化工学报 ›› 2023, Vol. 74 ›› Issue (5): 2013-2021.DOI: 10.11949/0438-1157.20221542
蔺彩虹1( ), 王丽1, 吴瑜2, 刘鹏2, 杨江峰1(
), 王丽1, 吴瑜2, 刘鹏2, 杨江峰1( ), 李晋平1
), 李晋平1
收稿日期:2022-11-30
修回日期:2023-04-27
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
杨江峰
作者简介:蔺彩虹(1997—),女,硕士研究生,626803611@qq.com
Caihong LIN1( ), Li WANG1, Yu WU2, Peng LIU2, Jiangfeng YANG1(
), Li WANG1, Yu WU2, Peng LIU2, Jiangfeng YANG1( ), Jinping LI1
), Jinping LI1
Received:2022-11-30
Revised:2023-04-27
Online:2023-05-05
Published:2023-06-29
Contact:
Jiangfeng YANG
摘要:
氧化亚氮(N2O)是仅次于CO2和CH4的第三大温室气体,排放后加剧温室效应,而N2O具有广泛的用途,因此对其进行回收具有重要意义。苯酚法和环己烷法生产己二酸时排放的尾气中CO2与N2O共存,二者具有相同的动力学直径和相似的物理性质,它们的分离具有很大的挑战。研究了A型沸石中一价碱金属阳离子(Li+、Na+、K+、Cs+)对CO2/N2O吸附分离性能的影响,通过XRD、ICP-OES、SEM和TGA进行表征,并对吸附性能、IAST选择性以及分离性能进行了研究。研究结果表明,随着碱金属阳离子半径的增大,CO2和N2O的吸附容量逐渐降低,CsA几乎对CO2和N2O均不吸附;但CO2/N2O选择性却呈相反的趋势,其中KA的选择性达到最高(2.6),混合气体(
中图分类号:
蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021.
Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O[J]. CIESC Journal, 2023, 74(5): 2013-2021.
| 样品 | 质量浓度/(mg/L) | ELi/% | ENa/% | EK/% | ECs/% | 分子式 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSi | CAl | CLi | CNa | CK | CCs | |||||||
| LiA | 67.41 | 72.43 | 9.88 | 25.59 | — | — | 1.80 | 56.15 | — | — | — | Na44Li57Al101Si91O384 |
| NaA | 58.57 | 48.46 | — | 58.57 | — | — | 1.82 | — | 100.00 | — | — | Na101Al101Si91O384 |
| KA | 46.61 | 52.98 | — | 29.26 | 23.06 | — | 1.78 | — | — | 31.70 | — | Na69K32Al101Si91O384 |
| CsA | 52.78 | 55.70 | — | 27.16 | — | 112.2 | 1.82 | — | — | — | 42.50 | Na59Cs42Al101Si91O384 |
表1 A型沸石的元素含量和阳离子交换度
Table 1 Element content and cation exchange degree on A-type zeolite
| 样品 | 质量浓度/(mg/L) | ELi/% | ENa/% | EK/% | ECs/% | 分子式 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CSi | CAl | CLi | CNa | CK | CCs | |||||||
| LiA | 67.41 | 72.43 | 9.88 | 25.59 | — | — | 1.80 | 56.15 | — | — | — | Na44Li57Al101Si91O384 |
| NaA | 58.57 | 48.46 | — | 58.57 | — | — | 1.82 | — | 100.00 | — | — | Na101Al101Si91O384 |
| KA | 46.61 | 52.98 | — | 29.26 | 23.06 | — | 1.78 | — | — | 31.70 | — | Na69K32Al101Si91O384 |
| CsA | 52.78 | 55.70 | — | 27.16 | — | 112.2 | 1.82 | — | — | — | 42.50 | Na59Cs42Al101Si91O384 |
