化工学报 ›› 2022, Vol. 73 ›› Issue (11): 4838-4849.DOI: 10.11949/0438-1157.20221032
收稿日期:2022-07-26
修回日期:2022-10-13
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
夏淑倩
作者简介:高亚慧(1995—),女,博士研究生,yahuigao@tju.edu.cn
基金资助:Received:2022-07-26
Revised:2022-10-13
Online:2022-11-05
Published:2022-12-06
Contact:
Shuqian XIA
摘要:
近年来,二氧化碳(CO2)提高原油采收率(CO2-EOR)技术得到了广泛的研究。充分了解CO2-烃类混合物的热容性质是该项目能够成功实施的基础,但是目前对此性质的研究非常有限。基于绝热量热法,搭建了一个绝热量热计,测量了温度为317.15~353.15 K,压力高达29.49 MPa时,CO2-碳氢化合物(正己烷、正辛烷、十一烷、环己烷、乙苯)二元液相混合物的比定容热容(Cv )数据,共获得335个数据点。此外,采用预测模型(结合热力学关系的PR状态方程)和经验模型计算Cv,计算平均绝对相对偏差(AARD)分别为0.81%~3.66%和小于2.56%。经验模型的形式简单,但PR模型有较强的预测能力。实验测得的Cv 数据以及建立的两种计算模型对CO2-EOR项目的实施至关重要。
中图分类号:
高亚慧, 夏淑倩. CO2-烃类液相混合物比定容热容的实验与模型研究[J]. 化工学报, 2022, 73(11): 4838-4849.
Yahui GAO, Shuqian XIA. Experiment and model for isochoric heat capacity of CO2-hydrocarbon liquid phase mixtures[J]. CIESC Journal, 2022, 73(11): 4838-4849.
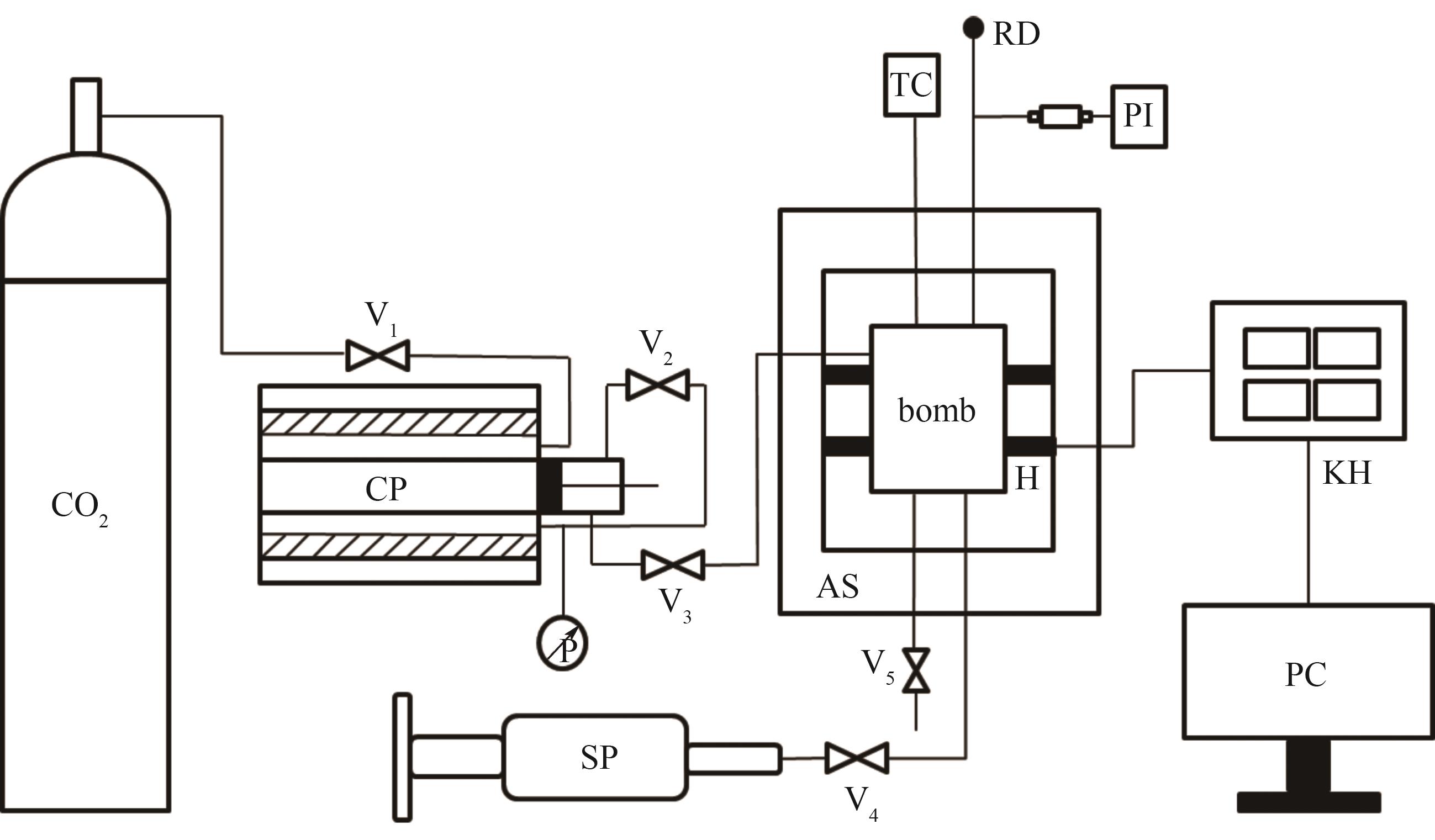
图1 仪器原理图CP—CO2注射泵;SP—液体注射泵;TC—温度控制器;PI—压力指示器;bomb—内釜;H—加热器;AS—外层隔热罩;RD—爆破片;KH—无纸记录仪;PC—计算机
Fig.1 Schematic diagram of the apparatusCP—CO2 syringe pump; SP—liquid syringe pump; TC—temperature controller; PI—pressure indicator; bomb—internal bomb; H—heaters; AS—adiabatic shield; RD—rupture disk; KH—paperless recorder; PC—computer
| 参数 | 回归值 |
|---|---|
| a×10-2 | 7.5665 |
| b×10-2 | -3.0678 |
| c×10-2 | 2.3821 |
表1 式(5)参数
Table 1 Coefficients of Eq. (5)
| 参数 | 回归值 |
|---|---|
| a×10-2 | 7.5665 |
| b×10-2 | -3.0678 |
| c×10-2 | 2.3821 |
| T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) | T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) |
|---|---|---|---|---|---|
| 317.15 | 4.69 | 4.108 | 337.15 | 9.82 | 3.921 |
| 319.15 | 5.15 | 4.079 | 339.15 | 10.46 | 3.912 |
| 321.15 | 5.60 | 4.039 | 341.15 | 11.16 | 3.904 |
| 323.15 | 6.07 | 4.017 | 343.15 | 11.86 | 3.892 |
| 325.15 | 6.54 | 4.010 | 345.15 | 12.58 | 3.885 |
| 327.15 | 7.03 | 3.987 | 347.15 | 13.40 | 3.883 |
| 329.15 | 7.58 | 3.958 | 349.15 | 14.70 | 3.881 |
| 331.15 | 9.09 | 3.939 | 351.15 | 15.00 | 3.877 |
| 333.15 | 8.60 | 3.928 | 353.15 | 15.87 | 3.866 |
| 335.15 | 9.20 | 3.919 |
表2 去离子水的实验比定容热容(Cv )数据
Table 2 Experimental isochoric heat capacity data (Cv ) of deionized water
| T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) | T/K | p/MPa | Cv-exp/(J∙g-1∙K-1) |
|---|---|---|---|---|---|
| 317.15 | 4.69 | 4.108 | 337.15 | 9.82 | 3.921 |
| 319.15 | 5.15 | 4.079 | 339.15 | 10.46 | 3.912 |
| 321.15 | 5.60 | 4.039 | 341.15 | 11.16 | 3.904 |
| 323.15 | 6.07 | 4.017 | 343.15 | 11.86 | 3.892 |
| 325.15 | 6.54 | 4.010 | 345.15 | 12.58 | 3.885 |
| 327.15 | 7.03 | 3.987 | 347.15 | 13.40 | 3.883 |
| 329.15 | 7.58 | 3.958 | 349.15 | 14.70 | 3.881 |
| 331.15 | 9.09 | 3.939 | 351.15 | 15.00 | 3.877 |
| 333.15 | 8.60 | 3.928 | 353.15 | 15.87 | 3.866 |
| 335.15 | 9.20 | 3.919 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.5678 x1=0.1834 | 343.15 | 8.26 | 1.792 | 329.15 | 10.68 | 1.633 | ||
| 317.15 | 3.22 | 1.749 | 345.15 | 9.00 | 1.812 | 331.15 | 11.27 | 1.642 |
| 319.65 | 3.74 | 1.759 | 347.15 | 9.78 | 1.815 | 333.15 | 11.94 | 1.644 |
| 321.15 | 4.07 | 1.765 | 349.15 | 10.70 | 1.819 | 335.15 | 12.74 | 1.649 |
| 325.15 | 5.15 | 1.774 | 351.15 | 11.30 | 1.836 | 337.15 | 13.54 | 1.651 |
| 327.65 | 6.38 | 1.779 | 353.15 | 12.03 | 1.865 | 339.15 | 14.35 | 1.658 |
| 329.65 | 6.87 | 1.787 | ρ/(g∙cm-3)=0.6045 x1=0.3351 | 341.15 | 15.17 | 1.661 | ||
| 331.15 | 7.40 | 1.794 | 317.15 | 9.98 | 1.687 | 343.15 | 16.00 | 1.663 |
| 333.15 | 8.14 | 1.803 | 319.15 | 10.78 | 1.688 | 345.15 | 16.78 | 1.665 |
| 335.15 | 8.87 | 1.816 | 321.15 | 11.63 | 1.689 | 347.15 | 17.72 | 1.669 |
| 337.15 | 9.67 | 1.831 | 323.15 | 12.49 | 1.694 | 349.15 | 18.57 | 1.670 |
| 339.15 | 10.38 | 1.835 | 325.15 | 13.29 | 1.700 | 351.15 | 19.42 | 1.673 |
| 341.15 | 11.21 | 1.842 | 327.15 | 14.07 | 1.705 | 353.15 | 19.97 | 1.681 |
