化工学报 ›› 2022, Vol. 73 ›› Issue (3): 1343-1350.DOI: 10.11949/0438-1157.20211344
收稿日期:2021-09-16
修回日期:2021-11-23
出版日期:2022-03-15
发布日期:2022-03-14
通讯作者:
王保伟
作者简介:王小西(1992—),男,硕士研究生,助理工程师,基金资助:
Xiaoxi WANG( ),Xiaoyan LI,Baowei WANG(
),Xiaoyan LI,Baowei WANG( )
)
Received:2021-09-16
Revised:2021-11-23
Online:2022-03-15
Published:2022-03-14
Contact:
Baowei WANG
摘要:
二氧化碳既是主要的温室气体之一,也是包含碳和氧的资源,把相对惰性的CO2转化为易于利用的CO是其利用的方法之一。采用介质阻挡微等离子体反应器通过单变量和正交实验探究了反应器参数(放电区长度、放电间距、介质厚度)和工艺参数(输入功率、放电频率和停留时间)对CO2分解为CO的转化率和能量效率的影响规律。研究结果表明,影响CO2转化率的大小顺序依次为:放电间距>放电长度>输入功率≈停留时间>介质厚度>放电频率;输入功率60.0 W、放电频率9.0 kHz和停留时间1.5 s、放电区长度60 mm、放电间距0.5 m、介质厚度1.6 mm时,CO2的转化率为10.6%,能量效率为4.1%。
中图分类号:
王小西, 李笑艳, 王保伟. 介质阻挡放电微等离子体分解二氧化碳研究[J]. 化工学报, 2022, 73(3): 1343-1350.
Xiaoxi WANG, Xiaoyan LI, Baowei WANG. Decomposition of carbon dioxide via dielectric barrier discharge microplasma[J]. CIESC Journal, 2022, 73(3): 1343-1350.
| 输入功率/W | 放电频率/kHz | 停留时间/s | 放电间距/mm | 放电长度/mm |
|---|---|---|---|---|
| 10.0~60.0 | 7.0~10.0 | 1.0~4.0 | 0.5~1.5 | 60.0~120.0 |
表1 DBD等离子体分解CO2实验过程中主要参数及其取值范围
Table 1 The main parameters and range of CO2 decomposition by DBD plasma
| 输入功率/W | 放电频率/kHz | 停留时间/s | 放电间距/mm | 放电长度/mm |
|---|---|---|---|---|
| 10.0~60.0 | 7.0~10.0 | 1.0~4.0 | 0.5~1.5 | 60.0~120.0 |

图2 输入功率对电流波形图的影响
Fig.2 Influence of the input power on discharge current waveforms (frequency: 7.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
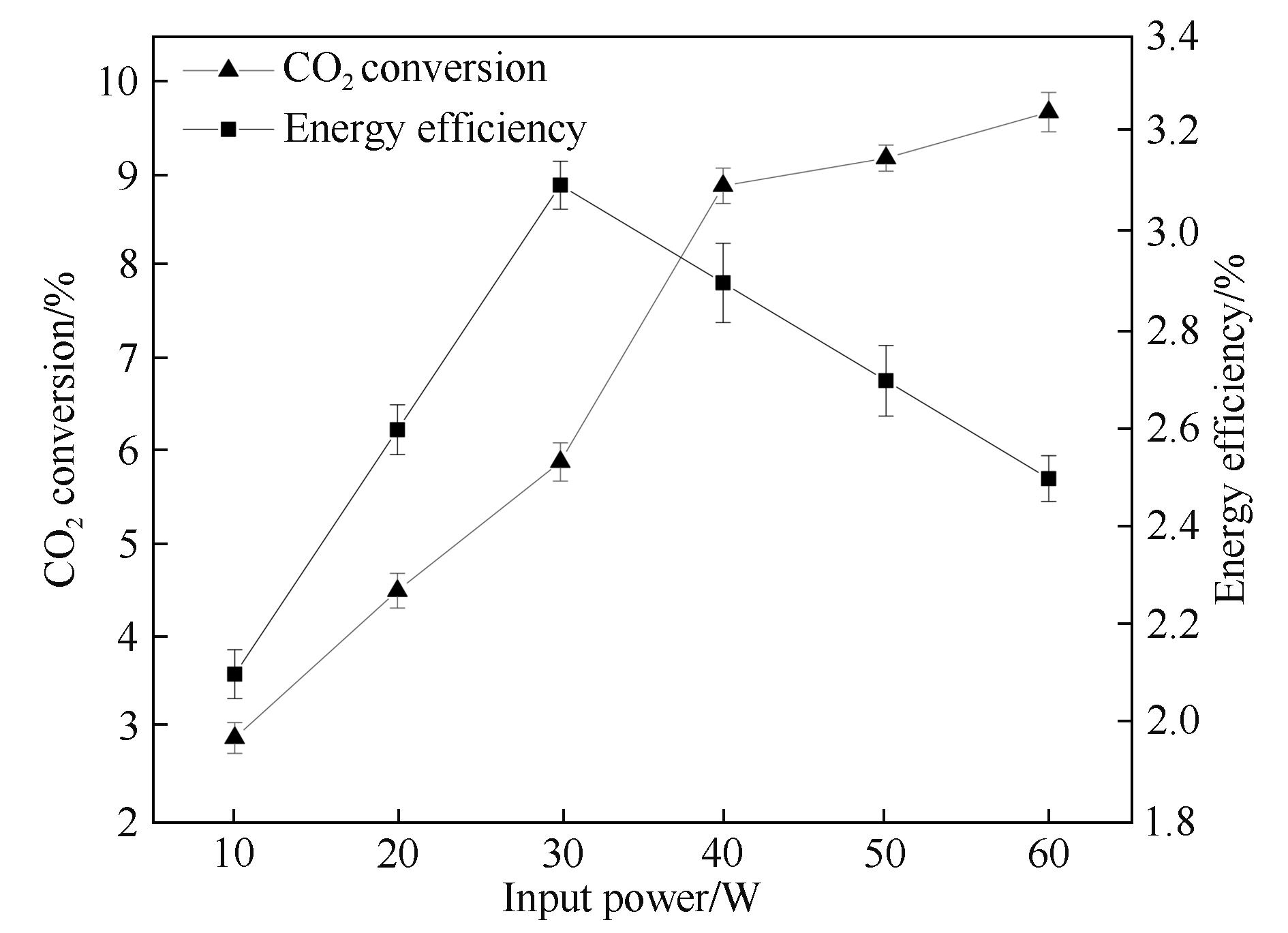
图3 输入功率对CO2转化率和能量效率的影响
Fig.3 Influence of the input power on CO2 conversion and energy efficiency (frequency: 7.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
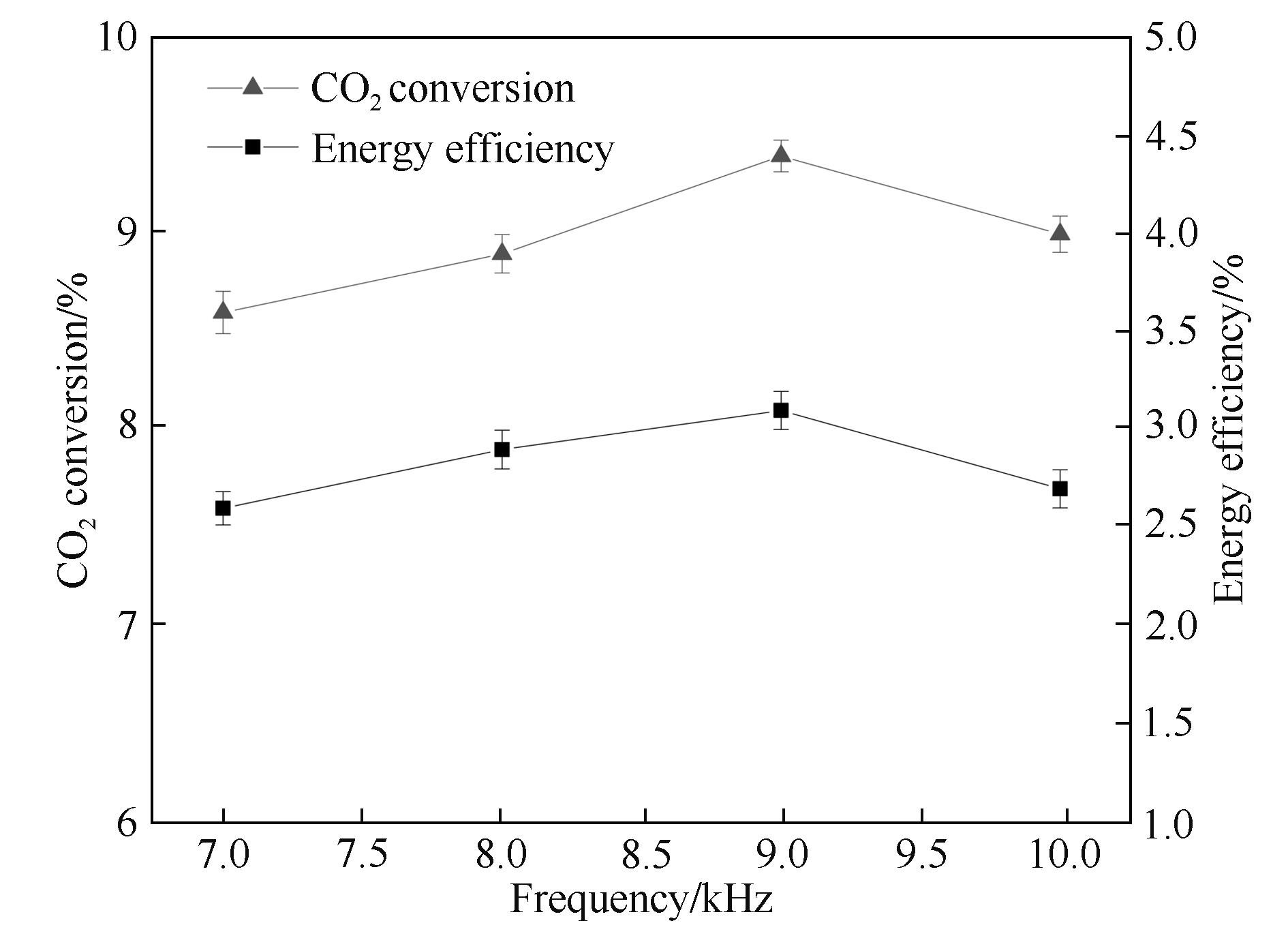
图4 放电频率对CO2转化率和能量效率的影响
Fig.4 Influence of the frequency on CO2 conversion and energy efficiency (input power: 40 W; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm; τ: 3.0 s)
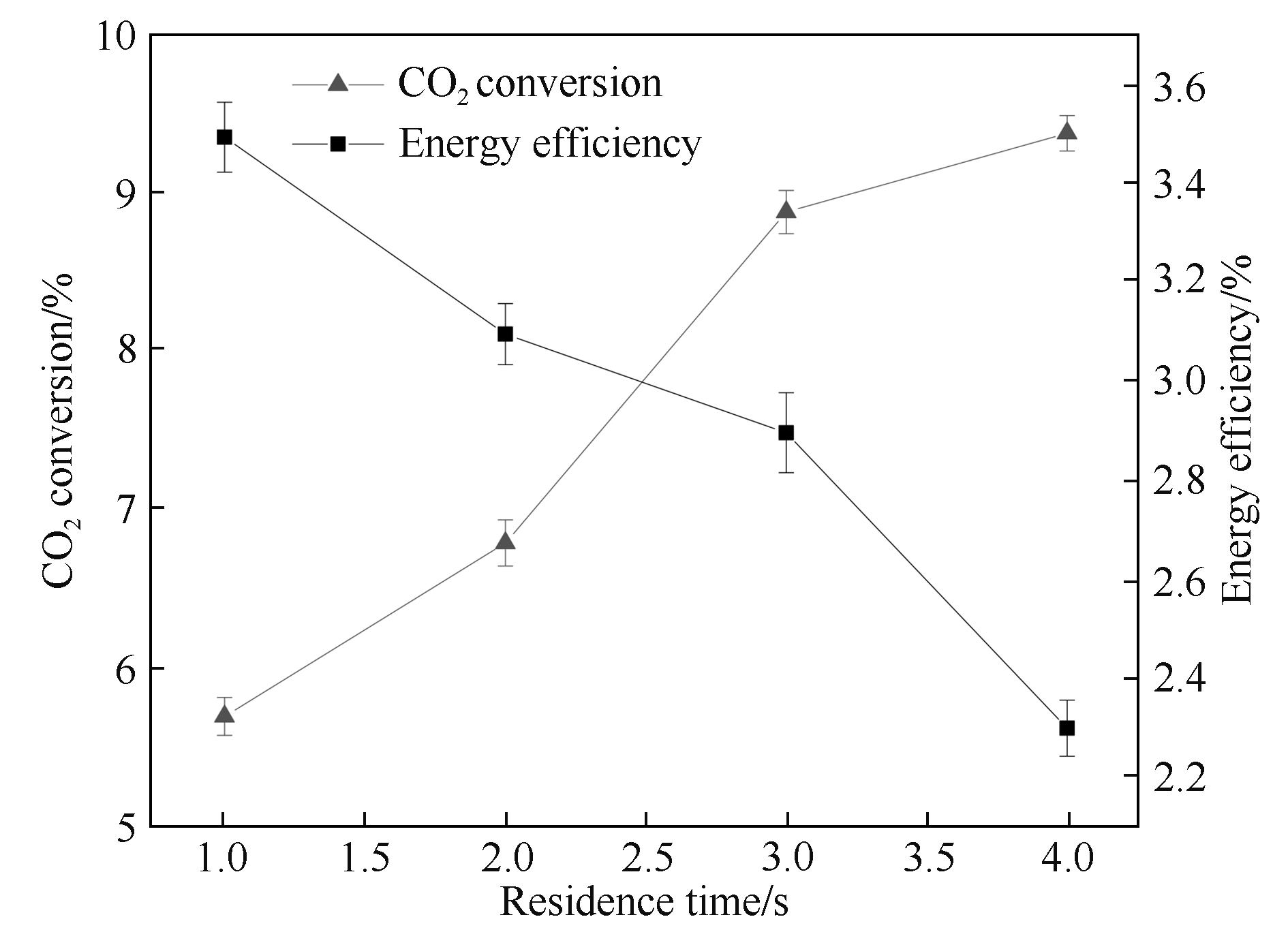
图5 停留时间对CO2转化率和能量效率的影响
Fig.5 Influence of the residence time on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; discharge length: 80.0 mm; discharge gap: 0.5 mm; barrier thickness: 1.6 mm)

