化工学报 ›› 2022, Vol. 73 ›› Issue (5): 2111-2119.DOI: 10.11949/0438-1157.20211848
陈冠益1,2( ),童图军1,3,李瑞1,王燕杉1,颜蓓蓓1,李宁1(
),童图军1,3,李瑞1,王燕杉1,颜蓓蓓1,李宁1( ),侯立安1
),侯立安1
收稿日期:2021-12-30
修回日期:2022-02-19
出版日期:2022-05-05
发布日期:2022-05-24
通讯作者:
李宁
作者简介:陈冠益(1970—),男,教授,基金资助:
Guanyi CHEN1,2( ),Tujun TONG1,3,Rui LI1,Yanshan WANG1,Beibei YAN1,Ning LI1(
),Tujun TONG1,3,Rui LI1,Yanshan WANG1,Beibei YAN1,Ning LI1( ),Li'an HOU1
),Li'an HOU1
Received:2021-12-30
Revised:2022-02-19
Online:2022-05-05
Published:2022-05-24
Contact:
Ning LI
摘要:
污泥热解制备生物炭是一种污泥有效处理处置与资源化利用方法。通过控制热解时间,调控污泥生物炭表面的活性位点,改变过一硫酸盐(PMS)体系中的活性物种组成,可实现环丙沙星(CIP)的高效降解。研究发现,热解温度为700℃、热解时间为120 min时,污泥生物炭具有较高的PMS活化性能,对CIP的去除率近90%。机理探究表明,1O2在体系中发挥主要作用。C
中图分类号:
陈冠益, 童图军, 李瑞, 王燕杉, 颜蓓蓓, 李宁, 侯立安. 热解时间对污泥生物炭活化过硫酸盐的影响研究[J]. 化工学报, 2022, 73(5): 2111-2119.
Guanyi CHEN, Tujun TONG, Rui LI, Yanshan WANG, Beibei YAN, Ning LI, Li'an HOU. Influence of pyrolysis time on sludge-derived biochar performance for peroxymonosulfate activation[J]. CIESC Journal, 2022, 73(5): 2111-2119.
| 时间/min | 流速/(ml/min) | A/%(体积) | B/%(体积) |
|---|---|---|---|
| 0 | 0.3 | 93 | 7 |
| 1 | 0.3 | 93 | 7 |
| 20 | 0.3 | 50 | 50 |
| 25 | 0.3 | 93 | 7 |
| 30 | 0.3 | 93 | 7 |
表1 UHPLC-MS/MS流动相配比
Table 1 UHPLC-MS/MS parameters
| 时间/min | 流速/(ml/min) | A/%(体积) | B/%(体积) |
|---|---|---|---|
| 0 | 0.3 | 93 | 7 |
| 1 | 0.3 | 93 | 7 |
| 20 | 0.3 | 50 | 50 |
| 25 | 0.3 | 93 | 7 |
| 30 | 0.3 | 93 | 7 |
| 位点 | 含量/% | |||
|---|---|---|---|---|
| 30 min | 120 min | 180 min | 300 min | |
| C—C/C | 29.5 | 29.2 | 29.9 | 29.0 |
| C—O | 13.0 | 12.8 | 12.1 | 11.3 |
| C—N | 4.6 | 7.6 | 7.8 | 6.5 |
| C | 4.6 | 3.5 | 5.1 | 4.1 |
| 吡啶氮 | 1.1 | 1.1 | 0.9 | 0.8 |
| 吡咯氮 | 1.3 | 0.3 | 0.8 | 1.1 |
| 石墨氮 | 1.3 | 2.9 | 2.1 | 2.1 |
| 氧化氮 | 1.3 | 0.7 | 0.5 | 0.5 |
| 晶格氧 | 11.1 | 10.3 | 9.9 | 9.7 |
| —OH | 14.0 | 14.3 | 14.4 | 14.5 |
| C | 14.8 | 13.8 | 13.0 | 17.1 |
| Fe(0) | 0.1 | 0.1 | 0.1 | 0.1 |
| Fe(Ⅱ) | 1.3 | 1.5 | 1.6 | 1.6 |
| Fe(Ⅲ) | 2.0 | 1.9 | 1.8 | 1.6 |
表2 不同热解时间下污泥生物炭表面活性位点相对含量
Table 2 Relative amount of functional active sites in SSB prepared at different pyrolysis time
| 位点 | 含量/% | |||
|---|---|---|---|---|
| 30 min | 120 min | 180 min | 300 min | |
