化工学报 ›› 2023, Vol. 74 ›› Issue (1): 313-329.DOI: 10.11949/0438-1157.20221268
李鑫1,2( ), 曾少娟2, 彭奎霖2, 袁磊2,3, 张香平1,2(
), 曾少娟2, 彭奎霖2, 袁磊2,3, 张香平1,2( )
)
收稿日期:2022-09-21
修回日期:2022-11-28
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
张香平
作者简介:李鑫(1996—),男,硕士研究生,lixin21@ipe.ac.cn
基金资助:
Xin LI1,2( ), Shaojuan ZENG2, Kuilin PENG2, Lei YUAN2,3, Xiangping ZHANG1,2(
), Shaojuan ZENG2, Kuilin PENG2, Lei YUAN2,3, Xiangping ZHANG1,2( )
)
Received:2022-09-21
Revised:2022-11-28
Online:2023-01-05
Published:2023-03-20
Contact:
Xiangping ZHANG
摘要:
可再生电能驱动CO2电催化合成化学品或燃料,具有反应条件温和、产物选择性可调且可利用分布式可再生能源优势。合成气作为一类重要的化工原料气,可制备甲醇、乙醇、烯烃等大宗化学品,是CO2电催化转化的重要途径,如何高电流密度、高选择性且精准调控碳氢比例(CO/H2)是需要解决的关键科学技术难题。本文从提升电流密度和效率、拓宽合成气比例角度出发,综述了CO2电催化还原制合成气的最新研究进展,包括电极材料设计、电解液开发、电解槽结构创新等;论述了利用原位表征和理论模拟(DFT、MD)方法对CO2电催化还原制合成气反应机理的研究进展。在此基础上,提出可通过催化剂多级形貌调控、多活性位点设计、CO2捕集与转化系统集成、CO2还原与阳极反应耦合等途径,提升CO2电催化还原制合成气效率的策略。最后,探讨和展望了实现CO2电催化还原制合成气工业化的挑战和问题。
中图分类号:
李鑫, 曾少娟, 彭奎霖, 袁磊, 张香平. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329.
Xin LI, Shaojuan ZENG, Kuilin PENG, Lei YUAN, Xiangping ZHANG. Research progress and tendency of CO2 electrocatalytic reduction to syngas[J]. CIESC Journal, 2023, 74(1): 313-329.
| 反应 | 标准电极电势(vs RHE)/V |
|---|---|
| 2H++2e- | -0.42 |
| CO2 + 8H++ 8e- | -0.24 |
| CO2 + 6H++ 6e- | -0.38 |
| CO2 + 4H++ 4e- | -0.51 |
| CO2 + 2H++ 2e- | -0.52 |
| CO2 + 2H++ 2e- | -0.61 |
| 2CO2 + 12H++ 12e- | 0.064 |
| 2CO2 + 12H++ 12e- | 0.084 |
表1 CO2电催化还原半反应电极电位[17-20]
Table 1 Electrode potential for semi-reaction of CO2 electrocatalytic reduction [17-20]
| 反应 | 标准电极电势(vs RHE)/V |
|---|---|
| 2H++2e- | -0.42 |
| CO2 + 8H++ 8e- | -0.24 |
| CO2 + 6H++ 6e- | -0.38 |
| CO2 + 4H++ 4e- | -0.51 |
| CO2 + 2H++ 2e- | -0.52 |
| CO2 + 2H++ 2e- | -0.61 |
| 2CO2 + 12H++ 12e- | 0.064 |
| 2CO2 + 12H++ 12e- | 0.084 |
| CO/H2 | 下游产物 |
|---|---|
| 纯CO | CO电子特气 |
| 约1 | 氢甲酰化产品 |
| 0.5~1.0 | 费托合成品 |
| 约0.5 | 甲醇 |
| 0.3~0.5 | 甲烷 |
表2 不同CO/H2比对应下游化学品[19,21-24]
Table 2 CO/H2 ratios and corresponding downstream chemical products[19,21-24]
| CO/H2 | 下游产物 |
|---|---|
| 纯CO | CO电子特气 |
| 约1 | 氢甲酰化产品 |
| 0.5~1.0 | 费托合成品 |
| 约0.5 | 甲醇 |
| 0.3~0.5 | 甲烷 |
| 电极 | 电解液 | 电解槽 | 电势(vs RHE)/V | 电流密度/(mA/cm2) | CO/H2 | 文献 |
|---|---|---|---|---|---|---|
| Cu-In | 0.1 mol/L KHCO3 | H | -1.1 | 20 | 2~9 | [ |
| CuZnAl | 0.5 mol/L NaHCO3 | H | -2.4 | 90 | 0.14~0.5 | [ |
| PdAg | 0.5 mol/L NaHCO3 | H | -0.9 | — | 2.7 | [ |
| PdCu | 0.5 mol/L NaHCO3 | H | -0.9 | — | 1.2 | [ |
| Zn-1P | 0.5 mol/L NaHCO3 | H | -1.27 | 90.4 | 0.09~11.4 | [ |
| Co3O4-CDots-C3N4 | 0.5 mol/L KHCO3 | H | -1.0 | 15 | 0.25~14.2 | [ |
| 3D N-CNTs/SS | 0.1 mol/L KHCO3 | H | -1.1 | 2 | 0.3~3 | [ |
| ZnO-C | 0.5 mol/L KHCO3 | H | -1.2 | 27.07 | 0.73~2 | [ |
| 4.3Pd-SnO2 | 0.5 mol/L KHCO3 | H | -0.6 | 10 | 0.28~4.2 | [ |
| Zn/Cu | 0.5 mol/L KHCO3 | H | -1.53 | 20.4 | 0.25~0.84 | [ |
| HPC-Co/CoPc | 1.0 mol/L KHCO3 | H | -0.86 | 225 | 0.26~0.95 | [ |
| CF-120 | 0.1 mol/L KHCO3 | H | -0.6 | ~8.5 | 0.33~2 | [ |
| Ag | 18%(mol)[Emim][BF4] | H | — | — | — | [ |
| Ru | Bu4NH2PO4 /MeCN | H | -1.2 | — | 0.45~49 | [ |
| MoO2 | MeCN/0.3 mol/L [Bmim][PF4] | H | -2.45 | 20 | 2~5 | [ |
| In2Se3/CP | [Bmim]PF6 | H | -2.3 | 90.1 | 0.33~24 | [ |
| Ag/TiO2 | 1 mol/L NaOH | GDE | -0.56 | ~-83 | 0.5~1.5 | [ |
| Cu-In | 1 mol/L KOH | GDE | -1.17 | ∼200 | 1.49~14.77 | [ |
| 4.3Pd-SnO2 | 0.5 mol/L KHCO3 | GDE | -0.9 | 100 | 0.26~9.2 | [ |
| HPC-Co/CoPc | 1.0 mol/L KOH | GDE | -0.6 | 880 | — | [ |