图6 放电长度对CO2转化率和能量效率的影响
Fig.6 Influence of the discharge length on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; τ: 2.5 s; discharge gap: 0.5 mm; barrier thickness: 1.6 mm)
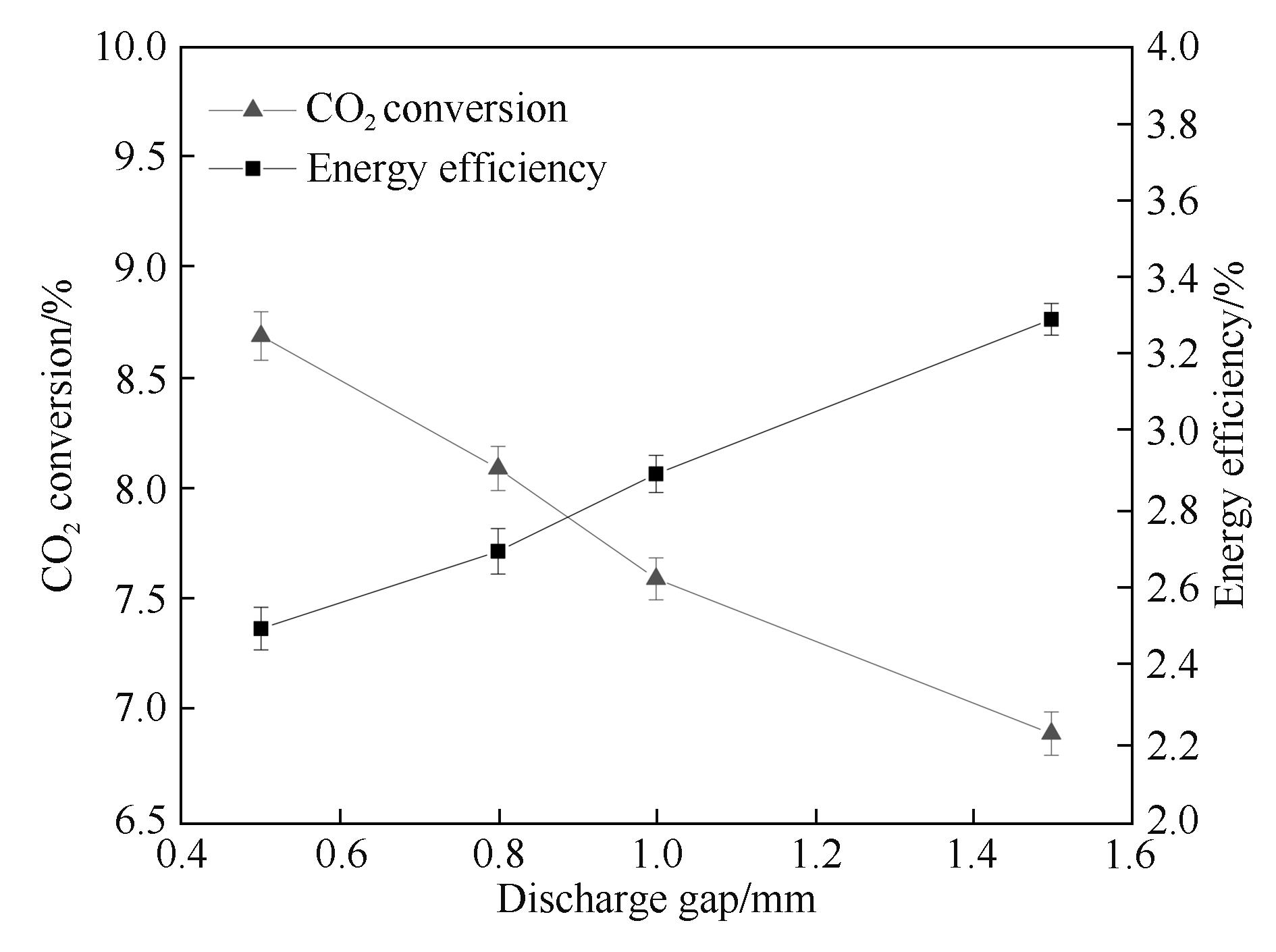
图7 放电间距对CO2转化率和能量效率的影响
Fig.7 Influence of the discharge gap on CO2 conversion and energy efficiency (input power: 40.0 W; frequency: 9.0 kHz; τ: 2.5 s; discharge length: 80 mm; barrier thickness: 1.6 mm)
| 水平 | 因素 | |||||
|---|---|---|---|---|---|---|
| 输入功率/W | 放电间距/mm | 放电频率/kHz | 停留时间/s | 放电长度/mm | 介质厚度/mm | |
| 1 | 40.0 | 1.0 | 8.0 | 1.5 | 100 | 1.0 |
| 2 | 50.0 | 0.8 | 9.0 | 2.5 | 80 | 1.6 |
| 3 | 60.0 | 0.5 | 10.0 | 3.5 | 60 | 2.1 |
表2 因素及水平
Table 2 Factors and levels
| 水平 | 因素 | |||||
|---|---|---|---|---|---|---|
| 输入功率/W | 放电间距/mm | 放电频率/kHz | 停留时间/s | 放电长度/mm | 介质厚度/mm | |
| 1 | 40.0 | 1.0 | 8.0 | 1.5 | 100 | 1.0 |
| 2 | 50.0 | 0.8 | 9.0 | 2.5 | 80 | 1.6 |
| 3 | 60.0 | 0.5 | 10.0 | 3.5 | 60 | 2.1 |
| 序号 | A | B | C | 空白 | D | E | F | χCO2/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5.6 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 7.1 |
| 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 8.5 |
| 4 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 7.7 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 7.3 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 8.7 |
| 7 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 7.9 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 9.3 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 8.3 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 4.9 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 7.4 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 6.9 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 8.2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 6.1 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 9.6 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 7.5 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 6.6 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 9.1 |
