化工学报 ›› 2023, Vol. 74 ›› Issue (12): 4764-4776.DOI: 10.11949/0438-1157.20231155
收稿日期:2023-11-09
修回日期:2023-12-26
出版日期:2023-12-25
发布日期:2024-02-19
通讯作者:
施志聪
作者简介:郑立涵(2000—),男,硕士研究生,15560520693@163.com
基金资助:
Lihan ZHENG( ), Zhichuan SHEN, Zhicong SHI(
), Zhichuan SHEN, Zhicong SHI( )
)
Received:2023-11-09
Revised:2023-12-26
Online:2023-12-25
Published:2024-02-19
Contact:
Zhicong SHI
摘要:
固体电解质界面(solid electrolyte interphase, SEI)是锂金属电池在首次充放电时,锂金属负极表面形成的一层钝化膜,对电池的循环性能和安全性能等具有至关重要的影响。充分地认识SEI膜将有助于开发具有更长循环寿命和更高安全性能的锂金属电池。综述了锂金属电池SEI膜的研究进展,介绍了SEI膜的组成和结构,梳理了各种常见组分的形成条件和作用,论证了两种用于描述SEI膜结构的模型,即马赛克模型和层状模型,总结了影响SEI膜组成和结构的关键因素,如电解质添加剂、电极电位、温度和电流密度等。并介绍了通过引入电解质添加剂和构建人工SEI膜实现界面稳定调控的最新研究进展,最后展望了SEI膜未来的研究方向。
中图分类号:
郑立涵, 沈之川, 施志聪. 金属锂固体电解质界面膜的研究进展[J]. 化工学报, 2023, 74(12): 4764-4776.
Lihan ZHENG, Zhichuan SHEN, Zhicong SHI. Research progress on solid electrolyte interphase of lithium metal[J]. CIESC Journal, 2023, 74(12): 4764-4776.
| 成分 | 来源 | 作用 | 文献 |
|---|---|---|---|
| Li2O | 在碳酸酯类或醚类电解液中产生 | 提高SEI膜的稳定性、离子电导率和机械强度 | [ |
| Li2CO3 | 由碳酸脂类电解质中的烷基碳酸锂与痕量水反应产生 | 提高SEI膜的机械强度,是所有成分中吸湿性最小的稳定化合物 | [ |
| LiN x O y | 由LiNO3或ISDN分解产生 | 改善SEI膜的均匀性,有效抑制了电解质和锂金属负极之间的副反应 | [ |
| Li3N | LiNO3添加剂或电解质中的 | 具有高电导率,可以促进SEI膜中Li+的运输 | [ |
| Li2S | 由电解质中的多硫化物与Li+反应生成或从含有LiTFSI 或LiFTFSI的电解质中还原形成 | 可以提高SEI膜的稳定性,改善Li+在SEI膜的扩散,促进均匀的Li沉积 | [ |
| LiF | 由电解质中的含氟锂盐(如LiPF6、LiTFSI、LiFSI等) 或添加剂(如FEC等)产生 | 具有高的化学稳定性和机械强度以及低的Li+扩散势垒,可以抑制负极表面的锂枝晶生长 | [ |
| LiH | 由氢和沉积Li反应生成或者通过溶剂、H2O和LiOH产生 | 消耗活性Li,破坏锂金属负极的循环稳定性 | [ |
| 有机成分 | 由电解质分解产生 | 调节SEI膜的力学性能,提高柔韧性,降低SEI膜的致密性,影响Li+扩散 | [ |
表1 SEI膜中常见成分的来源以及其作用
Table 1 The sources and functions of common components in SEI
| 成分 | 来源 | 作用 | 文献 |
|---|---|---|---|
| Li2O | 在碳酸酯类或醚类电解液中产生 | 提高SEI膜的稳定性、离子电导率和机械强度 | [ |
| Li2CO3 | 由碳酸脂类电解质中的烷基碳酸锂与痕量水反应产生 | 提高SEI膜的机械强度,是所有成分中吸湿性最小的稳定化合物 | [ |
| LiN x O y | 由LiNO3或ISDN分解产生 | 改善SEI膜的均匀性,有效抑制了电解质和锂金属负极之间的副反应 | [ |
| Li3N | LiNO3添加剂或电解质中的 | 具有高电导率,可以促进SEI膜中Li+的运输 | [ |
| Li2S | 由电解质中的多硫化物与Li+反应生成或从含有LiTFSI 或LiFTFSI的电解质中还原形成 | 可以提高SEI膜的稳定性,改善Li+在SEI膜的扩散,促进均匀的Li沉积 | [ |
| LiF | 由电解质中的含氟锂盐(如LiPF6、LiTFSI、LiFSI等) 或添加剂(如FEC等)产生 | 具有高的化学稳定性和机械强度以及低的Li+扩散势垒,可以抑制负极表面的锂枝晶生长 | [ |
| LiH | 由氢和沉积Li反应生成或者通过溶剂、H2O和LiOH产生 | 消耗活性Li,破坏锂金属负极的循环稳定性 | [ |