| PdH | 0.5 mol/L NaHCO3 | MEA | -0.9 | 200 | 0.25~1 | [ |
| BiO x /[Bmim]OTf | — | MEA | -3.8 V(full cell) | 200 | — | [ |
| Ni SA | — | MEA | -2.78 V(full cell) | ~50 | — | [ |
| NiO | — | SOEC | -1.3 | 620 | 0.5~2 | [ |
表3 不同CO2电催化还原制合成气体系
Table 3 Different system of CO2 electrocatalytic reduction to syngas
| 电极 | 电解液 | 电解槽 | 电势(vs RHE)/V | 电流密度/(mA/cm2) | CO/H2 | 文献 |
|---|---|---|---|---|---|---|
| Cu-In | 0.1 mol/L KHCO3 | H | -1.1 | 20 | 2~9 | [ |
| CuZnAl | 0.5 mol/L NaHCO3 | H | -2.4 | 90 | 0.14~0.5 | [ |
| PdAg | 0.5 mol/L NaHCO3 | H | -0.9 | — | 2.7 | [ |
| PdCu | 0.5 mol/L NaHCO3 | H | -0.9 | — | 1.2 | [ |
| Zn-1P | 0.5 mol/L NaHCO3 | H | -1.27 | 90.4 | 0.09~11.4 | [ |
| Co3O4-CDots-C3N4 | 0.5 mol/L KHCO3 | H | -1.0 | 15 | 0.25~14.2 | [ |
| 3D N-CNTs/SS | 0.1 mol/L KHCO3 | H | -1.1 | 2 | 0.3~3 | [ |
| ZnO-C | 0.5 mol/L KHCO3 | H | -1.2 | 27.07 | 0.73~2 | [ |
| 4.3Pd-SnO2 | 0.5 mol/L KHCO3 | H | -0.6 | 10 | 0.28~4.2 | [ |
| Zn/Cu | 0.5 mol/L KHCO3 | H | -1.53 | 20.4 | 0.25~0.84 | [ |
| HPC-Co/CoPc | 1.0 mol/L KHCO3 | H | -0.86 | 225 | 0.26~0.95 | [ |
| CF-120 | 0.1 mol/L KHCO3 | H | -0.6 | ~8.5 | 0.33~2 | [ |
| Ag | 18%(mol)[Emim][BF4] | H | — | — | — | [ |
| Ru | Bu4NH2PO4 /MeCN | H | -1.2 | — | 0.45~49 | [ |
| MoO2 | MeCN/0.3 mol/L [Bmim][PF4] | H | -2.45 | 20 | 2~5 | [ |
| In2Se3/CP | [Bmim]PF6 | H | -2.3 | 90.1 | 0.33~24 | [ |
| Ag/TiO2 | 1 mol/L NaOH | GDE | -0.56 | ~-83 | 0.5~1.5 | [ |
| Cu-In | 1 mol/L KOH | GDE | -1.17 | ∼200 | 1.49~14.77 | [ |
| 4.3Pd-SnO2 | 0.5 mol/L KHCO3 | GDE | -0.9 | 100 | 0.26~9.2 | [ |
| HPC-Co/CoPc | 1.0 mol/L KOH | GDE | -0.6 | 880 | — | [ |
| PdH | 0.5 mol/L NaHCO3 | MEA | -0.9 | 200 | 0.25~1 | [ |
| BiO x /[Bmim]OTf | — | MEA | -3.8 V(full cell) | 200 | — | [ |
| Ni SA | — | MEA | -2.78 V(full cell) | ~50 | — | [ |
| NiO | — | SOEC | -1.3 | 620 | 0.5~2 | [ |

图1 (a)碳纳米管破壁开链[31];(b)不同尺度纳米Zn对CO/H2的影响[50];(c)快速火焰法制备氧空位N-ZnO[35];(d)Pd-SnO2界面构建示意图[36];(e)双Co单原子催化剂催化CO2RR和HER[38]
Fig.1 (a) Wall breaking and ring opening of carbon nanotube[31]; (b) Effect of different sizes nano Zn on CO/H2[50]; (c) Preparation of oxygen vacancy N-ZnO by rapid flame method[35]; (d) Construction of Pd-SnO2 interface[36]; (e) Dual single-cobalt atom-based carbon electrocatalyst for CO2RR and HER[38]
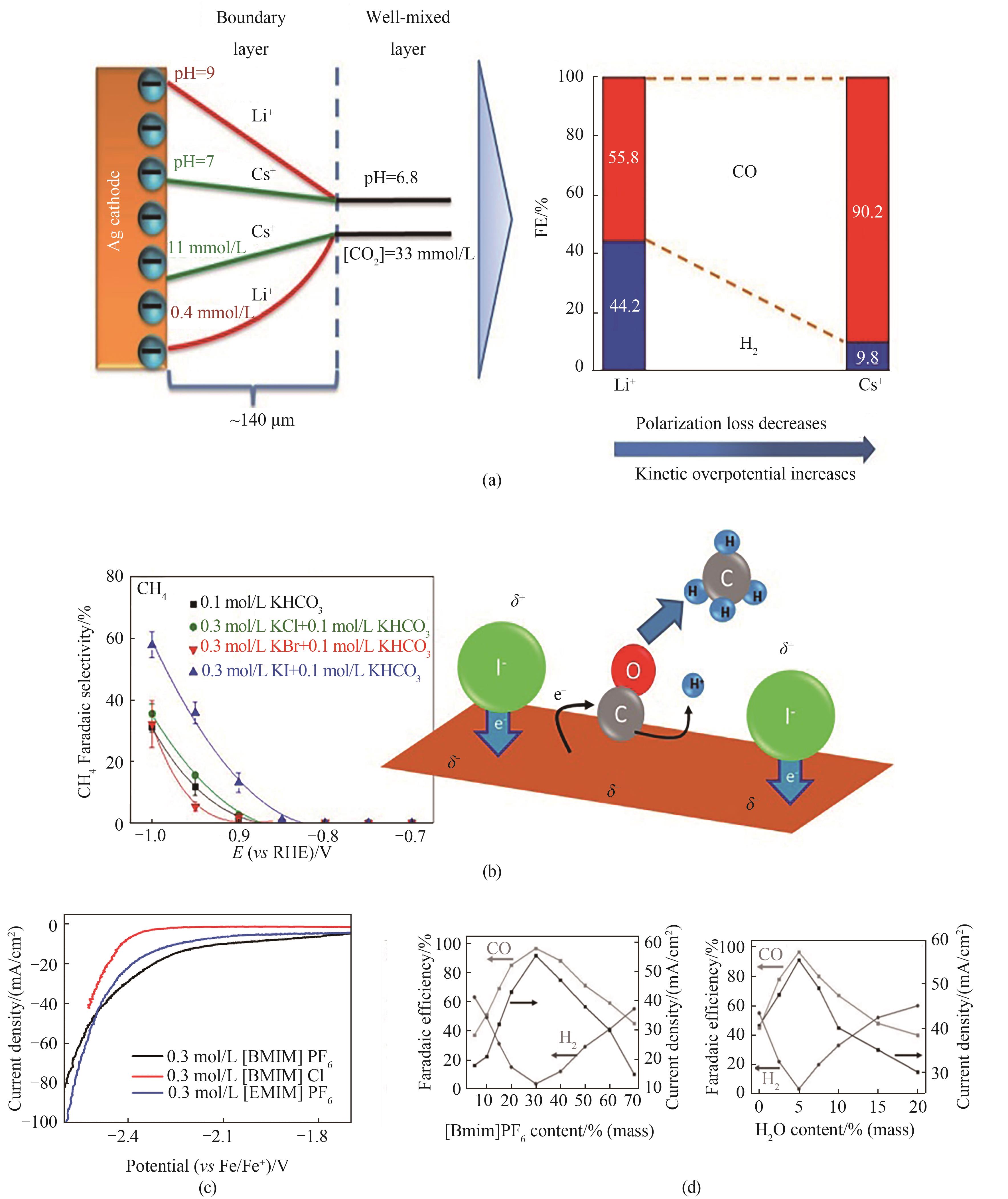
图2 (a)阳离子与水解pKa的关系[64];(b)卤素离子对产物选择性的影响[66];(c)不同离子液体类型对电流密度的影响[42];(d)调节IL浓度与H2O含量调控CO/H2[43]
Fig.2 (a) Relationship between cation and pKa[64]; (b) Effect of halogen ion on product selectivity[66]; (c) Effect of different ionic liquids on current density[42]; (d) Adjust IL concentration and H2O content to regulate CO/H2 ratio[43]
| 1 | Global monitoring laboratory, earth system research laboratories[EB/OL]. . |
| 2 | Goeppert A, Czaun M, Surya Prakash G K, et al. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere[J]. Energy & Environmental Science, 2012, 5(7): 7833. |
| 3 | Nguyen T N, Dinh C T. Gas diffusion electrode design for electrochemical carbon dioxide reduction[J]. Chemical Society Reviews, 2020, 49(21): 7488-7504. |
| 4 | Tomboc G M, Choi S, Kwon T, et al. Potential link between Cu surface and selective CO2 electroreduction: perspective on future electrocatalyst designs[J]. Advanced Materials, 2020, 32(17): 1908398. |
| 5 | Rubin E S, Chen C, Rao A B. Cost and performance of fossil fuel power plants with CO2 capture and storage[J]. Energy Policy, 2007, 35(9): 4444-4454. |