| 样品 | ELi/% | ENa/% | EK/% | ECs/% | |
|---|---|---|---|---|---|
| LiA | 1.90 | — | — | — | — |
| NaA | 1.86 | — | 100.0 | — | — |
| KA | 1.80 | — | — | 30.5 | — |
| CsA | 1.82 | — | — | — | 54.3 |
表2 不同阳离子的A型沸石的EDS元素分布
Table 2 EDS elemental distribution of A-type zeolites with different cations
| 样品 | ELi/% | ENa/% | EK/% | ECs/% | |
|---|---|---|---|---|---|
| LiA | 1.90 | — | — | — | — |
| NaA | 1.86 | — | 100.0 | — | — |
| KA | 1.80 | — | — | 30.5 | — |
| CsA | 1.82 | — | — | — | 54.3 |
| 吸附剂 | 吸附质 | q1/(cm3/g) | q2/(cm3/g) | b1 | b2 | R2 |
|---|---|---|---|---|---|---|
| LiA | CO2 | 60.20 | 63.35 | 46.17 | 3.79 | 0.999 |
| N2O | 66.25 | 40.68 | 34.32 | 1.37 | 0.999 | |
| NaA | CO2 | 47.31 | 26.54 | 54.49 | 0.70 | 0.999 |
| N2O | 58.03 | 1.92 | 44.29 | 44.40 | 0.999 | |
| KA | CO2 | 29.55 | 36.53 | 63.67 | 9.06 | 0.999 |
| N2O | 86.72 | 32.71 | 2.94 | 32.71 | 0.999 |
表3 A型沸石在25℃下对CO2和N2O的吸附等温线的Dual-Site Langmuir拟合参数
Table 3 Dual-Site Langmuir parameters for CO2 and N2O at 25℃ on A-type zeolite
| 吸附剂 | 吸附质 | q1/(cm3/g) | q2/(cm3/g) | b1 | b2 | R2 |
|---|---|---|---|---|---|---|
| LiA | CO2 | 60.20 | 63.35 | 46.17 | 3.79 | 0.999 |
| N2O | 66.25 | 40.68 | 34.32 | 1.37 | 0.999 | |
| NaA | CO2 | 47.31 | 26.54 | 54.49 | 0.70 | 0.999 |
| N2O | 58.03 | 1.92 | 44.29 | 44.40 | 0.999 | |
| KA | CO2 | 29.55 | 36.53 | 63.67 | 9.06 | 0.999 |
| N2O | 86.72 | 32.71 | 2.94 | 32.71 | 0.999 |
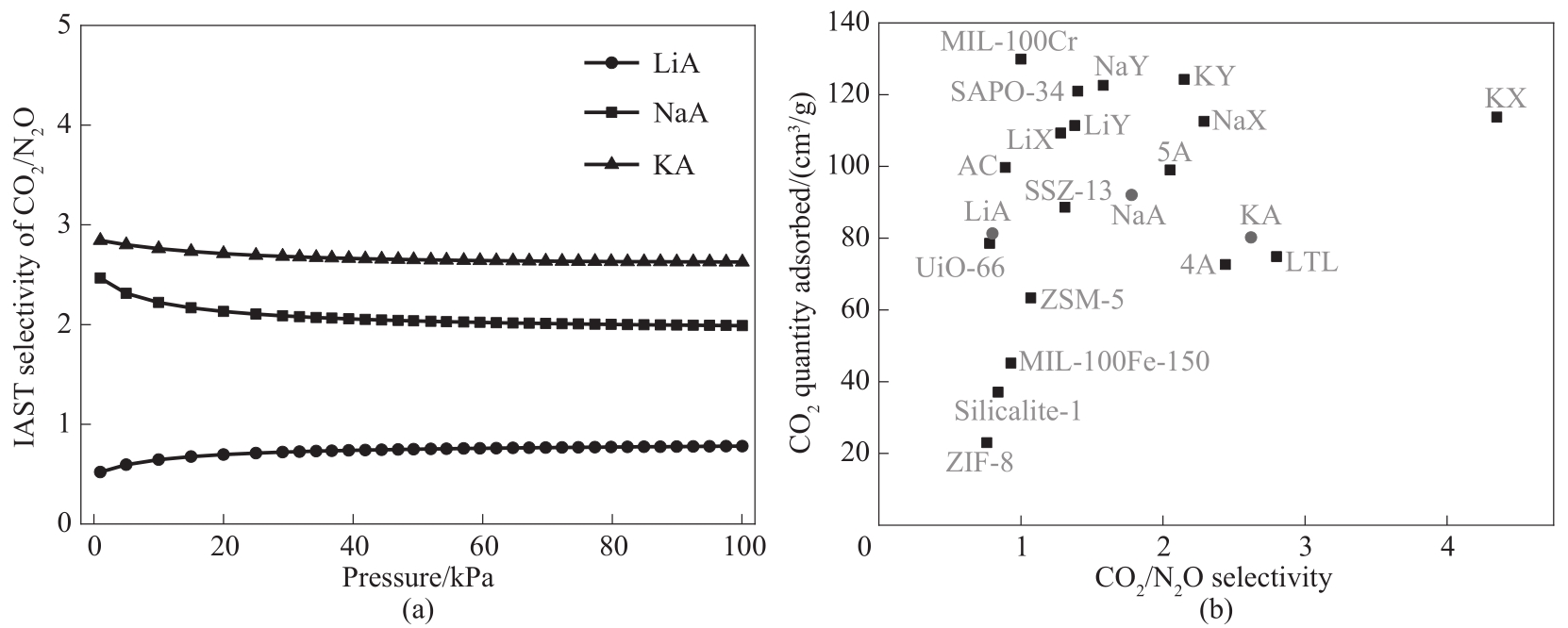
图6 A型沸石对CO2/N2O的IAST选择性(a);部分材料[21,43]对CO2/N2O选择性的比较(b)
Fig.6 IAST selectivity of CO2/N2O on A-type zeolite (a);Comparison of CO2/N2O selectivity on some materials[21,43] (b)
| 样品 | CO2吸附量/(cm3/g) | N2O吸附量/(cm3/g) | CO2/N2O选择性 |
|---|---|---|---|
| LiA | 81.31 | 81.04 | 0.8 |
| NaA | 92.02 | 87.82 | 2.0 |
| KA | 80.21 | 67.55 | 2.6 |
| CsA | 0.48 | 1.22 | — |
表4 A型沸石在25℃时的吸附量和IAST选择性
Table 4 Adsorption capacity and IAST selectivity of A-type zeolite at 25℃
| 样品 | CO2吸附量/(cm3/g) | N2O吸附量/(cm3/g) | CO2/N2O选择性 |
|---|---|---|---|
| LiA | 81.31 | 81.04 | 0.8 |
| NaA | 92.02 | 87.82 | 2.0 |
| KA | 80.21 | 67.55 | 2.6 |
| CsA | 0.48 | 1.22 | — |
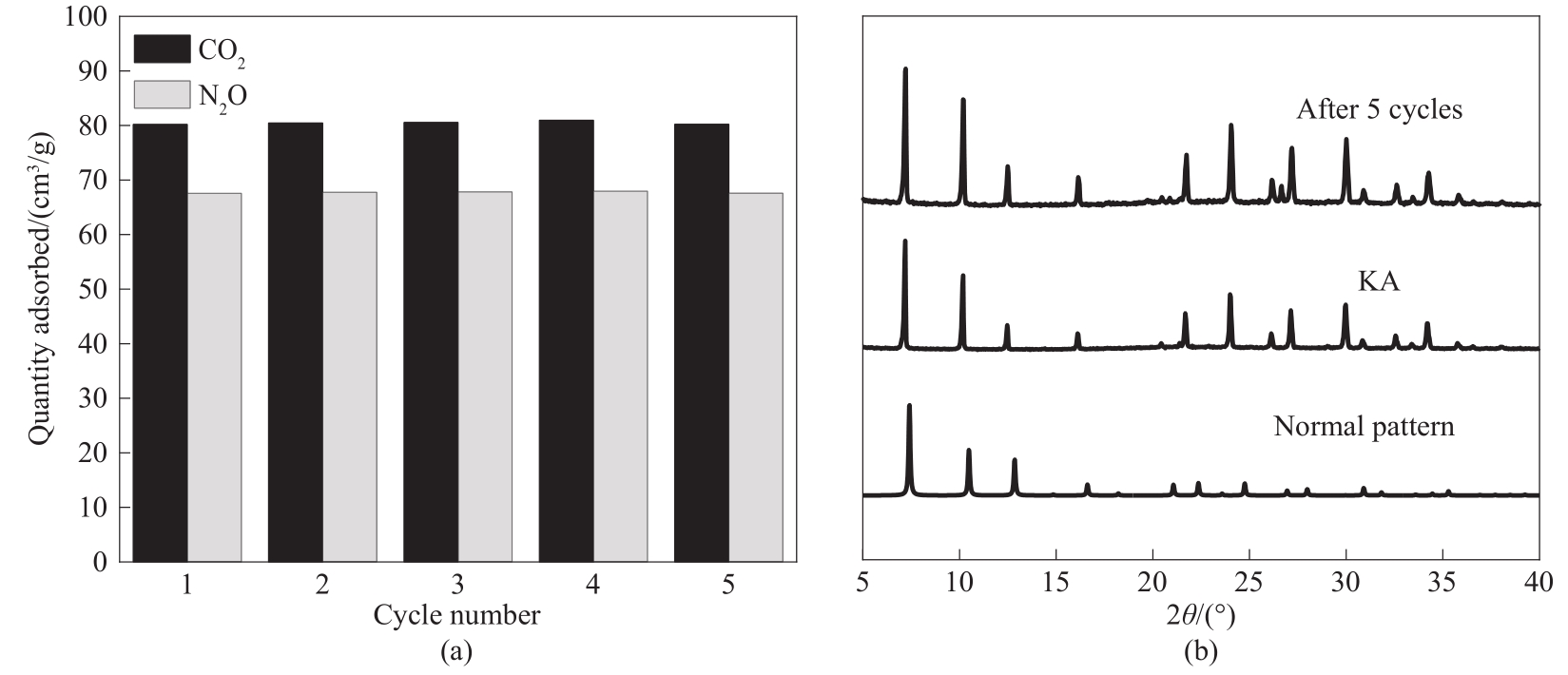
图9 25℃、100 kPa下KA对CO2和N2O吸附循环(a)以及吸附循环前后样品的XRD谱图(b)