| K1 | 30.381 | 26.895 | 35.691 | 35.019 | 31.674 | 31.53 | 33.549 | |
| K2 | 36.576 | 30.444 | 36.06 | 34.665 | 34.326 | 33.126 | 37.77 | |
| K3 | 37.005 | 46.62 | 32.211 | 34.275 | 37.959 | 39.306 | 32.64 | |
| K1 | 10.127 | 8.965 | 11.897 | 11.673 | 10.558 | 10.51 | 11.183 | |
| K2 | 12.192 | 10.148 | 12.02 | 11.555 | 11.442 | 11.042 | 12.59 | |
| K3 | 12.335 | 15.54 | 10.737 | 11.425 | 12.653 | 13.102 | 10.88 | |
| R1 | 2.208 | 6.575 | 1.283 | 0.248 | 2.095 | 2.592 | 1.71 |
表3 正交实验结果和极差分析
Table 3 The result and range analysis of orthogonal experiment
| 序号 | A | B | C | 空白 | D | E | F | χCO2/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 5.6 |
| 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 7.1 |
| 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 8.5 |
| 4 | 2 | 1 | 1 | 2 | 2 | 3 | 3 | 7.7 |
| 5 | 2 | 2 | 2 | 3 | 3 | 1 | 1 | 7.3 |
| 6 | 2 | 3 | 3 | 1 | 1 | 2 | 2 | 8.7 |
| 7 | 3 | 1 | 2 | 1 | 3 | 2 | 3 | 7.9 |
| 8 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 9.3 |
| 9 | 3 | 3 | 1 | 3 | 2 | 1 | 2 | 8.3 |
| 10 | 1 | 1 | 3 | 3 | 2 | 2 | 1 | 4.9 |
| 11 | 1 | 2 | 1 | 1 | 3 | 3 | 2 | 7.4 |
| 12 | 1 | 3 | 2 | 2 | 1 | 1 | 3 | 6.9 |
| 13 | 2 | 1 | 2 | 3 | 1 | 3 | 2 | 8.2 |
| 14 | 2 | 2 | 3 | 1 | 2 | 1 | 3 | 6.1 |
| 15 | 2 | 3 | 1 | 2 | 3 | 2 | 1 | 9.6 |
| 16 | 3 | 1 | 3 | 2 | 3 | 1 | 2 | 7.5 |
| 17 | 3 | 2 | 1 | 3 | 1 | 2 | 3 | 6.6 |
| 18 | 3 | 3 | 2 | 1 | 2 | 3 | 1 | 9.1 |
| K1 | 30.381 | 26.895 | 35.691 | 35.019 | 31.674 | 31.53 | 33.549 | |
| K2 | 36.576 | 30.444 | 36.06 | 34.665 | 34.326 | 33.126 | 37.77 | |
| K3 | 37.005 | 46.62 | 32.211 | 34.275 | 37.959 | 39.306 | 32.64 | |
| K1 | 10.127 | 8.965 | 11.897 | 11.673 | 10.558 | 10.51 | 11.183 | |
| K2 | 12.192 | 10.148 | 12.02 | 11.555 | 11.442 | 11.042 | 12.59 | |
| K3 | 12.335 | 15.54 | 10.737 | 11.425 | 12.653 | 13.102 | 10.88 | |
| R1 | 2.208 | 6.575 | 1.283 | 0.248 | 2.095 | 2.592 | 1.71 |
| 参数 | 局部方差总和 | 自由度 | 方差比 | F临界值 | 重要性 |
|---|---|---|---|---|---|
| 输入功率 | 18.323 | 2 | 99.043 | 99 | * |
| 放电间距 | 147.402 | 2 | 796.768 | 99 | * |
| 放电频率 | 6.016 | 2 | 32.519 | 99 | |