| 6 | Hao L, Kang L, Huang H W, et al. Surface-halogenation-induced atomic-site activation and local charge separation for superb CO2 photoreduction[J]. Advanced Materials, 2019, 31(25): 1900546. |
| 7 | Li Y, Li B H, Zhang D N, et al. Crystalline carbon nitride supported copper single atoms for photocatalytic CO2 reduction with nearly 100% CO selectivity[J]. ACS Nano, 2020, 14(8): 10552-10561. |
| 8 | Heenemann M, Millet M M, Girgsdies F, et al. The mechanism of interfacial CO2 activation on Al doped Cu/ZnO[J]. ACS Catalysis, 2020, 10(10): 5672-5680. |
| 9 | Wang S W, Wu T J, Lin J, et al. Iron-potassium on single-walled carbon nanotubes as efficient catalyst for CO2 hydrogenation to heavy olefins[J]. ACS Catalysis, 2020, 10(11): 6389-6401. |
| 10 | Xu D, Ding M Y, Hong X L, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catalysis, 2020, 10(9): 5250-5260. |
| 11 | Ebaid M, Jiang K, Zhang Z M, et al. Production of C2/C3 oxygenates from planar copper nitride-derived mesoporous copper via electrochemical reduction of CO2 [J]. Chemistry of Materials, 2020, 32(7): 3304-3311. |
| 12 | Li J, Wu D H, Malkani A S, et al. Hydroxide is not a promoter of C2+ product formation in the electrochemical reduction of CO on copper[J]. Angewandte Chemie International Edition, 2020, 59(11): 4464-4469. |
| 13 | Ma L S, Hu W B, Mei B B, et al. Covalent triazine framework confined copper catalysts for selective electrochemical CO2 reduction: operando diagnosis of active sites[J]. ACS Catalysis, 2020, 10(8): 4534-4542. |
| 14 | Bouzon M, Perret A, Loreau O, et al. A synthetic alternative to canonical one-carbon metabolism[J]. ACS Synthetic Biology, 2017, 6(8): 1520-1533. |
| 15 | Döring V, Darii E, Yishai O, et al. Implementation of a reductive route of one-carbon assimilation in Escherichia coli through directed evolution[J]. ACS Synthetic Biology, 2018, 7(9): 2029-2036. |
| 16 | Zhang W J, Hu Y, Ma L B, et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2017, 5(1): 1700275. |
| 17 | 张少阳, 商阳阳, 赵瑞花, 等. 电催化还原二氧化碳制一氧化碳催化剂研究进展[J]. 化工进展, 2022, 41(4): 1848-1857. |
| Zhang S Y, Shang Y Y, Zhao R H, et al. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to carbon monoxide[J]. Chemical Industry and Engineering Progress, 2022, 41(4): 1848-1857. | |
| 18 | 孙睿, 徐跃, 刘建芳, 等. CO2催化还原转化为高附加值化学品[J]. 中国科学: 化学, 2018, 48(6): 547-561. |
| Sun R, Xu Y, Liu J F, et al. Recent progress in CO2 catalytic reduction to high value-added chemicals[J]. Scientia Sinica Chimica, 2018, 48(6): 547-561. | |
| 19 | 华亚妮, 冯少广, 党欣悦, 等. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240. |
| Hua Y N, Feng S G, Dang X Y, et al. Research progress of CO2 electrocatalytic reduction to syngas[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1224-1240. | |
| 20 | 邵斌, 孙哲毅, 章云, 等. 二氧化碳转化为合成气及高附加值产品的研究进展[J]. 化工进展, 2022, 41(3): 1136-1151. |
| Shao B, Sun Z Y, Zhang Y, et al. Recent progresses in CO2 to syngas and high value-added products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1136-1151. | |
| 21 | Wenzel M, Rihko-Struckmann L, Sundmacher K. Thermodynamic analysis and optimization of RWGS processes for solar syngas production from CO2 [J]. AIChE Journal, 2017, 63(1): 15-22. |
| 22 | Ma S C, Huang S D, Liu Z P. Dynamic coordination of cations and catalytic selectivity on zinc-chromium oxide alloys during syngas conversion[J]. Nature Catalysis, 2019, 2(8): 671-677. |
| 23 | 王茹洁, 赵华君, 齐彩娆, 等. 电催化还原CO2制单碳产物反应机理及催化剂研究进展[J]. 天然气化工—C1化学与化工, 2022, 47(2): 11-17. |