Fig.9 CO2 and N2O adsorption recycles of KA at 25℃ and 100 kPa (a) and XRD patterns of samples before and after adsorption recycles (b)
| 1 | Wuebbles D J. Nitrous oxide: no laughing matter[J]. Science, 2009, 326(5949): 56-57. |
| 2 | Highton M P, Bakken L R, Dörsch P, et al. Soil N2O emission potential falls along a denitrification phenotype gradient linked to differences in microbiome, rainfall and carbon availability[J]. Soil Biology and Biochemistry, 2020, 150: 108004. |
| 3 | Ravishankara A R, Daniel J S, Portmann R W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century[J]. Science, 2009, 326(5949): 123-125. |
| 4 | Hu Z, Lee J W, Chandran K, et al. Nitrous oxide (N2O) emission from aquaculture: a review[J]. Environmental Science & Technology, 2012, 46(12): 6470-6480. |
| 5 | Lashof D A, Ahuja D R. Relative contributions of greenhouse gas emissions to global warming[J]. Nature, 1990, 344(6266): 529-531. |
| 6 | Bongaarts J. Climate change: the IPCC scientific assessment[J]. Population and Development Review, 1992, 18: 191. |
| 7 | Schilt A, Brook E J, Bauska T K, et al. Isotopic constraints on marine and terrestrial N2O emissions during the last deglaciation[J]. Nature, 2014, 516(7530): 234-237. |
| 8 | Montzka S A, Dlugokencky E J, Butler J H. Non-CO2 greenhouse gases and climate change[J]. Nature, 2011, 476(7358): 43-50. |
| 9 | Kay S. Synthetic chemistry with nitrous oxide[J]. Chemical Society Reviews, 2015, 44(17): 6375-6386. |
| 10 | Tskhovrebov A G, Vuichoud B, Solari E, et al. Adducts of nitrous oxide and N-heterocyclic carbenes: syntheses, structures, and reactivity[J]. Journal of the American Chemical Society, 2013, 135(25): 9486-9492. |
| 11 | Liu N, Chen B H, Li Y P, et al. Charge transfer analysis on the direct decomposition of nitrous oxide over Fe-BEA zeolite: an experimental and density functional study[J]. Journal of Physical Chemistry C, 2011, 115(26): 12883-12890. |
| 12 | Syakila A, Kroeze C. The global nitrous oxide budget revisited[J]. Greenhouse Gas Measurement and Management, 2011, 1(1): 17-26. |
| 13 | Shimizu A, Tanaka K, Fujimori M. Abatement technologies for N2O emissions in the adipic acid industry[J]. Chemosphere-Global Change Science, 2000, 2(3/4): 425-434. |