| 停留时间 | 13.275 | 2 | 71.757 | 99 | |
| 放电长度 | 22.486 | 2 | 121.546 | 99 | * |
| 介质厚度 | 9.99 | 2 | 54 | 99 | |
| 误差 | 0.18 | 2 | — | — |
表4 方差分析
Table 4 The variance analysis
| 参数 | 局部方差总和 | 自由度 | 方差比 | F临界值 | 重要性 |
|---|---|---|---|---|---|
| 输入功率 | 18.323 | 2 | 99.043 | 99 | * |
| 放电间距 | 147.402 | 2 | 796.768 | 99 | * |
| 放电频率 | 6.016 | 2 | 32.519 | 99 | |
| 停留时间 | 13.275 | 2 | 71.757 | 99 | |
| 放电长度 | 22.486 | 2 | 121.546 | 99 | * |
| 介质厚度 | 9.99 | 2 | 54 | 99 | |
| 误差 | 0.18 | 2 | — | — |
| 输入 功率/W | 放电 间距/mm | 放电 频率/kHz | 停留 时间/s | 放电 长度/mm | 介质 厚度/mm |
|---|---|---|---|---|---|
| 60.0 | 0.5 | 9.0 | 1.5 | 60 | 1.6 |
表5 最佳因素水平组合
Table 5 The best factor level combination
| 输入 功率/W | 放电 间距/mm | 放电 频率/kHz | 停留 时间/s | 放电 长度/mm | 介质 厚度/mm |
|---|---|---|---|---|---|
| 60.0 | 0.5 | 9.0 | 1.5 | 60 | 1.6 |
| CO2转化技术 | 转化率/% | 文献 |
|---|---|---|
| 热催化法 | 0.5 | [ |
| 电化学法 | 16.1 | [ |
| 滑动弧光放电 | <15 | [ |
| 电晕放电 | 15.2 | [ |
| DBD等离子体技术 | 10.6 | 本实验 |
表6 不同方法转化CO2对比
Table 6 Comparison of CO2 conversion with different methods
| CO2转化技术 | 转化率/% | 文献 |
|---|---|---|
| 热催化法 | 0.5 | [ |
| 电化学法 | 16.1 | [ |
| 滑动弧光放电 | <15 | [ |
| 电晕放电 | 15.2 | [ |
| DBD等离子体技术 | 10.6 | 本实验 |
| 1 | Zhou W, Zhou C, Yin H R, et al. Direct conversion of syngas into aromatics over a bifunctional catalyst: inhibiting net CO2 release[J]. Chemical Communications, 2020, 56(39): 5239-5242. |
| 2 | 刘昌俊, 郭秋婷, 叶静云, 等. 二氧化碳转化催化剂研究进展及相关问题思考[J]. 化工学报, 2016, 67(1): 6-13. |
| Liu C J, Guo Q T, Ye J Y, et al. Perspective on catalyst investigation for CO2 conversion and related issues[J]. CIESC Journal, 2016, 67(1): 6-13. | |
| 3 | Thomas H, Bozec Y, Elkalay K, et al. Enhanced open ocean storage of CO2 from shelf sea pumping[J]. Science, 2004, 304(5673): 1005-1008. |
| 4 | Mac Dowell N, Fennell P S, Shah N, et al. The role of CO2 capture and utilization in mitigating climate change[J]. Nature Climate Change, 2017, 7(4): 243-249. |
| 5 | Sun Y, Lin Z, Peng S H, et al. A critical perspective on CO₂ conversions into chemicals and fuels[J]. Journal of Nanoscience and Nanotechnology, 2019, 19(6): 3097-3109. |