| Wang R J, Zhao H J, Qi C R, et al. Research progress on reaction mechanism and catalysts of electrocatalytic reduction of CO2 to single-carbon compounds[J]. Natural Gas Chemical Industry, 2022, 47(2): 11-17. | |
| 24 | Hua Y N, Wang J Y, Min T, et al. Electrochemical CO2 conversion towards syngas: recent catalysts and improving strategies for ratio-tunable syngas[J]. Journal of Power Sources, 2022, 535: 231453. |
| 25 | Scarpa D, Sarno M. Single-atom catalysts for the electro-reduction of CO2 to syngas with a tunable CO/H2 ratio:a review[J]. Catalysts, 2022, 12(3): 275-284. |
| 26 | Gao D F, Arán-Ais R M, Jeon H S, et al. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products[J]. Nature Catalysis, 2019, 2(3): 198-210. |
| 27 | Chu M G, Chen C J, Wu Y H, et al. Enhanced CO2 electroreduction to ethylene via strong metal-support interaction[J]. Green Energy & Environment, 2022, 7(4): 792-798. |
| 28 | Clark E L, Ringe S, Tang M, et al. Influence of atomic surface structure on the activity of Ag for the electrochemical reduction of CO2 to CO[J]. ACS Catalysis, 2019, 9(5): 4006-4014. |
| 29 | Mahyoub S A, Qaraah F A, Yan S L, et al. 3D Cu/In nanocones by morphological and interface engineering design in achieving a high current density for electroreduction of CO2 to syngas under elevated pressure[J]. Journal of CO2 Utilization, 2022, 61: 102033. |
| 30 | Guzmán H, Roldán D, Sacco A, et al. CuZnAl-oxide nanopyramidal mesoporous materials for the electrocatalytic CO2 reduction to syngas: tuning of H2/CO ratio[J]. Nanomaterials, 2021, 11(11): 3052. |
| 31 | Pan F P, Li B Y, Sarnello E, et al. Atomically dispersed iron-nitrogen sites on hierarchically mesoporous carbon nanotube and graphene nanoribbon networks for CO2 reduction[J]. ACS Nano, 2020, 14(5): 5506-5516. |
| 32 | Li P S, Liu J Y, Bi J H, et al. Tuning the efficiency and product composition for electrocatalytic CO2 reduction to syngas over zinc films by morphology and wettability[J]. Green Chemistry, 2022, 24(4): 1439-1444. |
| 33 | Guo S J, Zhao S Q, Wu X Q, et al. A Co3O4-CDots-C3N4 three component electrocatalyst design concept for efficient and tunable CO2 reduction to syngas[J]. Nature Communications, 2017, 8: 1828. |
| 34 | Liu K H, Zhong H X, Yang X Y, et al. Composition-tunable synthesis of “clean” syngas via a one-step synthesis of metal-free pyridinic-N-enriched self-supported CNTs: the synergy of electrocatalyst pyrolysis temperature and potential[J]. Green Chemistry, 2017, 19(18): 4284-4288. |
| 35 | Ma C, Zou X Y, Li A, et al. Rapid flame synthesis of carbon doped defective ZnO for electrocatalytic CO2 reduction to syngas[J]. Electrochimica Acta, 2022, 411: 140098. |
| 36 | He H C, Xia D, Yu X, et al. Pd-SnO2 interface enables synthesis of syngas with controllable H2/CO ratios by electrocatalytic reduction of CO2 [J]. Applied Catalysis B: Environmental, 2022, 312: 121392. |
| 37 | Chen P, Jiao Y, Zhu Y H, et al. Syngas production from electrocatalytic CO2 reduction with high energetic efficiency and current density[J]. Journal of Materials Chemistry A, 2019, 7(13): 7675-7682. |
| 38 | Ni W P, Liu Z X, Guo X G, et al. Dual single-cobalt atom-based carbon electrocatalysts for efficient CO2 to syngas conversion with industrial current densities[J]. Applied Catalysis B: Environmental, 2021, 291: 120092. |
