tayibi H, Choura M, López F A, et al. Environmental impact and management of phosphogypsum [J]. Journal of Environmental Management, 2009, 90(8): 2377-2386. |
| 6 | 朱家骅, 曹耀峰, 王子宁, 等. 烟气CO2与磷石膏浆膜三相流化矿化方法: 102728285[P]. 2016. |
| Zhu J H, Cao Y F, Wang Z N, et al. Three phase fluidization and mineralization of flue gas CO2 and phosphogypsum serous film: 102728285[P]. 2016. | |
| 7 | 王子宁. 磷石膏与二氧化碳矿化反应体系钙离子转移过程研究[D]. 成都: 四川大学, 2014. |
| Wang Z N. Research on process of Ca2+ transfer in multi-phase reaction system of CO2 mineralized by phosphogypsum[D]. Chengdu: Sichuan University, 2014. | |
| 8 | Lu S Q, Lan P Q, Wu S F. Preparation of nano CaCO3 from phosphogypsum by gas-liquid-solid reaction for CO2 sorption[J]. Industrial & Engineering Chemistry Research, 2016, 55(38): 10172-10177. |
| 9 | 鲁厚芳. 杂质对石膏-碳酸铵转化过程的影响[D]. 成都: 四川大学, 2002. |
| Lu H F. Effect of impurities on gypsum - ammonium carbonate conversion process[D]. Chengdu: Sichuan University, 2002. | |
| 10 | 王子宁, 周加贝, 朱家骅, 等. 二水硫酸钙溶解动力学[J]. 化工学报, 2015, 66(3): 1001-1006. |
| Wang Z N, Zhou J B, Zhu J H, et al. Dissolution kinetics of calcium sulfate dihydrate[J]. CIESC Journal, 2015, 66(3): 1001-1006. | |
| 11 | Xie H P, Yue H R, Zhu J H. Scientific and engineering progress in CO2 mineralization using industrial waste and natural minerals[J]. Engineering, 2015, 1(1): 150-157. |
| 12 | Raines M A, Dewers T A. Mixed transport/reaction control of gypsum dissolution kinetics in aqueous solutions and initiation of gypsum karst[J]. Chemical Geology, 1997, 140(1): 29-48. |
| 13 | Christoffersen M R. The kinetics of dissolution of calcium sulphate dihydrate in water[J]. Journal of Crystal Growth, 1976, 35(1): 79-88. |
| 14 | Dewers T, Raines M. Reply to comment on: mixed transport/reaction control of gypsum dissolution kinetics[J]. Chemical Geology, 2000, 168(3/4): 275-278. |
| 15 | Feng P, Brand A S, Chen L, et al. In situ nanoscale observations of gypsum dissolution by digital holographic microscopy[J]. Chemical Geology, 2017, 460(1): 25-36. |
| 16 | 关润铎, 刘涛, 张方坤, 等. 基于PBM的L-谷氨酸粒度分布控制优化[J]. 化工学报, 2017, 68(3): 956-963. |
| Guan R D, Liu T, Zhang F K, et al. Optimal control of L-glutamic acid crystal size distribution based on population balance model[J]. CIESC Journal, 2017, 68(3): 956-963. | |
| 17 | Xu D, Liu Z, Cai L, et al. A CFD-PBM approach for modeling ice slurry flow in horizontal pipes[J]. Chemical Engineering Science, 2018, 176(2): 546-559. |
| 18 | Pedersen B F, Semmingsen D. Neutron diffraction refinement of the structure of gypsum, CaSO4·2H2O[J]. Acta Crystallographica, 1982, 38(4): 1074-1077. |
| 19 | Glemza A J, Koehler J A, Brune B J, et al. Selective adsorption of methoxyphenol positional somers[J]. Industrial & Engineering Chemistry Research, 1998, 37(9): 3685-3690. |
| 20 | 曾维伟. 菱锌矿和石英的浮选分离研究[D]. 长沙: 中南大学, 2011. |
| Zeng W W. Flotation separation of quartzite and quartzite[D]. Changsha: Central South University, 2011. | |
| 21 | 张建新. 天然硬石膏水化硬化及活性激发研究[D]. 重庆: 重庆大学, 2009. |
| Zhang J X. Study on hydration hardening and activation of natural anhydrite[D]. Chongqing: Chongqing University, 2009. | |
| 22 | 李晔, 刘奇, 许时. 淀粉类多糖在方解石和萤石表面吸附特性及作用机理[J]. 有色金属, 1996, 48(1): 26. |
| Li Y, Liu Q, Xu S. Adsorption properties and interaction mechanism of starch-type polysaccharides onto fluorite and calcite[J]. Nonferrous Metals, 1996, 48(1): 26. | |
| 23 | Leblanc S E, Fogler H S. Population balance modeling of the dissolution of polydisperse solids: rate limiting regimes[J]. AIChE Journal, 1987, 33(1): 54-63. |
| 24 | Hanchen M, Krevor S, Mazzotti M, et al. Validation of a population balance model for olivine dissolution[J]. Chemical Engineering Science,2007, 62(22): 6412-6422. |
| 25 | 宫源, 罗安安, 朱家骅, 等. 传质控制下宽分布石膏颗粒群溶解特性[J]. 化工学报, 2018, 69(10): 4177-4183. |
| Gong Y, Luo A A, Zhu J H, et al. Dissolution characteristic of gypsum particles with wide size distribution under mass transfer controlling[J]. CIESC Journal, 2018, 69(10): 4177-4183. | |
| 26 | Jeschke A A, Vosbeck K, Dreybrodt W. Surface controlled dissolution rates of gypsum in aqueous solutions exhibit nonlinear dissolution kinetics[J]. Geochimica Et Cosmochimica Acta, 2011, 65(1): 27-34. |
| 27 | Mangin D, Garcia E, Gerard S, et al. Modeling of the dissolution of a pharmaceutical compound[J]. Journal of Crystal Growth, 2005, 286(1): 121-125. |
| 28 | Vučak M, Perić J, Žmikić A,et al. A study of carbon dioxide absorption into aqueous monoethanolamine solution containing calcium nitrate in the gas-liquid reactive precipitation of calcium carbonate[J]. Chemical Engineering Journal, 2002, 87(2): 171-179. |
| 29 | Randolph A. Theory of Particulate Processes: Analysis and Techniques of Continuous Crystallization [M]. Salt Lake City: Academic Press, 2012. |
| 30 | Hounslow M J, Reynolds G K. Product engineering for crystal size distribution[J]. AIChE Journal, 2006, 52(7): 2507-2517. |
| 31 | Nchen M, Krevor S, Mazzotti M, et al. Validation of a population balance model for olivine dissolution[J]. Chemical Engineering Science, 2007, 62(22): 6412-6422. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [4] | 袁佳琦, 刘政, 黄锐, 张乐福, 贺登辉. 泡状入流条件下旋流泵能量转换特性研究[J]. 化工学报, 2023, 74(9): 3807-3820. |
| [5] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [6] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [7] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [8] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [9] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [10] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [11] | 王帅, 杨富凯, 徐新宇. 阻燃型全生物基多元醇聚氨酯泡沫的制备及性能研究[J]. 化工学报, 2023, 74(3): 1399-1408. |
| [12] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| [13] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [14] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [15] | 王永倩, 王平, 程康, 毛晨林, 刘文锋, 尹智成, Ferrante Antonio. 氨气/甲烷贫预混旋转湍流火焰稳定性及NO生成[J]. 化工学报, 2022, 73(9): 4087-4094. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号