| 343.15 | 11.92 | 1.856 | 329.15 | 14.73 | 1.707 | ρ/(g∙cm-3)=0.6276 x1=0.5020 | ||
| 345.15 | 12.73 | 1.865 | 331.15 | 15.45 | 1.714 | 317.15 | 5.43 | 1.531 |
| 347.15 | 13.52 | 1.871 | 333.15 | 16.25 | 1.721 | 319.65 | 5.92 | 1.540 |
| 349.15 | 14.28 | 1.884 | 335.15 | 17.09 | 1.723 | 321.15 | 6.39 | 1.557 |
| 351.15 | 15.06 | 1.892 | 337.15 | 18.02 | 1.725 | 323.15 | 7.15 | 1.569 |
| 353.15 | 15.85 | 1.908 | 339.15 | 18.89 | 1.734 | 325.15 | 7.94 | 1.588 |
| ρ/(g∙cm-3)=0.5807 x1=0.2417 | 341.15 | 19.76 | 1.736 | 327.15 | 8.68 | 1.601 | ||
| 317.15 | 3.66 | 1.697 | 343.15 | 20.68 | 1.738 | 329.15 | 9.37 | 1.617 |
| 319.15 | 3.71 | 1.709 | 345.65 | 21.87 | 1.748 | 331.15 | 9.96 | 1.626 |
| 321.15 | 3.86 | 1.713 | 347.15 | 22.37 | 1.751 | 333.15 | 10.61 | 1.631 |
| 324.15 | 4.19 | 1.721 | 349.15 | 23.16 | 1.764 | 335.15 | 11.48 | 1.636 |
| 326.15 | 4.38 | 1.727 | 351.15 | 23.99 | 1.772 | 337.15 | 12.27 | 1.640 |
| 327.15 | 4.46 | 1.728 | 353.15 | 24.83 | 1.776 | 339.15 | 12.97 | 1.642 |
| 329.15 | 4.72 | 1.739 | ρ/(g∙cm-3)=0.6174 x1=0.4476 | 341.15 | 13.78 | 1.654 | ||
| 331.15 | 5.01 | 1.749 | 317.15 | 5.93 | 1.557 | 343.15 | 14.52 | 1.655 |
| 333.15 | 5.33 | 1.758 | 319.15 | 6.68 | 1.567 | 345.15 | 15.34 | 1.659 |
| 335.15 | 5.65 | 1.763 | 321.15 | 7.53 | 1.583 | 347.15 | 16.08 | 1.660 |
| 337.15 | 6.11 | 1.769 | 323.15 | 8.32 | 1.596 | 350.15 | 17.31 | 1.665 |
| 339.15 | 6.73 | 1.771 | 325.15 | 9.12 | 1.618 | 351.15 | 17.70 | 1.668 |
| 341.15 | 7.58 | 1.787 | 327.15 | 9.84 | 1.622 | 353.15 | 18.44 | 1.670 |
表3 CO2-正己烷液相混合物的实验Cv 数据
Table 3 Experimental isochoric heat capacity data (Cv ) of CO2-n-hexane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.5678 x1=0.1834 | 343.15 | 8.26 | 1.792 | 329.15 | 10.68 | 1.633 | ||
| 317.15 | 3.22 | 1.749 | 345.15 | 9.00 | 1.812 | 331.15 | 11.27 | 1.642 |
| 319.65 | 3.74 | 1.759 | 347.15 | 9.78 | 1.815 | 333.15 | 11.94 | 1.644 |
| 321.15 | 4.07 | 1.765 | 349.15 | 10.70 | 1.819 | 335.15 | 12.74 | 1.649 |
| 325.15 | 5.15 | 1.774 | 351.15 | 11.30 | 1.836 | 337.15 | 13.54 | 1.651 |
| 327.65 | 6.38 | 1.779 | 353.15 | 12.03 | 1.865 | 339.15 | 14.35 | 1.658 |
| 329.65 | 6.87 | 1.787 | ρ/(g∙cm-3)=0.6045 x1=0.3351 | 341.15 | 15.17 | 1.661 | ||
| 331.15 | 7.40 | 1.794 | 317.15 | 9.98 | 1.687 | 343.15 | 16.00 | 1.663 |
| 333.15 | 8.14 | 1.803 | 319.15 | 10.78 | 1.688 | 345.15 | 16.78 | 1.665 |
| 335.15 | 8.87 | 1.816 | 321.15 | 11.63 | 1.689 | 347.15 | 17.72 | 1.669 |
| 337.15 | 9.67 | 1.831 | 323.15 | 12.49 | 1.694 | 349.15 | 18.57 | 1.670 |
| 339.15 | 10.38 | 1.835 | 325.15 | 13.29 | 1.700 | 351.15 | 19.42 | 1.673 |
| 341.15 | 11.21 | 1.842 | 327.15 | 14.07 | 1.705 | 353.15 | 19.97 | 1.681 |
| 343.15 | 11.92 | 1.856 | 329.15 | 14.73 | 1.707 | ρ/(g∙cm-3)=0.6276 x1=0.5020 | ||
| 345.15 | 12.73 | 1.865 | 331.15 | 15.45 | 1.714 | 317.15 | 5.43 | 1.531 |
| 347.15 | 13.52 | 1.871 | 333.15 | 16.25 | 1.721 | 319.65 | 5.92 | 1.540 |
| 349.15 | 14.28 | 1.884 | 335.15 | 17.09 | 1.723 | 321.15 | 6.39 | 1.557 |
| 351.15 | 15.06 | 1.892 | 337.15 | 18.02 | 1.725 | 323.15 | 7.15 | 1.569 |
| 353.15 | 15.85 | 1.908 | 339.15 | 18.89 | 1.734 | 325.15 | 7.94 | 1.588 |
| ρ/(g∙cm-3)=0.5807 x1=0.2417 | 341.15 | 19.76 | 1.736 | 327.15 | 8.68 | 1.601 | ||
| 317.15 | 3.66 | 1.697 | 343.15 | 20.68 | 1.738 | 329.15 | 9.37 | 1.617 |
| 319.15 | 3.71 | 1.709 | 345.65 | 21.87 | 1.748 | 331.15 | 9.96 | 1.626 |
| 321.15 | 3.86 | 1.713 | 347.15 | 22.37 | 1.751 | 333.15 | 10.61 | 1.631 |
| 324.15 | 4.19 | 1.721 | 349.15 | 23.16 | 1.764 | 335.15 | 11.48 | 1.636 |
| 326.15 | 4.38 | 1.727 | 351.15 | 23.99 | 1.772 | 337.15 | 12.27 | 1.640 |
| 327.15 | 4.46 | 1.728 | 353.15 | 24.83 | 1.776 | 339.15 | 12.97 | 1.642 |
| 329.15 | 4.72 | 1.739 | ρ/(g∙cm-3)=0.6174 x1=0.4476 | 341.15 | 13.78 | 1.654 | ||
| 331.15 | 5.01 | 1.749 | 317.15 | 5.93 | 1.557 | 343.15 | 14.52 | 1.655 |