| 39 | Li H Q, Xiao N, Wang Y W, et al. Nitrogen-doped tubular carbon foam electrodes for efficient electroreduction of CO2 to syngas with potential-independent CO/H2 ratios[J]. Journal of Materials Chemistry A, 2019, 7(32): 18852-18860. |
| 40 | Rosen B A, Salehi-Khojin A, Thorson M R, et al. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials[J]. Science, 2011, 334(6056): 643-644. |
| 41 | Chen Z F, Kang P, Zhang M T, et al. Making syngas electrocatalytically using a polypyridyl ruthenium catalyst[J]. Chemical Communications, 2014, 50(3): 335-337. |
| 42 | Oh Y, Hu X L. Ionic liquids enhance the electrochemical CO2 reduction catalyzed by MoO2 [J]. Chemical Communications, 2015, 51(71): 13698-13701. |
| 43 | Yang D X, Zhu Q G, Sun X F, et al. Electrosynthesis of a defective indium selenide with 3D structure on a substrate for tunable CO2 electroreduction to syngas[J]. Angewandte Chemie International Edition, 2020, 59(6): 2354-2359. |
| 44 | Kim Y E, Kim B, Lee W, et al. Highly tunable syngas production by electrocatalytic reduction of CO2 using Ag/TiO2 catalysts[J]. Chemical Engineering Journal, 2021, 413: 127448. |
| 45 | Xiang H, Rasul S, Hou B, et al. Copper-indium binary catalyst on a gas diffusion electrode for high-performance CO2 electrochemical reduction with record CO production efficiency[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 601-608. |
| 46 | Li Y C, Zhou D K, Yan Z F, et al. Electrolysis of CO2 to syngas in bipolar membrane-based electrochemical cells[J]. ACS Energy Letters, 2016, 1(6): 1149-1153. |
| 47 | Jiang K, Siahrostami S, Zheng T T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 48 | Song Y F, Zhou Z W, Zhang X M, et al. Pure CO2 electrolysis over an Ni/YSZ cathode in a solid oxide electrolysis cell[J]. Journal of Materials Chemistry A, 2018, 6(28): 13661-13667. |
| 49 | Zhu W L, Zhang Y J, Zhang H Y, et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires[J]. Journal of the American Chemical Society, 2014, 136(46): 16132-16135. |
| 50 | Han K, Ngene P, De Jongh P. Structure dependent product selectivity for CO2 electroreduction on ZnO derived catalysts[J]. ChemCatChem, 2021, 13(8): 1998-2004. |
| 51 | Qin B H, Li Y H, Fu H Q, et al. Electrochemical reduction of CO2 into tunable syngas production by regulating the crystal facets of earth-abundant Zn catalyst[J]. ACS Applied Materials & Interfaces, 2018, 10(24): 20530-20539. |
| 52 | Xiong B, Yang Y J, Liu J, et al. Crystal orientation effects on the electrochemical conversion of CO2 to syngas over Cu-M (M=Ag, Ni, Zn, Cd, and Pd) bimetal catalysts[J]. Applied Surface Science, 2021, 567: 150839. |
| 53 | Cao K L, Ji Y J, Bai S X, et al. A wide range of CO:H2 syngas ratios enabled by a tellurization-induced amorphous telluride–palladium surface[J]. Journal of Materials Chemistry A, 2021, 9(34): 18349-18355. |
| 54 | Xie C L, Niu Z Q, Kim D, et al. Surface and interface control in nanoparticle catalysis[J]. Chemical Reviews, 2020, 120(2): 1184-1249. |
| 55 | Luo W, Xie W, Mutschler R, et al. Selective and stable electroreduction of CO2 to CO at the copper/indium interface[J]. ACS Catalysis, 2018, 8(7): 6571-6581. |
| 56 | Wang X, Lv J, Zhang J X, et al. Hierarchical heterostructure of SnO2 confined on CuS nanosheets for efficient electrocatalytic CO2 reduction[J]. Nanoscale, 2020, 12(2): 772-784. |
| 57 | Zhang L L, Ren Y J, Liu W G, et al. Single-atom catalyst: a rising star for green synthesis of fine chemicals[J]. National Science Review, 2018, 5(5): 653-672. |