| 14 | Reed S, Hutchison J. Green chemistry in the organic teaching laboratory: an environmentally benign synthesis of adipic acid[J]. Journal of Chemical Education, 2000, 77: 1627. |
| 15 | van Duuren J B J H, Brehmer B, Mars A E, et al. A limited LCA of bio-adipic acid: manufacturing the nylon-6, 6 precursor adipic acid using the benzoic acid degradation pathway from different feedstocks[J]. Biotechnology and Bioengineering, 2011, 108(6): 1298-1306. |
| 16 | Xiao Q, Yang F F, Zhuang J, et al. Facile synthesis of uniform FeZSM-5 crystals with controlled size and their application to N2O decomposition[J]. Microporous and Mesoporous Materials, 2013, 167: 38-43. |
| 17 | Zhang F M, Chen X, Zhuang J, et al. Direct oxidation of benzene to phenol by N2O over meso-Fe-ZSM-5 catalysts obtained via alkaline post-treatment[J]. Catalysis Science & Technology, 2011, 1(7): 1250-1255. |
| 18 | Ouyang C, Li Y X, Li J W. The ZSM-5-catalyzed oxidation of benzene to phenol with N2O: effect of Lewis acid sites[J]. Catalysts, 2019, 9(1): 44. |
| 19 | Chen D L, Wang N W, Wang F F, et al. Utilizing the gate-opening mechanism in ZIF-7 for adsorption discrimination between N2O and CO2 [J]. Journal of Physical Chemistry C, 2014, 118(31): 17831-17837. |
| 20 | Yang J F, Bai H H, Shang H, et al. Experimental and simulation study on efficient CH4/N2 separation by pressure swing adsorption on silicalite-1 pellets[J]. Chemical Engineering Journal, 2020, 388: 124222. |
| 21 | Wang L, Liu J Q, Lin C H, et al. Effects of different alkali metal cations in FAU zeolites on the separation performance of CO2/N2O[J]. Chemical Engineering Journal, 2022, 431: 134257. |
| 22 | Eguchi R, Uchida S, Mizuno N. Inverse and high CO2/C2H2 sorption selectivity in flexible organic-inorganic ionic crystals[J]. Angewandte Chemie, 2012, 51(7): 1635-1639. |
| 23 | Wu T B, Niu Z Y, Feng L, et al. Performance analysis of VPSA process for separating N2O from adipic acid tail gas[J]. Separation and Purification Technology, 2021, 256: 117750. |
| 24 | Sircar S. Pressure swing adsorption[J]. Industrial & Engineering Chemistry Research, 2002, 41(6): 1389-1392. |