| C—C/C | 29.5 | 29.2 | 29.9 | 29.0 |
| C—O | 13.0 | 12.8 | 12.1 | 11.3 |
| C—N | 4.6 | 7.6 | 7.8 | 6.5 |
| C | 4.6 | 3.5 | 5.1 | 4.1 |
| 吡啶氮 | 1.1 | 1.1 | 0.9 | 0.8 |
| 吡咯氮 | 1.3 | 0.3 | 0.8 | 1.1 |
| 石墨氮 | 1.3 | 2.9 | 2.1 | 2.1 |
| 氧化氮 | 1.3 | 0.7 | 0.5 | 0.5 |
| 晶格氧 | 11.1 | 10.3 | 9.9 | 9.7 |
| —OH | 14.0 | 14.3 | 14.4 | 14.5 |
| C | 14.8 | 13.8 | 13.0 | 17.1 |
| Fe(0) | 0.1 | 0.1 | 0.1 | 0.1 |
| Fe(Ⅱ) | 1.3 | 1.5 | 1.6 | 1.6 |
| Fe(Ⅲ) | 2.0 | 1.9 | 1.8 | 1.6 |
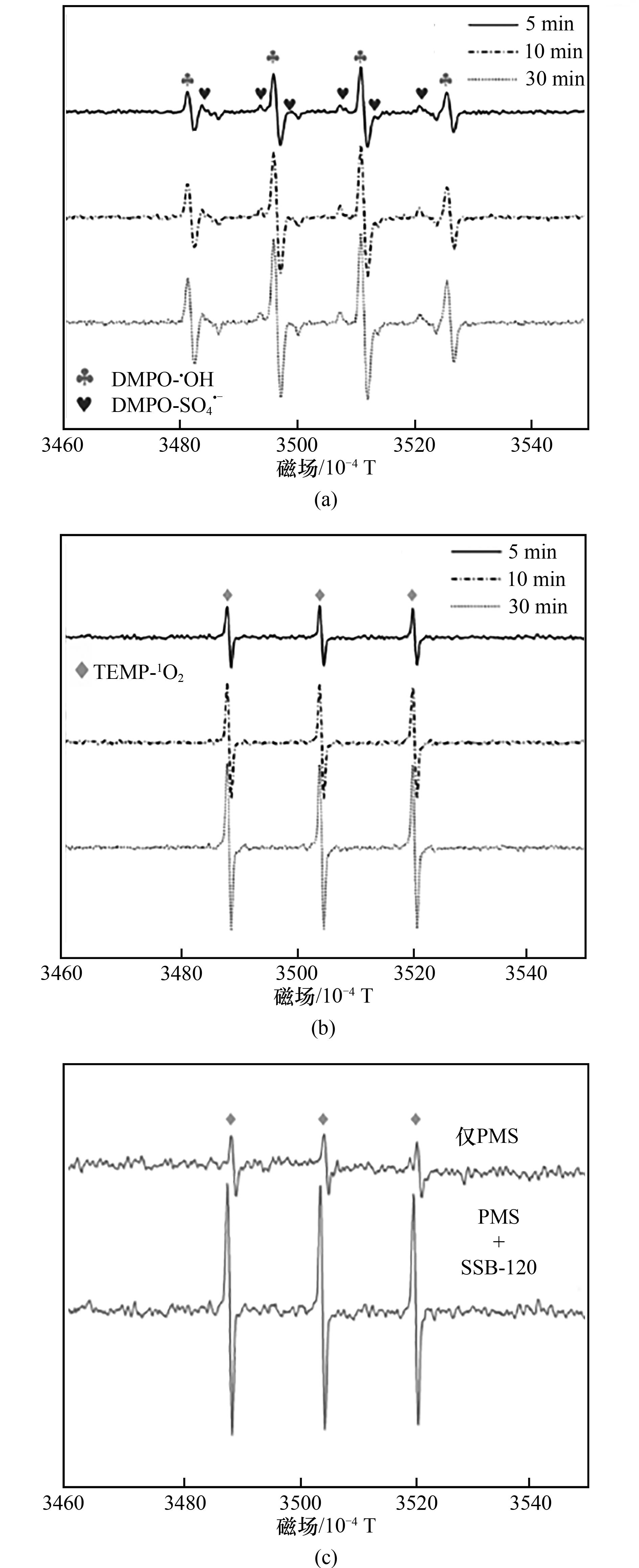
图3 SSB-120/PMS体系中?OH和SO4?-(a),1O2(b)在5、10和30 min的ESR谱图及有无SSB-120条件下1O2在PMS体系中的强度(c)
Fig.3 ESR spectra of ?OH and SO4?- (a), 1O2 (b) in 5, 10 and 30 min in SSB-120/PMS system and 1O2 in 5 min with/without SSB-120 in PMS system (c)
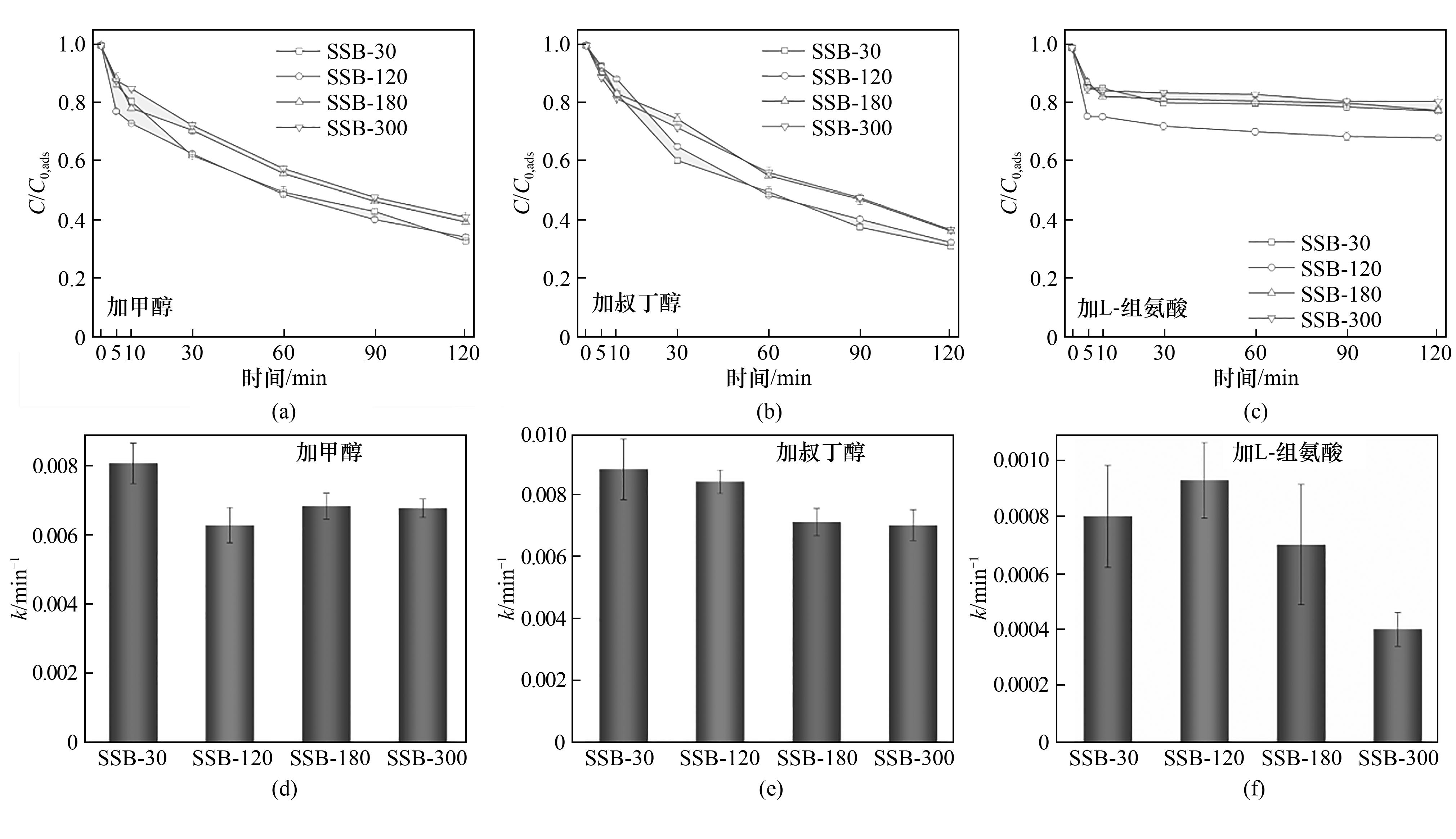
图4 不同热解时间制备的SSB在甲醇[(a),(d)],叔丁醇[(b),(e)]和L-组氨酸[(c),(f)]捕捉剂作用下活化PMS降解CIP的性能和氧化反应速率常数
Fig.4 CIP degradation efficiency and reaction rate constant during oxidation process catalyzed by SSB prepared under different pyrolysis time with methanol [(a),(d)], TBA [(b),(e)] and L-histidine [(c),(f)]
| 1 | Antoniou M G, Cruz A A, Dionysiou D D. Degradation of microcystin-LR using sulfate radicals generated through photolysis, thermolysis and e- transfer mechanisms[J]. Applied Catalysis B: Environmental, 2010, 96(3/4): 290-298. |
| 2 | Ouyang D, Chen Y, Yan J C, et al. Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1, 4-dioxane: important role of biochar defect structures[J]. Chemical Engineering Journal, 2019, 370: 614-624. |
| 3 | Yang Z, Wang Z W, Liang G W, et al. Catalyst bridging-mediated electron transfer for nonradical degradation of bisphenol A via natural manganese ore-cornstalk biochar composite activated peroxymonosulfate[J]. Chemical Engineering Journal, 2021, 426: 131777. |
| 4 | Ghanbari F, Moradi M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| 5 | Gao Y, Wang Q, Ji G Z, et al. Degradation of antibiotic pollutants by persulfate activated with various carbon materials[J]. Chemical Engineering Journal, 2022, 429: 132387. |
| 6 | Xing J, Xu G R, Li G B. Comparison of pyrolysis process, various fractions and potential soil applications between sewage sludge-based biochars and lignocellulose-based biochars[J]. Ecotoxicology and Environmental Safety, 2021, 208: 111756. |