| 58 | Zhang L L, Zhou M X, Wang A Q, et al. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms[J]. Chemical Reviews, 2020, 120(2): 683-733. |
| 59 | He Q, Liu D B, Lee J H, et al. Electrochemical conversion of CO2 to syngas with controllable CO/H2 ratios over Co and Ni single-atom catalysts[J]. Angewandte Chemie International Edition, 2020, 59(8): 3033-3037. |
| 60 | Deng B W, Huang M, Zhao X L, et al. Interfacial electrolyte effects on electrocatalytic CO2 reduction[J]. ACS Catalysis, 2022, 12(1): 331-362. |
| 61 | Sa Y J, Lee C W, Lee S Y, et al. Catalyst-electrolyte interface chemistry for electrochemical CO2 reduction[J]. Chemical Society Reviews, 2020, 49(18): 6632-6665. |
| 62 | Gutiérrez-Sánchez O, Daems N, Offermans W, et al. The inhibition of the proton donor ability of bicarbonate promotes the electrochemical conversion of CO2 in bicarbonate solutions[J]. Journal of CO2 Utilization, 2021, 48: 101521. |
| 63 | Zhong Y, Xu Y, Ma J, et al. An artificial electrode/electrolyte interface for CO2 electroreduction by cation surfactant self-assembly[J]. Angewandte Chemie International Edition, 2020, 59(43): 19095-19101. |
| 64 | Singh M R, Kwon Y, Lum Y, et al. Hydrolysis of electrolyte cations enhances the electrochemical reduction of CO2 over Ag and Cu[J]. Journal of the American Chemical Society, 2016, 138(39): 13006-13012. |
| 65 | Malkani A S, Anibal J, Xu B J. Cation effect on interfacial CO2 concentration in the electrochemical CO2 reduction reaction[J]. ACS Catalysis, 2020, 10(24): 14871-14876. |
| 66 | Varela A S, Ju W, Reier T, et al. Tuning the catalytic activity and selectivity of Cu for CO2 electroreduction in the presence of halides[J]. ACS Catalysis, 2016, 6(4): 2136-2144. |
| 67 | 张香平, 曾少娟, 冯佳奇, 等.CO2化工:离子微环境调控的CO2绿色高效转化[J]. 中国科学:化学, 2020, 50(2): 282-298. |
| Zhang X P, Zeng S J, Feng J Q, et al. CO2 chemical engineering: CO2 green conversion enhanced by ionic liquid microhabitat[J]. Scientia Sinica Chimica, 2020, 50(2): 282-298. | |
| 68 | Dong K, Liu X M, Dong H F, et al. Multiscale studies on ionic liquids[J]. Chemical Reviews, 2017, 117(10): 6636-6695. |
| 69 | Vasilyev D V, Shyshkanov S, Shirzadi E, et al. Principal descriptors of ionic liquid co-catalysts for the electrochemical reduction of CO2 [J]. ACS Applied Energy Materials, 2020, 3(5): 4690-4698. |
| 70 | Tanner E E L, Batchelor-Mcauley C, Compton R G. Carbon dioxide reduction in room-temperature ionic liquids: the effect of the choice of electrode material, cation, and anion[J]. The Journal of Physical Chemistry C, 2016, 120(46): 26442-26447. |
| 71 | Monroe M M, Lobaccaro P, Lum Y, et al. Membraneless laminar flow cell for electrocatalytic CO2 reduction with liquid product separation[J]. Journal of Physics D: Applied Physics, 2017, 50(15): 154006. |
| 72 | Zhu P, Wang H T. High-purity and high-concentration liquid fuels through CO2 electroreduction[J]. Nature Catalysis, 2021, 4(11): 943-951. |
| 73 | Mohd Adli N, Shan W T, Hwang S, et al. Engineering atomically dispersed FeN4 active sites for CO2 electroreduction[J]. Angewandte Chemie International Edition, 2021, 60(2): 1022-1032. |
| 74 | Sheng X D, Ge W X, Jiang H L, et al. Engineering the Ni-N-C catalyst microenvironment enabling CO2 electroreduction with nearly 100% CO selectivity in acid[J]. Advanced Materials, 2022, 34(38): e2201295. |