| 333.15 | 5.33 | 1.758 | 319.15 | 6.68 | 1.567 | 345.15 | 15.34 | 1.659 |
| 335.15 | 5.65 | 1.763 | 321.15 | 7.53 | 1.583 | 347.15 | 16.08 | 1.660 |
| 337.15 | 6.11 | 1.769 | 323.15 | 8.32 | 1.596 | 350.15 | 17.31 | 1.665 |
| 339.15 | 6.73 | 1.771 | 325.15 | 9.12 | 1.618 | 351.15 | 17.70 | 1.668 |
| 341.15 | 7.58 | 1.787 | 327.15 | 9.84 | 1.622 | 353.15 | 18.44 | 1.670 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.6153 x1=0.1434 | 345.15 | 16.99 | 1.928 | 343.15 | 18.86 | 1.801 | ||
| 331.15 | 5.07 | 1.913 | 347.15 | 17.81 | 1.933 | 345.15 | 19.75 | 1.809 |
| 333.15 | 5.87 | 1.920 | 349.15 | 18.65 | 1.945 | 347.15 | 20.55 | 1.813 |
| 335.15 | 6.64 | 1.925 | 351.15 | 19.48 | 1.957 | 349.15 | 21.34 | 1.817 |
| 337.15 | 7.40 | 1.939 | 353.15 | 20.27 | 1.962 | 351.15 | 22.20 | 1.825 |
| 339.15 | 8.18 | 1.946 | ρ/(g∙cm-3)=0.6410 x1=0.3228 | 353.15 | 22.98 | 1.829 | ||
| 341.65 | 9.15 | 1.949 | 340.15 | 7.04 | 1.821 | ρ/(g∙cm-3)=0.6639 x1=0.5337 | ||
| 343.15 | 9.73 | 1.960 | 341.65 | 7.39 | 1.829 | 317.65 | 6.64 | 1.600 |
| 345.15 | 10.42 | 1.964 | 344.15 | 8.36 | 1.838 | 319.15 | 7.17 | 1.614 |
| 347.15 | 11.16 | 1.971 | 345.15 | 8.65 | 1.842 | 321.15 | 7.90 | 1.626 |
| 349.15 | 11.97 | 1.986 | 347.15 | 9.39 | 1.854 | 323.15 | 8.61 | 1.639 |
| 351.15 | 12.72 | 1.993 | 349.15 | 10.15 | 1.868 | 325.15 | 9.39 | 1.656 |
| 353.15 | 13.51 | 2.001 | 351.15 | 10.89 | 1.880 | 327.15 | 10.08 | 1.666 |
| ρ/(g∙cm-3)=0.6240 x1=0.2209 | 353.15 | 11.68 | 1.889 | 329.15 | 10.84 | 1.671 | ||
| 317.15 | 6.09 | 1.814 | ρ/(g∙cm-3)=0.6584 x1=0.4160 | 331.15 | 11.62 | 1.674 | ||
| 319.15 | 6.72 | 1.830 | 317.15 | 8.57 | 1.739 | 333.15 | 12.39 | 1.679 |
| 321.15 | 7.50 | 1.837 | 319.15 | 9.39 | 1.742 | 335.15 | 13.04 | 1.686 |
| 323.15 | 8.25 | 1.846 | 321.15 | 10.22 | 1.741 | 337.15 | 13.66 | 1.688 |
| 325.15 | 9.05 | 1.852 | 323.15 | 10.99 | 1.747 | 339.15 | 14.35 | 1.694 |
| 327.15 | 9.86 | 1.865 | 325.15 | 11.82 | 1.751 | 341.15 | 15.10 | 1.698 |
| 329.15 | 10.61 | 1.871 | 327.15 | 12.66 | 1.754 | 343.15 | 15.68 | 1.700 |
| 331.15 | 11.31 | 1.876 | 329.15 | 13.58 | 1.762 | 345.15 | 16.71 | 1.702 |
| 333.65 | 12.34 | 1.886 | 331.15 | 14.37 | 1.766 | 347.15 | 17.61 | 1.706 |
| 335.15 | 12.95 | 1.889 | 333.15 | 15.03 | 1.773 | 349.15 | 18.39 | 1.712 |
| 337.15 | 13.75 | 1.895 | 335.15 | 15.84 | 1.776 | 351.15 | 19.18 | 1.718 |
| 339.15 | 14.59 | 1.902 | 337.15 | 16.54 | 1.782 | 353.15 | 19.99 | 1.723 |
| 341.15 | 15.42 | 1.915 | 339.15 | 17.25 | 1.786 | |||
| 343.15 | 15.98 | 1.924 | 341.15 | 18.02 | 1.792 | |||
表4 CO2-正辛烷液相混合物的实验Cv 数据
Table 4 Experimental isochoric heat capacity data (Cv ) of CO2-n-octane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.6153 x1=0.1434 | 345.15 | 16.99 | 1.928 | 343.15 | 18.86 | 1.801 | ||
| 331.15 | 5.07 | 1.913 | 347.15 | 17.81 | 1.933 | 345.15 | 19.75 | 1.809 |
| 333.15 | 5.87 | 1.920 | 349.15 | 18.65 | 1.945 | 347.15 | 20.55 | 1.813 |
| 335.15 | 6.64 | 1.925 | 351.15 | 19.48 | 1.957 | 349.15 | 21.34 | 1.817 |
| 337.15 | 7.40 | 1.939 | 353.15 | 20.27 | 1.962 | 351.15 | 22.20 | 1.825 |
| 339.15 | 8.18 | 1.946 | ρ/(g∙cm-3)=0.6410 x1=0.3228 | 353.15 | 22.98 | 1.829 | ||
| 341.65 | 9.15 | 1.949 | 340.15 | 7.04 | 1.821 | ρ/(g∙cm-3)=0.6639 x1=0.5337 | ||
| 343.15 | 9.73 | 1.960 | 341.65 | 7.39 | 1.829 | 317.65 | 6.64 | 1.600 |
| 345.15 | 10.42 | 1.964 | 344.15 | 8.36 | 1.838 | 319.15 | 7.17 | 1.614 |
| 347.15 | 11.16 | 1.971 | 345.15 | 8.65 | 1.842 | 321.15 | 7.90 | 1.626 |
| 349.15 | 11.97 | 1.986 | 347.15 | 9.39 | 1.854 | 323.15 | 8.61 | 1.639 |
| 351.15 | 12.72 | 1.993 | 349.15 | 10.15 | 1.868 | 325.15 | 9.39 | 1.656 |
| 353.15 | 13.51 | 2.001 | 351.15 | 10.89 | 1.880 | 327.15 | 10.08 | 1.666 |
| ρ/(g∙cm-3)=0.6240 x1=0.2209 | 353.15 | 11.68 | 1.889 | 329.15 | 10.84 | 1.671 | ||