| 6 | Ahmed R, Liu G J, Yousaf B, et al. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—a review[J]. Journal of Cleaner Production, 2020, 242: 118409. |
| 7 | Kozák T, Bogaerts A. Splitting of CO2 by vibrational excitation in non-equilibrium plasmas: a reaction kinetics model[J]. Plasma Sources Science & Technology, 2014, 23(4): 045004. |
| 8 | Kondratenko E V, Mul G, Baltrusaitis J, et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes[J]. Energy & Environmental Science, 2013, 6(11): 3112. |
| 9 | Appel A M, Bercaw J E, Bocarsly A B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chemical Reviews, 2013, 113(8): 6621-6658. |
| 10 | Whipple D T, Kenis P J A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction[J]. The Journal of Physical Chemistry Letters, 2010, 1(24): 3451-3458. |
| 11 | Saravanan A, Senthilkumar P, Vo D V N, et al. A comprehensive review on different approaches for CO2 utilization and conversion pathways[J]. Chemical Engineering Science, 2021, 236: 116515. |
| 12 | Kamkeng A D N, Wang M H, Hu J, et al. Transformation technologies for CO2 utilisation: current status, challenges and future prospects[J]. Chemical Engineering Journal, 2021, 409: 128138. |
| 13 | Nigara Y, Cales B. Production of CO by direct thermal splitting of CO2 at high temperature[J]. Bulletin of the Chemical Society of Japan, 1986, 59(6): 1997-2002. |
| 14 | Ganesh I. Conversion of carbon dioxide into methanol—a potential liquid fuel: fundamental challenges and opportunities (a review)[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 221-257. |
| 15 | Liu J L, Wang X, Li X S, et al. CO2 conversion, utilisation and valorisation in gliding arc plasma reactors[J]. Journal of Physics D:Applied Physics, 2020, 53(25): 253001. |
| 16 | Zhang S, Fan Q, Xia R, et al. CO2 reduction: from homogeneous to heterogeneous electrocatalysis[J]. Accounts of Chemical Research, 2020, 53(1): 255-264. |
| 17 | Das S, Wan Daud W M A. A review on advances in photocatalysts towards CO2 conversion[J]. RSC Advances, 2014, 4(40): 20856-20893. |