| 7 | Wang X P, Gu L, Zhou P, et al. Pyrolytic temperature dependent conversion of sewage sludge to carbon catalyst and their performance in persulfate degradation of 2-naphthol[J]. Chemical Engineering Journal, 2017, 324: 203-215. |
| 8 | Yu J F, Tang L, Pang Y, et al. Hierarchical porous biochar from shrimp shell for persulfate activation: a two-electron transfer path and key impact factors[J]. Applied Catalysis B: Environmental, 2020, 260: 118160. |
| 9 | 李辉, 吴晓芙, 蒋龙波, 等. 城市污泥脱水干化技术进展[J]. 环境工程, 2014, 32(11): 102-107, 101. |
| Li H, Wu X F, Jiang L B, et al. Progress on the dewatering and drying technology of municipal sludge[J]. Environmental Engineering, 2014, 32(11): 102-107, 101. | |
| 10 | 向晓黎, 党富民, 罗力力, 等. 城市污水污泥成分测定及资源化利用途径可行性研究: 以新疆石河子市为例[J]. 农产品加工, 2015(6): 51-53, 57. |
| Xiang X L, Dang F M, Luo L L, et al. Composition determined and feasibility study on resource utilization way of sewage sludge: taking Shihezi city for example[J]. Farm Products Processing, 2015(6): 51-53, 57. | |
| 11 | Bai X, Zhang Y C, Shi J, et al. A new application pattern for sludge-derived biochar adsorbent: ideal persulfate activator for the high-efficiency mineralization of pollutants[J]. Journal of Hazardous Materials, 2021, 419: 126343. |
| 12 | Liu C, Chen L W, Ding D H, et al. From rice straw to magnetically recoverable nitrogen doped biochar: efficient activation of peroxymonosulfate for the degradation of metolachlor[J]. Applied Catalysis B: Environmental, 2019, 254: 312-320. |
| 13 | Zang T C, Wang H, Liu Y H, et al. Fe-doped biochar derived from waste sludge for degradation of rhodamine B via enhancing activation of peroxymonosulfate[J]. Chemosphere, 2020, 261: 127616. |
| 14 | Graat P C J, Somers M A J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra[J]. Applied Surface Science, 1996, 100/101: 36-40. |
| 15 | Keller P, Strehblow H H. XPS investigations of electrochemically formed passive layers on Fe/Cr-alloys in 0.5 M H2SO4 [J]. Corrosion Science, 2004, 46(8): 1939-1952. |