| 317.15 | 6.09 | 1.814 | ρ/(g∙cm-3)=0.6584 x1=0.4160 | 331.15 | 11.62 | 1.674 | ||
| 319.15 | 6.72 | 1.830 | 317.15 | 8.57 | 1.739 | 333.15 | 12.39 | 1.679 |
| 321.15 | 7.50 | 1.837 | 319.15 | 9.39 | 1.742 | 335.15 | 13.04 | 1.686 |
| 323.15 | 8.25 | 1.846 | 321.15 | 10.22 | 1.741 | 337.15 | 13.66 | 1.688 |
| 325.15 | 9.05 | 1.852 | 323.15 | 10.99 | 1.747 | 339.15 | 14.35 | 1.694 |
| 327.15 | 9.86 | 1.865 | 325.15 | 11.82 | 1.751 | 341.15 | 15.10 | 1.698 |
| 329.15 | 10.61 | 1.871 | 327.15 | 12.66 | 1.754 | 343.15 | 15.68 | 1.700 |
| 331.15 | 11.31 | 1.876 | 329.15 | 13.58 | 1.762 | 345.15 | 16.71 | 1.702 |
| 333.65 | 12.34 | 1.886 | 331.15 | 14.37 | 1.766 | 347.15 | 17.61 | 1.706 |
| 335.15 | 12.95 | 1.889 | 333.15 | 15.03 | 1.773 | 349.15 | 18.39 | 1.712 |
| 337.15 | 13.75 | 1.895 | 335.15 | 15.84 | 1.776 | 351.15 | 19.18 | 1.718 |
| 339.15 | 14.59 | 1.902 | 337.15 | 16.54 | 1.782 | 353.15 | 19.99 | 1.723 |
| 341.15 | 15.42 | 1.915 | 339.15 | 17.25 | 1.786 | |||
| 343.15 | 15.98 | 1.924 | 341.15 | 18.02 | 1.792 | |||
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7285 x1=0.1609 | ρ/(g∙cm-3)=0.7292 x1=0.2602 | ρ/(g∙cm-3)=0.7363 x1=0.4350 | ||||||
| 317.15 | 13.39 | 1.896 | 317.15 | 9.52 | 1.896 | 323.15 | 7.10 | 1.761 |
| 319.15 | 14.21 | 1.906 | 319.65 | 10.52 | 1.902 | 325.15 | 7.84 | 1.772 |
| 321.15 | 14.99 | 1.913 | 321.15 | 11.06 | 1.903 | 327.15 | 8.64 | 1.783 |
| 323.15 | 15.78 | 1.926 | 323.15 | 11.80 | 1.912 | 329.15 | 9.33 | 1.793 |
| 325.15 | 16.64 | 1.931 | 325.15 | 12.60 | 1.918 | 331.15 | 10.07 | 1.803 |
| 327.15 | 17.42 | 1.931 | 327.15 | 13.39 | 1.921 | 333.15 | 10.81 | 1.811 |
| 329.15 | 18.22 | 1.944 | 329.15 | 14.20 | 1.928 | 335.35 | 11.69 | 1.819 |
| 331.15 | 19.10 | 1.946 | 331.15 | 15.03 | 1.936 | 337.15 | 12.33 | 1.824 |
| 333.15 | 19.97 | 1.956 | 333.15 | 15.86 | 1.941 | 339.15 | 13.00 | 1.830 |
| 335.15 | 20.80 | 1.967 | 335.15 | 16.72 | 1.951 | 341.15 | 13.74 | 1.836 |
| 337.15 | 21.72 | 1.971 | 337.15 | 17.57 | 1.958 | 343.15 | 14.58 | 1.840 |
| 339.15 | 22.61 | 1.978 | 339.15 | 18.37 | 1.963 | 345.15 | 15.27 | 1.846 |
| 341.15 | 23.47 | 1.986 | 341.15 | 19.24 | 1.972 | 347.15 | 16.08 | 1.852 |
| 343.15 | 24.31 | 1.994 | 343.15 | 20.12 | 1.979 | 349.15 | 16.88 | 1.856 |
| 345.15 | 25.22 | 1.997 | 345.15 | 20.96 | 1.991 | 351.15 | 17.60 | 1.861 |
| 347.15 | 26.03 | 2.000 | 347.15 | 21.75 | 1.995 | 353.15 | 18.32 | 1.869 |
| 349.15 | 26.96 | 2.019 | 349.15 | 22.63 | 2.008 | |||
| 351.15 | 23.48 | 2.018 | ||||||
| 353.15 | 24.28 | 2.021 | ||||||
表5 CO2-十一烷液相混合物的实验Cv 数据
Table 5 Experimental isochoric heat capacity data (Cv ) of CO2-undecane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7285 x1=0.1609 | ρ/(g∙cm-3)=0.7292 x1=0.2602 | ρ/(g∙cm-3)=0.7363 x1=0.4350 | ||||||
| 317.15 | 13.39 | 1.896 | 317.15 | 9.52 | 1.896 | 323.15 | 7.10 | 1.761 |
| 319.15 | 14.21 | 1.906 | 319.65 | 10.52 | 1.902 | 325.15 | 7.84 | 1.772 |
| 321.15 | 14.99 | 1.913 | 321.15 | 11.06 | 1.903 | 327.15 | 8.64 | 1.783 |
| 323.15 | 15.78 | 1.926 | 323.15 | 11.80 | 1.912 | 329.15 | 9.33 | 1.793 |
| 325.15 | 16.64 | 1.931 | 325.15 | 12.60 | 1.918 | 331.15 | 10.07 | 1.803 |
| 327.15 | 17.42 | 1.931 | 327.15 | 13.39 | 1.921 | 333.15 | 10.81 | 1.811 |
| 329.15 | 18.22 | 1.944 | 329.15 | 14.20 | 1.928 | 335.35 | 11.69 | 1.819 |
| 331.15 | 19.10 | 1.946 | 331.15 | 15.03 | 1.936 | 337.15 | 12.33 | 1.824 |
| 333.15 | 19.97 | 1.956 | 333.15 | 15.86 | 1.941 | 339.15 | 13.00 | 1.830 |
| 335.15 | 20.80 | 1.967 | 335.15 | 16.72 | 1.951 | 341.15 | 13.74 | 1.836 |
| 337.15 | 21.72 | 1.971 | 337.15 | 17.57 | 1.958 | 343.15 | 14.58 | 1.840 |
| 339.15 | 22.61 | 1.978 | 339.15 | 18.37 | 1.963 | 345.15 | 15.27 | 1.846 |
| 341.15 | 23.47 | 1.986 | 341.15 | 19.24 | 1.972 | 347.15 | 16.08 | 1.852 |
| 343.15 | 24.31 | 1.994 | 343.15 | 20.12 | 1.979 | 349.15 | 16.88 | 1.856 |