| 18 | Brennan L, Owende P. Biofuels from microalgae—a review of technologies for production, processing, and extractions of biofuels and co-products[J]. Renewable and Sustainable Energy Reviews, 2010, 14(2): 557-577. |
| 19 | George A, Shen B X, Craven M, et al. A review of non-thermal plasma technology: a novel solution for CO2 conversion and utilization[J]. Renewable and Sustainable Energy Reviews, 2021, 135: 109702. |
| 20 | Sun S R, Wang H X, Mei D H, et al. CO2 conversion in a gliding arc plasma: performance improvement based on chemical reaction modeling[J]. Journal of CO2 Utilization, 2017, 17: 220-234. |
| 21 | Li L, Zhang H, Li X D, et al. Magnetically enhanced gliding arc discharge for CO2 activation[J]. Journal of CO2 Utilization, 2020, 35: 28-37. |
| 22 | Nagassou D, Mohsenian S, Nallar M, et al. Decomposition of CO2 in a solar-gliding arc plasma reactor: effects of water, nitrogen, methane, and process optimization[J]. Journal of CO2 Utilization, 2020, 38: 39-48. |
| 23 | Ramakers M, Medrano J A, Trenchev G, et al. Revealing the arc dynamics in a gliding arc plasmatron: a better insight to improve CO2 conversion[J]. Plasma Sources Science & Technology, 2017, 26(12): 125002. |
| 24 | Li L, Zhang H, Li X D, et al. Plasma-assisted CO2 conversion in a gliding arc discharge: improving performance by optimizing the reactor design[J]. Journal of CO2 Utilization, 2019, 29: 296-303. |
| 25 | Liu J L, Park H W, Chung W J, et al. High-efficient conversion of CO2 in AC-pulsed tornado gliding arc plasma[J]. Plasma Chemistry and Plasma Processing, 2016, 36(2): 437-449. |
| 26 | Ramakers M, Heijkers S, Tytgat T, et al. Combining CO2 conversion and N2 fixation in a gliding arc plasmatron[J]. Journal of CO2 Utilization, 2019, 33: 121-130. |
| 27 | Liu J B, Li X S, Liu J L, et al. Insight into gliding arc (GA) plasma reduction of CO2 with H2: GA characteristics and reaction mechanism[J]. Journal of Physics D-Applied Physics, 2019, 52(28): 284001. |