| 16 | Li X W, Liu X T, Lin C Y, et al. Catalytic oxidation of contaminants by Fe0 activated peroxymonosulfate process: Fe(Ⅳ) involvement, degradation intermediates and toxicity evaluation[J]. Chemical Engineering Journal, 2020, 382: 123013. |
| 17 | Fang G D, Liu C, Gao J, et al. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation[J]. Environmental Science & Technology, 2015, 49(9): 5645-5653. |
| 18 | Yu Y, Li N, Lu X K, et al. Co/N co-doped carbonized wood sponge with 3D porous framework for efficient peroxymonosulfate activation: performance and internal mechanism[J]. Journal of Hazardous Materials, 2022, 421: 126735. |
| 19 | Chen X, Zhou J, Yang H W, et al. PMS activation by magnetic cobalt-N-doped carbon composite for ultra-efficient degradation of refractory organic pollutant: mechanisms and identification of intermediates[J]. Chemosphere, 2022, 287: 132074. |
| 20 | Kohantorabi M, Giannakis S, Moussavi G, et al. An innovative, highly stable Ag/ZIF-67@GO nanocomposite with exceptional peroxymonosulfate (PMS) activation efficacy, for the destruction of chemical and microbiological contaminants under visible light[J]. Journal of Hazardous Materials, 2021, 413: 125308. |
| 21 | Wang L H, Xu H D, Jiang N, et al. Trace cupric species triggered decomposition of peroxymonosulfate and degradation of organic pollutants: Cu(Ⅲ) being the primary and selective intermediate oxidant[J]. Environmental Science & Technology, 2020, 54(7): 4686-4694. |
| 22 | Wang Y B, Cao D, Zhao X. Heterogeneous degradation of refractory pollutants by peroxymonosulfate activated by CoO x -doped ordered mesoporous carbon[J]. Chemical Engineering Journal, 2017, 328: 1112-1121. |
| 23 | Rodgers M A J. The relaxation of O2(1Δg) in condensed systems[J]. Journal of Photochemistry, 1984, 25(2/3/4): 127-129. |
| 24 | Yun E T, Lee J H, Kim J, et al. Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer[J]. Environmental Science & Technology, 2018, 52(12): 7032-7042. |
| 25 | Wang S Z, Wang J L. Synergistic effect of PMS activation by Fe0@Fe3O4 anchored on N, S, O co-doped carbon composite for degradation of sulfamethoxazole[J]. Chemical Engineering Journal, 2022, 427: 131960. |