| 345.15 | 25.22 | 1.997 | 345.15 | 20.96 | 1.991 | 351.15 | 17.60 | 1.861 |
| 347.15 | 26.03 | 2.000 | 347.15 | 21.75 | 1.995 | 353.15 | 18.32 | 1.869 |
| 349.15 | 26.96 | 2.019 | 349.15 | 22.63 | 2.008 | |||
| 351.15 | 23.48 | 2.018 | ||||||
| 353.15 | 24.28 | 2.021 | ||||||
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7771 x1=0.0966 | ρ/(g∙cm-3)=0.7783 x1=0.1516 | 335.15 | 8.07 | 1.394 | ||||
| 317.15 | 5.46 | 1.414 | 343.15 | 6.39 | 1.516 | 337.15 | 8.74 | 1.398 |
| 319.15 | 5.92 | 1.421 | 345.15 | 6.96 | 1.524 | 339.15 | 9.45 | 1.411 |
| 321.15 | 6.53 | 1.435 | 347.15 | 7.85 | 1.542 | 341.15 | 10.22 | 1.421 |
| 323.15 | 7.41 | 1.441 | 349.15 | 8.68 | 1.561 | 343.15 | 10.98 | 1.432 |
| 325.15 | 8.27 | 1.459 | 351.15 | 9.52 | 1.563 | 345.15 | 11.66 | 1.440 |
| 327.15 | 9.12 | 1.472 | 353.15 | 10.41 | 1.573 | 347.15 | 12.44 | 1.446 |
| 329.15 | 10.00 | 1.482 | ρ/(g∙cm-3)=0.7870 x1=0.2928 | 349.15 | 13.20 | 1.454 | ||
| 331.15 | 10.92 | 1.491 | 337.15 | 6.66 | 1.434 | 351.15 | 13.97 | 1.460 |
| 333.15 | 11.68 | 1.511 | 339.15 | 7.22 | 1.439 | 353.15 | 14.77 | 1.472 |
| 335.15 | 12.62 | 1.521 | 341.15 | 7.99 | 1.446 | ρ/(g∙cm-3)=0.7923 x1=0.4150 | ||
| 337.15 | 13.53 | 1.537 | 343.15 | 8.77 | 1.457 | 337.15 | 6.60 | 1.352 |
| 339.15 | 14.45 | 1.546 | 345.15 | 9.46 | 1.460 | 339.15 | 7.12 | 1.357 |
| 342.15 | 15.88 | 1.556 | 347.15 | 10.23 | 1.472 | 341.15 | 7.75 | 1.369 |
| 343.15 | 16.20 | 1.561 | 349.15 | 11.02 | 1.483 | 343.15 | 8.34 | 1.384 |
| 345.15 | 17.19 | 1.569 | 351.15 | 11.86 | 1.496 | 345.15 | 8.99 | 1.395 |
| 347.15 | 18.08 | 1.578 | 353.15 | 12.60 | 1.505 | 347.15 | 9.65 | 1.399 |
| 349.15 | 19.02 | 1.587 | ρ/(g∙cm-3)=0.7891 x1=0.3646 | 349.15 | 10.30 | 1.416 | ||
| 351.15 | 19.93 | 1.601 | 331.15 | 6.69 | 1.377 | 351.15 | 11.02 | 1.425 |
| 353.15 | 20.88 | 1.613 | 333.15 | 7.30 | 1.385 | 353.15 | 11.98 | 1.432 |
表6 CO2-环己烷液相混合物的实验Cv 数据
Table 6 Experimental isochoric heat capacity data (Cv ) of CO2-cyclohexane liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.7771 x1=0.0966 | ρ/(g∙cm-3)=0.7783 x1=0.1516 | 335.15 | 8.07 | 1.394 | ||||
| 317.15 | 5.46 | 1.414 | 343.15 | 6.39 | 1.516 | 337.15 | 8.74 | 1.398 |
| 319.15 | 5.92 | 1.421 | 345.15 | 6.96 | 1.524 | 339.15 | 9.45 | 1.411 |
| 321.15 | 6.53 | 1.435 | 347.15 | 7.85 | 1.542 | 341.15 | 10.22 | 1.421 |
| 323.15 | 7.41 | 1.441 | 349.15 | 8.68 | 1.561 | 343.15 | 10.98 | 1.432 |
| 325.15 | 8.27 | 1.459 | 351.15 | 9.52 | 1.563 | 345.15 | 11.66 | 1.440 |
| 327.15 | 9.12 | 1.472 | 353.15 | 10.41 | 1.573 | 347.15 | 12.44 | 1.446 |
| 329.15 | 10.00 | 1.482 | ρ/(g∙cm-3)=0.7870 x1=0.2928 | 349.15 | 13.20 | 1.454 | ||
| 331.15 | 10.92 | 1.491 | 337.15 | 6.66 | 1.434 | 351.15 | 13.97 | 1.460 |
| 333.15 | 11.68 | 1.511 | 339.15 | 7.22 | 1.439 | 353.15 | 14.77 | 1.472 |
| 335.15 | 12.62 | 1.521 | 341.15 | 7.99 | 1.446 | ρ/(g∙cm-3)=0.7923 x1=0.4150 | ||
| 337.15 | 13.53 | 1.537 | 343.15 | 8.77 | 1.457 | 337.15 | 6.60 | 1.352 |
| 339.15 | 14.45 | 1.546 | 345.15 | 9.46 | 1.460 | 339.15 | 7.12 | 1.357 |
| 342.15 | 15.88 | 1.556 | 347.15 | 10.23 | 1.472 | 341.15 | 7.75 | 1.369 |
| 343.15 | 16.20 | 1.561 | 349.15 | 11.02 | 1.483 | 343.15 | 8.34 | 1.384 |
| 345.15 | 17.19 | 1.569 | 351.15 | 11.86 | 1.496 | 345.15 | 8.99 | 1.395 |
| 347.15 | 18.08 | 1.578 | 353.15 | 12.60 | 1.505 | 347.15 | 9.65 | 1.399 |
| 349.15 | 19.02 | 1.587 | ρ/(g∙cm-3)=0.7891 x1=0.3646 | 349.15 | 10.30 | 1.416 | ||
| 351.15 | 19.93 | 1.601 | 331.15 | 6.69 | 1.377 | 351.15 | 11.02 | 1.425 |
| 353.15 | 20.88 | 1.613 | 333.15 | 7.30 | 1.385 | 353.15 | 11.98 | 1.432 |
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.8215 x1=0.1088 | ρ/(g∙cm-3)=0.8295 x1=0.2122 | ρ/(g∙cm-3)=0.8326 x1=0.3466 | ||||||
| 317.65 | 12.07 | 1.390 | 317.15 | 9.64 | 1.372 | 317.15 | 7.58 | 1.350 |
| 319.15 | 12.96 | 1.394 | 319.65 | 10.91 | 1.378 | 319.15 | 8.51 | 1.355 |