| 28 | Ramakers M, Trenchev G, Heijkers S, et al. Gliding arc plasmatron: providing an alternative method for carbon dioxide conversion[J]. ChemSusChem, 2017, 10(12): 2642-2652. |
| 29 | Nunnally T, Gutsol K, Rabinovich A, et al. Dissociation of CO2 in a low current gliding arc plasmatron[J]. Journal of Physics D-Applied Physics, 2011, 44(27): 274009. |
| 30 | Kim S C, Lim M S, Chun Y N. Reduction characteristics of carbon dioxide using a plasmatron[J]. Plasma Chemistry and Plasma Processing, 2014, 34(1): 125-143. |
| 31 | Xu W, Li M W, Xu G H, et al. Decomposition of CO2 using DC corona discharge at atmospheric pressure[J]. Japanese Journal of Applied Physics, 2004, 43(12): 8310-8311. |
| 32 | Wen Y Z, Jiang X Z. Decomposition of CO2 using pulsed corona discharges combined with catalyst[J]. Plasma Chemistry and Plasma Processing, 2001, 21(4): 665-678. |
| 33 | 代斌, 宫为民, 张秀玲, 等. 脉冲电晕等离子体活化纯CO2的反应[J]. 中国环境科学, 1999, 19(5): 410-412. |
| Dai B, Gong W M, Zhang X L, et al. Investigation on the conversion of pure CO2 by pulse corona plasma[J]. China Environmental Science, 1999, 19(5): 410-412 | |
| 34 | 李明伟, 许根慧, 刘昌俊, 等. 电晕放电二氧化碳冷等离子体转化特性研究[J]. 燃料化学学报, 2001, 29(3): 243-246. |
| Li M W, Xu G H, Liu C J, et al. Study on corona discharge for carbon dioxide conversion using cold plasma reaction[J]. Journal of Fuel Chemistry, 2001, 29(3): 243-246. | |
| 35 | Aerts R, Somers W, Bogaerts A. Carbon dioxide splitting in a dielectric barrier discharge plasma: a combined experimental and computational study[J]. ChemSusChem, 2015, 8(4): 702-716. |
| 36 | Ozkan A, Bogaerts A, Reniers F. Routes to increase the conversion and the energy efficiency in the splitting of CO2 by a dielectric barrier discharge[J]. Journal of Physics D: Applied Physics, 2017, 50(8): 084004. |
| 37 | Duan X F, Li Y P, Ge W J, et al. Degradation of CO2 through dielectric barrier discharge microplasma[J]. Greenhouse Gases: Science and Technology, 2015, 5(2): 131-140. |
| 38 | Duan X F, Hu Z Y, Li Y P, et al. Effect of dielectric packing materials on the decomposition of carbon dioxide using DBD microplasma reactor[J]. AIChE Journal, 2015, 61(3): 898-903. |