| 25 | Abd A A, Othman M R, Naji S Z, et al. Methane enrichment in biogas mixture using pressure swing adsorption: process fundamental and design parameters[J]. Materials Today Sustainability, 2021, 11/12: 100063. |
| 26 | 徐如人. 分子筛与多孔材料化学[M]. 北京: 科学出版社, 2004. |
| Xu R R. Molecular Sieves and Porous Materials Chemistry[M]. Beijing: Science Press, 2004. | |
| 27 | 范延臻, 王宝贞. 活性炭表面化学[J]. 煤炭转化, 2000, 23(4): 26-30. |
| Fan Y Z, Wang B Z. Surface chemistry of activated carbon[J]. Coal Conversion, 2000, 23(4): 26-30. | |
| 28 | Zhou H C J, Kitagawa S. Metal-organic frameworks (MOFs)[J]. Chemical Society Reviews, 2014, 43(16): 5415-5418. |
| 29 | Wang L, Zhang F F, Wang C, et al. Ethylenediamine-functionalized metal organic frameworks MIL-100(Cr) for efficient CO2/N2O separation[J]. Separation and Purification Technology, 2020, 235: 116219. |
| 30 | McKinstry C, Cathcart R J, Cussen E J, et al. Scalable continuous solvothermal synthesis of metal organic framework (MOF-5) crystals[J]. Chemical Engineering Journal, 2016, 285: 718-725. |
| 31 | 冯爱虎, 于洋, 于云, 等. 沸石分子筛及其负载型催化剂去除VOCs研究进展[J]. 化学学报, 2018, 76(10): 757-773. |
| Feng A H, Yu Y, Yu Y, et al. Recent progress in the removal of volatile organic compounds by zeolite and its supported catalysts[J]. Acta Chimica Sinica, 2018, 76(10): 757-773. | |
| 32 | 孙静, 董一霖, 李法齐, 等. Co3O4改性USY分子筛吸附和催化氧化甲苯特性研究[J]. 化工学报, 2021, 72(6): 3306-3315. |
| Sun J, Dong Y L, Li F Q, et al. Study on adsorption and catalytic oxidation characteristics of toluene on Co3O4 modified USY molecular sieve[J]. CIESC Journal, 2021, 72(6): 3306-3315. | |
| 33 | Primo A, Garcia H. Zeolites as catalysts in oil refining[J]. Chemical Society Reviews, 2014, 43(22): 7548-7561. |
| 34 | Dipendu S, Deng S G. Adsorption equilibrium, kinetics, and enthalpy of N2O on zeolite 4A and 13X[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3312-3317. |
| 35 | Wu T B, Shen Y H, Feng L, et al. Adsorption properties of N2O on zeolite 5A, 13X, activated carbon, ZSM-5, and silica gel[J]. Journal of Chemical & Engineering Data, 2019, 64(8): 3473-3482. |
| 36 | Groen J C, Pérez-Ramírez J, Zhu W D. Adsorption of nitrous oxide on silicalite-1[J]. Journal of Chemical & Engineering Data, 2002, 47(3): 587-589. |