| 26 | Zhou Y B, Zhang Y L, Hu X M. Novel zero-valent Co-Fe encapsulated in nitrogen-doped porous carbon nanocomposites derived from CoFe2O4@ZIF-67 for boosting 4-chlorophenol removal via coupling peroxymonosulfate[J]. Journal of Colloid and Interface Science, 2020, 575: 206-219. |
| 27 | Li J, Wan Y J, Li Y J, et al. Surface Fe(Ⅲ)/Fe(Ⅱ) cycle promoted the degradation of atrazine by peroxymonosulfate activation in the presence of hydroxylamine[J]. Applied Catalysis B: Environmental, 2019, 256: 117782. |
| 28 | Dai H W, Zhou W J, Wang W. Co/N co-doped carbonaceous polyhedron as efficient peroxymonosulfate activator for degradation of organic pollutants: role of cobalt[J]. Chemical Engineering Journal, 2021, 417: 127921. |
| 29 | Chen X, Oh W D, Hu Z T, et al. Enhancing sulfacetamide degradation by peroxymonosulfate activation with N-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes[J]. Applied Catalysis B: Environmental, 2018, 225: 243-257. |
| 30 | Yang S S, Duan X D, Liu J Q, et al. Efficient peroxymonosulfate activation and bisphenol A degradation derived from mineral-carbon materials: key role of double mineral-templates[J]. Applied Catalysis B: Environmental, 2020, 267: 118701. |
| [1] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [4] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [5] | 杨峥豪, 何臻, 常玉龙, 靳紫恒, 江霞. 生物质快速热解下行式流化床反应器研究进展[J]. 化工学报, 2023, 74(6): 2249-2263. |
| [6] | 陈宇豪, 陈晓平, 马吉亮, 梁财. 市政污泥回转窑焚烧气态污染物排放特性研究[J]. 化工学报, 2023, 74(5): 2170-2178. |
| [7] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [8] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [9] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [10] | 闫新龙, 黄志刚, 胡清勋, 张新, 胡晓燕. Cu/Co掺杂多孔炭活化过硫酸盐降解水中硝基酚研究[J]. 化工学报, 2023, 74(3): 1102-1112. |
| [11] | 徐银, 蔡洁, 陈露, 彭宇, 刘夫珍, 张晖. 异相可见光催化耦合过硫酸盐活化技术在水污染控制中的研究进展[J]. 化工学报, 2023, 74(3): 995-1009. |
| [12] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [13] | 张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860. |
| [14] | 姜家豪, 黄笑乐, 任纪云, 朱正荣, 邓磊, 车得福. 生物炭吸附溶液中Pb2+的定性及定量研究[J]. 化工学报, 2023, 74(2): 830-842. |
| [15] | 郝泽光, 张乾, 高增林, 张宏文, 彭泽宇, 杨凯, 梁丽彤, 黄伟. 生物质与催化裂化油浆共热解协同作用研究[J]. 化工学报, 2022, 73(9): 4070-4078. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号