| 39 | Belov I, Paulussen S, Bogaerts A. Appearance of a conductive carbonaceous coating in a CO2 dielectric barrier discharge and its influence on the electrical properties and the conversion efficiency[J]. Plasma Sources Science & Technology, 2016, 25(1): 015023. |
| 40 | Wang J Y, Xia G G, Huang A M, et al. CO2 decomposition using glow discharge plasmas[J]. Journal of Catalysis, 1999, 185(1): 152-159. |
| 41 | Snoeckx R, Heijkers S, van Wesenbeeck K, et al. CO2 conversion in a dielectric barrier discharge plasma: N2 in the mix as a helping hand or problematic impurity?[J]. Energy and Environmental Science, 2016, 9(3): 999-1011. |
| 42 | Li S R, Ongis M, Manzolini G, et al. Non-thermal plasma-assisted capture and conversion of CO2 [J]. Chemical Engineering Journal, 2021, 410: 128335. |
| 43 | Itoh N, Sanchez M A, Xu W C, et al. Application of a membrane reactor system to thermal decomposition of CO2 [J]. Journal of Membrane Science, 1993, 77(2/3): 245-253. |
| 44 | 宋爽, 裘建平, 何志桥, 等. CO2一步电化学转化技术的可行性研究[J]. 航天医学与医学工程, 2006, 19(3): 199-203. |
| Song S, Qiu J P, He Z Q, et al. Study on feasibility of one-step electrochemical conversion of CO2 [J]. Aerospace Medicine and Medical Engineering, 2006, 19(3): 199-203. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 刘文竹, 云和明, 王宝雪, 胡明哲, 仲崇龙. 基于场协同和 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. |
| [6] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [7] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [8] | 黄可欣, 李彤, 李桉琦, 林梅. 加装旋转叶轮T型通道流场的模态分解[J]. 化工学报, 2023, 74(7): 2848-2857. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [11] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [12] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [13] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [14] | 贾露凡, 王艺颖, 董钰漫, 李沁园, 谢鑫, 苑昊, 孟涛. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| [15] | 朱兵国, 何吉祥, 徐进良, 彭斌. 冷却条件下渐扩/渐缩管内超临界压力二氧化碳的传热特性[J]. 化工学报, 2023, 74(3): 1062-1072. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号