| 321.15 | 13.85 | 1.401 | 321.15 | 11.66 | 1.380 | 321.15 | 9.43 | 1.358 |
| 323.15 | 14.64 | 1.407 | 323.15 | 12.63 | 1.386 | 323.15 | 10.32 | 1.362 |
| 325.15 | 15.88 | 1.413 | 325.15 | 13.62 | 1.390 | 325.15 | 11.27 | 1.368 |
| 327.15 | 16.96 | 1.419 | 327.15 | 14.67 | 1.396 | 327.15 | 12.13 | 1.370 |
| 329.15 | 17.91 | 1.426 | 329.15 | 15.69 | 1.400 | 329.15 | 13.06 | 1.373 |
| 331.15 | 18.94 | 1.432 | 331.15 | 16.70 | 1.406 | 331.15 | 13.97 | 1.377 |
| 333.15 | 19.93 | 1.438 | 333.15 | 17.68 | 1.411 | 333.15 | 14.89 | 1.381 |
| 335.15 | 20.90 | 1.444 | 335.15 | 18.66 | 1.417 | 335.65 | 15.99 | 1.386 |
| 337.15 | 21.91 | 1.451 | 337.15 | 19.70 | 1.423 | 337.15 | 16.65 | 1.389 |
| 339.15 | 22.87 | 1.457 | 339.15 | 20.66 | 1.429 | 339.15 | 17.60 | 1.394 |
| 341.15 | 23.89 | 1.463 | 341.15 | 21.84 | 1.434 | 341.15 | 18.52 | 1.398 |
| 343.15 | 24.81 | 1.470 | 343.15 | 22.67 | 1.440 | 343.15 | 19.39 | 1.404 |
| 345.15 | 25.82 | 1.476 | 345.15 | 23.81 | 1.445 | 345.15 | 20.32 | 1.408 |
| 347.65 | 27.04 | 1.485 | 347.65 | 24.80 | 1.454 | 347.15 | 21.25 | 1.413 |
| 349.15 | 27.72 | 1.490 | 349.15 | 25.50 | 1.457 | 349.15 | 22.13 | 1.419 |
| 351.15 | 28.64 | 1.496 | 351.15 | 26.59 | 1.464 | 351.15 | 23.00 | 1.425 |
| 353.15 | 29.49 | 1.501 | 353.15 | 27.39 | 1.471 | 353.15 | 23.88 | 1.430 |
表7 CO2-乙苯液相混合物的实验Cv 数据
Table 7 Experimental isochoric heat capacity data (Cv ) of CO2-ethylbenzene liquid phase mixtures
| T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) | T/K | p/MPa | Cv/(J∙g-1∙K-1) |
|---|---|---|---|---|---|---|---|---|
| ρ/(g∙cm-3)=0.8215 x1=0.1088 | ρ/(g∙cm-3)=0.8295 x1=0.2122 | ρ/(g∙cm-3)=0.8326 x1=0.3466 | ||||||
| 317.65 | 12.07 | 1.390 | 317.15 | 9.64 | 1.372 | 317.15 | 7.58 | 1.350 |
| 319.15 | 12.96 | 1.394 | 319.65 | 10.91 | 1.378 | 319.15 | 8.51 | 1.355 |
| 321.15 | 13.85 | 1.401 | 321.15 | 11.66 | 1.380 | 321.15 | 9.43 | 1.358 |
| 323.15 | 14.64 | 1.407 | 323.15 | 12.63 | 1.386 | 323.15 | 10.32 | 1.362 |
| 325.15 | 15.88 | 1.413 | 325.15 | 13.62 | 1.390 | 325.15 | 11.27 | 1.368 |
| 327.15 | 16.96 | 1.419 | 327.15 | 14.67 | 1.396 | 327.15 | 12.13 | 1.370 |
| 329.15 | 17.91 | 1.426 | 329.15 | 15.69 | 1.400 | 329.15 | 13.06 | 1.373 |
| 331.15 | 18.94 | 1.432 | 331.15 | 16.70 | 1.406 | 331.15 | 13.97 | 1.377 |
| 333.15 | 19.93 | 1.438 | 333.15 | 17.68 | 1.411 | 333.15 | 14.89 | 1.381 |
| 335.15 | 20.90 | 1.444 | 335.15 | 18.66 | 1.417 | 335.65 | 15.99 | 1.386 |
| 337.15 | 21.91 | 1.451 | 337.15 | 19.70 | 1.423 | 337.15 | 16.65 | 1.389 |
| 339.15 | 22.87 | 1.457 | 339.15 | 20.66 | 1.429 | 339.15 | 17.60 | 1.394 |
| 341.15 | 23.89 | 1.463 | 341.15 | 21.84 | 1.434 | 341.15 | 18.52 | 1.398 |
| 343.15 | 24.81 | 1.470 | 343.15 | 22.67 | 1.440 | 343.15 | 19.39 | 1.404 |
| 345.15 | 25.82 | 1.476 | 345.15 | 23.81 | 1.445 | 345.15 | 20.32 | 1.408 |
| 347.65 | 27.04 | 1.485 | 347.65 | 24.80 | 1.454 | 347.15 | 21.25 | 1.413 |
| 349.15 | 27.72 | 1.490 | 349.15 | 25.50 | 1.457 | 349.15 | 22.13 | 1.419 |
| 351.15 | 28.64 | 1.496 | 351.15 | 26.59 | 1.464 | 351.15 | 23.00 | 1.425 |
| 353.15 | 29.49 | 1.501 | 353.15 | 27.39 | 1.471 | 353.15 | 23.88 | 1.430 |
| 组分 | a0 | a1×103 | a2×105 | a3×108 | a4×1011 |
|---|---|---|---|---|---|
| CO2 | 3.259 | 1.356 | 1.502 | -2.374 | 1.056 |
| 正己烷 | 8.831 | -0.166 | 14.302 | -18.314 | 7.124 |
| 正辛烷 | 10.824 | 4.983 | 17.751 | -23.137 | 8.980 |
| 十一烷 | 15.285 | 5.485 | 24.990 | -35.821 | 14.200 |
| 环己烷 | 4.035 | -4.443 | 16.834 | -20.775 | 7.746 |
| 乙苯 | 4.544 | 10.578 | 13.644 | -19.276 | 7.885 |
表8 纯化合物的理想气体热容系数[29]
Table 8 Ideal gas heat capacity coefficients of pure compound[29]
| 组分 | a0 | a1×103 | a2×105 | a3×108 | a4×1011 |
|---|---|---|---|---|---|
| CO2 | 3.259 | 1.356 | 1.502 | -2.374 | 1.056 |
| 正己烷 | 8.831 | -0.166 | 14.302 | -18.314 | 7.124 |
| 正辛烷 | 10.824 | 4.983 | 17.751 | -23.137 | 8.980 |
| 十一烷 | 15.285 | 5.485 | 24.990 | -35.821 | 14.200 |