| 75 | Wicks J, Jue M L, Beck V A, et al. 3D-printable fluoropolymer gas diffusion layers for CO2 electroreduction[J]. Advanced Materials, 2021, 33(7): 2003855. |
| 76 | Song Y F, Zhang X M, Xie K, et al. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects[J]. Advanced Materials, 2019, 31(50): 1902033. |
| 77 | Zhang X Y, O’Brien J E, O’Brien R C, et al. Improved durability of SOEC stacks for high temperature electrolysis[J]. International Journal of Hydrogen Energy, 2013, 38(1): 20-28. |
| 78 | Fan H, Han M F. Improved performance and stability of Ag-infiltrated nanocomposite La0.6Sr0.4Co0.2Fe0.8O3- δ -(Y2O3)0.08(ZrO2)0.92 oxygen electrode for H2O/CO2 co-electrolysis[J]. Journal of Power Sources, 2016, 336: 179-185. |
| 79 | Zhou Y J, Zhou Z W, Song Y F, et al. Enhancing CO2 electrolysis performance with vanadium-doped perovskite cathode in solid oxide electrolysis cell[J]. Nano Energy, 2018, 50: 43-51. |
| 80 | Jin S, Hao Z M, Zhang K, et al. Advances and challenges for the electrochemical reduction of CO2 to CO: from fundamentals to industrialization[J]. Angewandte Chemie International Edition, 2021, 60(38): 20627-20648. |
| 81 | Daiyan R, Chen R, Kumar P, et al. Tunable syngas production through CO2 electroreduction on cobalt-carbon composite electrocatalyst[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 9307-9315. |
| 82 | Charles D, John N. Mathematical modeling of CO2 reduction to CO in aqueous electrolytes (Ⅱ): Study of an electrolysis cell making syngas (CO+H2) from CO2 and H2O reduction at room temperature[J]. Joural of The Electrochemical Society, 2010, 157(12:) 1911-1926. |
| 83 | Pan F P, Yang Y. Designing CO2 reduction electrode materials by morphology and interface engineering[J]. Energy & Environmental Science, 2020, 13(8): 2275-2309. |
| 84 | Seifitokaldani A, Gabardo C M, Burdyny T, et al. Hydronium-induced switching between CO2 electroreduction pathways[J]. Journal of the American Chemical Society, 2018, 140(11): 3833-3837. |
| 85 | Ge W X, Chen Y X, Fan Y, et al. Dynamically formed surfactant assembly at the electrified electrode-electrolyte interface boosting CO2 electroreduction[J]. Journal of the American Chemical Society, 2022, 144(14): 6613-6622. |
| 86 | 江重阳, 冯佳奇, 曾少娟, 等.CO2电化学还原过程中电解质研究现状及趋势[J]. 科学通报, 2021, 66(7): 716-727. |
| Jiang C Y, Feng J Q, Zeng S J, et al. Research status and trend of electrolytes in the CO2 electrochemical reduction[J]. Chinese Science Bulletin, 2021, 66(7): 716-727. | |
| 87 | 冯建朋, 张香平, 尚大伟, 等.离子液体中电化学还原CO2研究评述与展望[J]. 化工学报, 2018, 69(1): 69-75. |
| Feng J P, Zhang X P, Shang D W, et al. Review and prospect of CO2 electro-reduction in ionic liquids[J]. CIESC Journal, 2018, 69(1): 69-75. | |
| 88 | Yang D W, Li Q Y, Shen F X, et al. Electrochemical impedance studies of CO2 reduction in ionic liquid/organic solvent electrolyte on Au electrode[J]. Electrochimica Acta, 2016, 189: 32-37. |
| 89 | Sun L Y, Ramesha G K, Kamat P V, et al. Switching the reaction course of electrochemical CO2 reduction with ionic liquids[J]. Langmuir, 2014, 30(21): 6302-6308. |
| 90 | DiMeglio J L, Rosenthal J. Selective conversion of CO2 to CO with high efficiency using an inexpensive bismuth-based electrocatalyst[J]. Journal of the American Chemical Society, 2013, 135(24): 8798-8801. |
| 91 | Lau G P S, Schreier M, Vasilyev D, et al. New insights into the role of imidazolium-based promoters for the electroreduction of CO2 on a silver electrode[J]. Journal of the American Chemical Society, 2016, 138(25): 7820-7823. |