| 37 | Inglezakis V J, Loizidou M M, Grigoropoulou H P. Ion exchange studies on natural and modified zeolites and the concept of exchange site accessibility[J]. Journal of Colloid and Interface Science, 2004, 275(2): 570-576. |
| 38 | Siperstein F R, Myers A L. Mixed-gas adsorption[J]. AIChE Journal, 2001, 47(5): 1141-1159. |
| 39 | Sethia G, Somani R S, Bajaj H. Sorption of methane and nitrogen on cesium exchanged zeolite-X: structure, cation position and adsorption relationship[J]. Industrial & Engineering Chemistry Research, 2014, 53(16): 6807-6814. |
| 40 | Hu S F, Song G Q, Xue D, et al. Influence of alkalinity on the synthesis of hierarchical LTA zeolite by using bridged polysilsesquioxane[J]. RSC Advances, 2019, 9(5): 2551-2558. |
| 41 | Lin G, Zhuang Q, Cui Q, et al. Synthesis and adsorption property of zeolite FAU/LTA from lithium slag with utilization of mother liquid[J]. Chinese Journal of Chemical Engineering, 2015, 23(11): 1768-1773. |
| 42 | Huang H L, Zhang W J, Liu D H, et al. Effect of temperature on gas adsorption and separation in ZIF-8: a combined experimental and molecular simulation study[J]. Chemical Engineering Science, 2011, 66(23): 6297-6305. |
| 43 | Wang L, Zhang F F, Yang J F, et al. The efficient separation of N2O/CO2 using unsaturated Fe2+ sites in MIL-100Fe[J]. Chemical Communications, 2021, 57(54): 6636-6639. |
| [1] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [6] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [7] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [8] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [9] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [10] | 陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494. |
| [11] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [12] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [13] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [14] | 朱兵国, 何吉祥, 徐进良, 彭斌. 冷却条件下渐扩/渐缩管内超临界压力二氧化碳的传热特性[J]. 化工学报, 2023, 74(3): 1062-1072. |
| [15] | 何仁初, 张朝晖, 杨明磊, 王聪, 奚桢浩. 考虑碳排放因素的汽油调合在线优化[J]. 化工学报, 2023, 74(2): 818-829. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号