| 环己烷 | 4.035 | -4.443 | 16.834 | -20.775 | 7.746 |
| 乙苯 | 4.544 | 10.578 | 13.644 | -19.276 | 7.885 |
| PR模型 | 经验模型 | ||||
|---|---|---|---|---|---|
| 体系 | AARD/% | 参数 | 数值 | 训练集 | 测试集 |
| CO2-正己烷 | 3.25 | a | 4.4095 | ||
| CO2-正辛烷 | 1.06 | b | 0.3246 | ||
| CO2-十一烷 | 0.81 | c | 0.1544 | ||
| CO2-环己烷 | 2.71 | d | -2.3119 | ||
| CO2-乙苯 | 3.66 | e | 0.0718 | ||
| f | -2.2543×10-4 | ||||
| g | 2.0609 | ||||
| R2 | 0.9438 | ||||
| RMSE/% | 4.58 | 5.47 | |||
| AARD/% | 2.45 | 2.56 | |||
表9 两种模型的计算结果
Table 9 The calculated results of the two models
| PR模型 | 经验模型 | ||||
|---|---|---|---|---|---|
| 体系 | AARD/% | 参数 | 数值 | 训练集 | 测试集 |
| CO2-正己烷 | 3.25 | a | 4.4095 | ||
| CO2-正辛烷 | 1.06 | b | 0.3246 | ||
| CO2-十一烷 | 0.81 | c | 0.1544 | ||
| CO2-环己烷 | 2.71 | d | -2.3119 | ||
| CO2-乙苯 | 3.66 | e | 0.0718 | ||
| f | -2.2543×10-4 | ||||
| g | 2.0609 | ||||
| R2 | 0.9438 | ||||
| RMSE/% | 4.58 | 5.47 | |||
| AARD/% | 2.45 | 2.56 | |||
| 1 | Sedaghat M, Rouhibakhsh K. Investigation of carbon dioxide capture and storage by a novel LSSVM-GA method[J]. Pet. Sci. Technol., 2020, 38(5): 421-427. |
| 2 | Marocco Stuardi F, MacPherson F, Leclaire J. Integrated CO2 capture and utilization: a priority research direction[J]. Curr. Opin. Green Sustainable Chem., 2019, 16: 71-76. |
| 3 | Rudyk S, Hussain S, Spirov P. Supercritical extraction of crude oil by methanol- and ethanol-modified carbon dioxide[J]. J. Supercrit. Fluids, 2013, 78: 63-69. |
| 4 | Jia B, Tsau J S, Barati R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs[J]. Fuel, 2019, 236: 404-427. |
| 5 | Gui X, Wang W, Gao Q, et al. Measurement and correlation of high pressure phase equilibria for CO2+ alkanes and CO2+ crude oil systems[J]. J. Chem. Eng. Data, 2017, 62(11): 3807-3822. |
| 6 | Mosavat N, Abedini A, Torabi F. Phase behaviour of CO2-brine and CO2-oil systems for CO2 storage and enhanced oil recovery: experimental studies[J]. Energy Procedia, 2014, 63: 5631-5645. |
| 7 | Zábranský M, Kolská Z, Růžička V, et al. Heat capacity of liquids: critical review and recommended values. Supplement Ⅱ[J]. J. Phys. Chem. Ref. Data, 2010, 39(1): 013103. |
| 8 | Yamaya K, Matsuguchi A, Kagawa N, et al. Isochoric specific heat capacity of trans-1,3,3,3-tetrafluoropropene (HFO-1234ze(E)) and the HFO-1234ze(E) + CO2 mixture in the liquid phase[J]. J. Chem. Eng. Data, 2011, 56(4): 1535-1539. |
| 9 | Xiao X, Al Ghafri S Z S, Rowland D, et al. Isobaric heat capacity measurements of natural gas model mixtures (methane + n-heptane) and (propane + n-heptane) by differential scanning calorimetry at temperatures from 313 K to 422 K and pressures up to 31 MPa[J]. Fuel, 2021, 296: 120668. |
| 10 | Xiao X, Oakley J, Al Ghafri S Z S, et al. Isobaric heat capacities of a methane (1) + propane (2) mixture by differential scanning calorimetry at near-critical and supercritical conditions[J]. Fuel, 2021, 289: 119840. |
| 11 | Dai Z X, Chen Y F, Liu C, et al. Prediction and verification of heat capacities for pure ionic liquids[J]. Chin. J. Chem. Eng., 2021, 31: 169-176. |
| 12 | Wang L, Song J, Sheng B W, et al. The isochoric specific heat capacity for R1234ze(E) at temperatures from (237 to 349) K and pressures up to 9.2 MPa[J]. J. Chem. Thermodyn., 2020, 141: 105936. |
| 13 | Li H P, Zhang X G, Han B X, et al. Effect of phase behavior and pressure on the constant-volume heat capacity and intermolecular interaction of CO2-ethanol and CO2-n-pentane mixtures in the critical region[J]. Chem. - Eur. J., 2002, 8(2): 451-456. |
| 14 | Zhang X F, Zhang X G, Han B X, et al. Determination of constant volume heat capacity of mixed supercritical fluids and study on the intermolecular interaction[J]. J. Supercrit. Fluids, 2002, 24(3): 193-201. |