| 92 | Liang S Y, Huang L, Gao Y S, et al. Electrochemical reduction of CO2 to CO over transition metal/N-doped carbon catalysts: the active sites and reaction mechanism[J]. Advanced Science, 2021, 8(24): 2102886. |
| 93 | Chen Z S, Zhang G X, Chen H R, et al. Multi-metallic catalysts for the electroreduction of carbon dioxide: recent advances and perspectives[J]. Renewable and Sustainable Energy Reviews, 2022, 155: 111922. |
| 94 | Gui J J, Zhang K F, Zhan X W, et al. Nitrogen-doped porous carbon nanosheets as a robust catalyst for tunable CO2 electroreduction to syngas[J]. Sustainable Energy & Fuels, 2022, 6(6): 1512-1518. |
| 95 | Yan W Y, Zhang C, Liu L. Hierarchically porous CuAg via 3D printing/dealloying for tunable CO2 reduction to syngas[J]. ACS Applied Materials & Interfaces, 2021, 13(38): 45385-45393. |
| 96 | Luo H Q, Li B, Ma J G, et al. Surface modification of nano-Cu2O for controlling CO2 electrochemical reduction to ethylene and syngas[J]. Angewandte Chemie International Edition, 2022, 61(11): e202116736. |
| 97 | Lu Q, Jiao F. Electrochemical CO2 reduction: electrocatalyst, reaction mechanism, and process engineering[J]. Nano Energy, 2016, 29: 439-456. |
| 98 | Yan X X, Gu M Y, Wang Y, et al. In-situ growth of Ni nanoparticle-encapsulated N-doped carbon nanotubes on carbon nanorods for efficient hydrogen evolution electrocatalysis[J]. Nano Research, 2020, 13(4): 975-982. |
| 99 | Ismail A M, Samu G F, Balog Á, et al. Composition-dependent electrocatalytic behavior of Au-Sn bimetallic nanoparticles in carbon dioxide reduction[J]. ACS Energy Letters, 2019, 4(1): 48-53. |
| 100 | Zou X Y, Ma C, Li A, et al. Nanoparticle-assisted Ni-Co binary single-atom catalysts supported on carbon nanotubes for efficient electroreduction of CO2 to syngas with controllable CO/H2 ratios[J]. ACS Applied Energy Materials, 2021, 4(9): 9572-9581. |
| 101 | Liang Z, Song L P, Sun M Z, et al. Tunable CO/H2 ratios of electrochemical reduction of CO2 through the Zn-La dual atomic catalysts[J]. Science Advances, 2021, 7(47): eabl4915. |
| 102 | Sullivan I, Goryachev A, Digdaya I A, et al. Coupling electrochemical CO2 conversion with CO2 capture[J]. Nature Catalysis, 2021, 4(11): 952-958. |
| 103 | Lee G, Li Y C, Kim J Y, et al. Electrochemical upgrade of CO2 from amine capture solution[J]. Nature Energy, 2021, 6(1): 46-53. |
| 104 | Huang Y Y, Yang R, Anandhababu G, et al. Cobalt/iron(oxides) heterostructures for efficient oxygen evolution and benzyl alcohol oxidation reactions[J]. ACS Energy Letters, 2018, 3(8): 1854-1860. |
| 105 | Verma S, Lu S, Kenis P J A. Co-electrolysis of CO2 and glycerol as a pathway to carbon chemicals with improved technoeconomics due to low electricity consumption[J]. Nature Energy, 2019, 4(6): 466-474. |
| 106 | Li T F, Cao Y, He J F, et al. Electrolytic CO2 reduction in tandem with oxidative organic chemistry[J]. ACS Central Science, 2017, 3(7): 778-783. |
| 107 | Wei X F, Li Y, Chen L S, et al. Formic acid electro-synthesis by concurrent cathodic CO2 reduction and anodic CH3OH oxidation[J]. Angewandte Chemie International Edition, 2021, 60(6): 3148-3155. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [10] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [11] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [12] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [15] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号