| 15 | Polikhronidi N G, Batyrova R G, Abdulagatov I M, et al. Isochoric heat capacity measurements for a CO2 + n-decane mixture in the near-critical and supercritical regions[J]. J. Supercrit. Fluids, 2005, 33(3): 209-222. |
| 16 | Yousefi Seyf J. Evaluation of the PR, tc-PR, CPA, PC-SAFT and IAPWS-95 models in the predicting thermodynamic properties of pure water at the supercooled, compressed liquid, saturated liquid-vapor and superheat regions[J]. J. Mol. Liq., 2019, 288: 111088. |
| 17 | Saeed A, Ghader S. Calculation of density, vapor pressure and heat capacity near the critical point by incorporating cubic SRK EoS and crossover translation[J]. Fluid Phase Equilib., 2019, 493: 10-25. |
| 18 | Liu Y, He X, Zhao X M, et al. Modeling heat capacity of saturated hydrocarbon in liquid phase over a wide range of temperature and pressure[J]. J. Mol. Liq., 2020, 319: 114068. |
| 19 | Zhong Q, Dong X Q, Zhao Y X, et al. A simple generalized equation for compressed liquid isochoric heat capacity of pure and mixture refrigerants[J]. Fluid Phase Equilib., 2019, 490: 33-38. |
| 20 | Kuroki T, Kagawa N, Endo H, et al. Specific heat capacity at constant volume for water, methanol, and their mixtures at temperatures from 300 K to 400 K and pressures to 20 MPa[J]. J. Chem. Eng. Data, 2001, 46(5): 1101-1106. |
| 21 | Zhong Q, Dong X Q, Zhao Y X, et al. Adiabatic calorimeter for isochoric specific heat capacity measurements and experimental data of compressed liquid R1234yf[J]. J. Chem. Thermodyn., 2018, 125: 86-92. |
| 22 | Japan Society of Mechanical Engineers. JSME Data Book: Heat Transfer[M]. 4th ed. Tokyo: JSME, 1994. |
| 23 | Lindstrom P J. NIST Standard Reference Database Number 69[DB/OL]. [2020-06-28]. . |
| 24 | Wagner W, Pruß A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use[J]. J. Phys. Chem. Ref. Data, 2002, 31(2): 387-535. |
| 25 | Shi Q Z, Jing L S, Qiao W H. Solubility of n-alkanes in supercritical CO2 at diverse temperature and pressure[J]. J. CO2 Util., 2015, 9: 29-38. |
| 26 | Mu T C, Zhang X G, Han B X, et al. Effect of phase behavior on the constant volume heat capacity of ethane + ethanol and ethane + acetone mixed fluids near the critical region and the intermolecular interaction[J]. Fluid Phase Equilib., 2003, 214(1): 53-65. |
| 27 | Kian K, Scurto A M. Heat transport properties of CO2-expanded liquids: n-hexane, n-decane, and n-tetradecane[J]. Ind. Eng. Chem. Res., 2017, 56(44): 12822-12832. |
| 28 | Yang Z H, Li M Y, Peng B, et al. Dispersion property of CO2 in oil (2): Volume expansion of CO2 + organic liquid at near-critical and supercritical conditions of CO2 [J]. J. Chem. Eng. Data, 2012, 57(4): 1305-1311. |
| 29 | Poling B E, Prausnitz J M, O’connell J P. The Properties of Gases and Liquids[M]. 5th ed. New York: McGraw-Hill Professional, 2001. |
| 30 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Ind. Eng. Chem. Fundam., 1976, 15(1): 59-64. |
| 31 | Hankinson R W, Thomson G H. A new correlation for saturated densities of liquids and their mixtures[J]. AIChE J., 1979, 25(4): 653-663. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [5] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [6] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [7] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [8] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [9] | 李晨曦, 刘永峰, 张璐, 刘海峰, 宋金瓯, 何旭. O2/CO2氛围下正庚烷的燃烧机理研究[J]. 化工学报, 2023, 74(5): 2157-2169. |
| [10] | 王皓, 唐思扬, 钟山, 梁斌. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| [11] | 朱兵国, 何吉祥, 徐进良, 彭斌. 冷却条件下渐扩/渐缩管内超临界压力二氧化碳的传热特性[J]. 化工学报, 2023, 74(3): 1062-1072. |
| [12] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [13] | 何仁初, 张朝晖, 杨明磊, 王聪, 奚桢浩. 考虑碳排放因素的汽油调合在线优化[J]. 化工学报, 2023, 74(2): 818-829. |
| [14] | 王煦清, 严圣林, 朱礼涛, 张希宝, 罗正鸿. 填料塔中有机胺吸收CO2气液传质的研究进展[J]. 化工学报, 2023, 74(1): 237-256. |
| [15] | 李鑫, 曾少娟, 彭奎霖, 袁磊, 张香平. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