| 有机成分 | 由电解质分解产生 | 调节SEI膜的力学性能,提高柔韧性,降低SEI膜的致密性,影响Li+扩散 | [ |

图2 不同电解液条件下形成的SEI膜的冷冻电镜图像以及结构示意图:(a)EC/DEC电解质中形成的SEI膜的原子分辨率图像;(b)EC/DEC电解质中形成的镶嵌状SEI膜的结构示意图;(c)FEC电解质中形成的SEI膜的原子分辨率图像;(d)FEC电解质中形成的层状SEI膜的结构示意图[56]
Fig.2 Cryo-electron microscopy images and structural diagrams of SEI under different electrolyte conditions: (a) Atomic-resolution image of the SEI formed in EC/DEC electrolyte; (b) Schematic of the mosaic-type SEI formed in EC/DEC electrolyte; (c) Atomic-resolution image of the SEI formed in FEC electrolyte; (d) Schematic of the multilayered SEI formed in FEC electrolyte[56]
| 影响因素 | 影响作用 | 文献 |
|---|---|---|
| 电解质添加剂 | 不同的添加剂与各种电解质盐具有不同的电化学稳定性窗口,可以促进或抑制电解质中某种反应的发生或某种物质的分解,又或者是自身参与反应,从而改变SEI膜的结构和成分 | [ |
| 电极电位 | 阳极的电极电位能够显著影响溶剂在阳极表面还原反应的ΔGm,当电位较低时,无机成分和有机成分还原反应的ΔGm不同,无机成分优先在电极表面还原,SEI膜呈层状结构。当电位较高时有机和无机化合物同时生成,形成的SEI膜呈镶嵌结构 | [ |
| 温度 | 当温度较低时,Li+的扩散速度和电解质体系的反应动力学减慢,容易产生锂枝晶;当温度较高时,由于反应动力学加快,生成的SEI膜更厚,并且某些组分可能发生自发分解或者倾向于在表面呈大晶粒状 | [ |
| 电流密度 | 不同的电流密度可以影响离子的浓度梯度、扩散速率以及电极表面的电化学反应速率。在低电流密度下形成的SEI膜主要由有机物组成,呈无定形结构;在高电流密度下形成的SEI膜是镶嵌结构,无机物嵌入在由有机物组成的无定形基质中,可能对SEI膜造成破坏 | [ |
| 交流电场 | 在SEI膜形成时对电极表面施加交流电场可以促进大量阴离子在锂金属负极表面聚集并分解,可形成由阴离子衍生的SEI膜 | [ |
| 压力 | 较低的压力会促进富含有机物的SEI膜和非均质、细丝状、分布有孔隙的锂沉积物生成,较高的压力可促进富含氟元素的无机SEI膜生成,能够形成更均匀、更致密的锂薄膜 | [ |
表2 影响SEI膜组成和形态结构的因素及其影响作用
Table 2 Factors and their effects on the composition and morphological structure of SEI
| 影响因素 | 影响作用 | 文献 |
|---|---|---|
| 电解质添加剂 | 不同的添加剂与各种电解质盐具有不同的电化学稳定性窗口,可以促进或抑制电解质中某种反应的发生或某种物质的分解,又或者是自身参与反应,从而改变SEI膜的结构和成分 | [ |
| 电极电位 | 阳极的电极电位能够显著影响溶剂在阳极表面还原反应的ΔGm,当电位较低时,无机成分和有机成分还原反应的ΔGm不同,无机成分优先在电极表面还原,SEI膜呈层状结构。当电位较高时有机和无机化合物同时生成,形成的SEI膜呈镶嵌结构 | [ |
| 温度 | 当温度较低时,Li+的扩散速度和电解质体系的反应动力学减慢,容易产生锂枝晶;当温度较高时,由于反应动力学加快,生成的SEI膜更厚,并且某些组分可能发生自发分解或者倾向于在表面呈大晶粒状 | [ |
| 电流密度 | 不同的电流密度可以影响离子的浓度梯度、扩散速率以及电极表面的电化学反应速率。在低电流密度下形成的SEI膜主要由有机物组成,呈无定形结构;在高电流密度下形成的SEI膜是镶嵌结构,无机物嵌入在由有机物组成的无定形基质中,可能对SEI膜造成破坏 | [ |
| 交流电场 | 在SEI膜形成时对电极表面施加交流电场可以促进大量阴离子在锂金属负极表面聚集并分解,可形成由阴离子衍生的SEI膜 | [ |
| 压力 | 较低的压力会促进富含有机物的SEI膜和非均质、细丝状、分布有孔隙的锂沉积物生成,较高的压力可促进富含氟元素的无机SEI膜生成,能够形成更均匀、更致密的锂薄膜 | [ |

图5 PyF添加剂加入碳酸酯电解液中形成的富阴离子溶剂化环境和SEI膜成分示意图[76]
Fig.5 Schematic diagram of anion-dominant solvation environment and SEI composition formed by PyF additive in carbonate electrolyte[76]
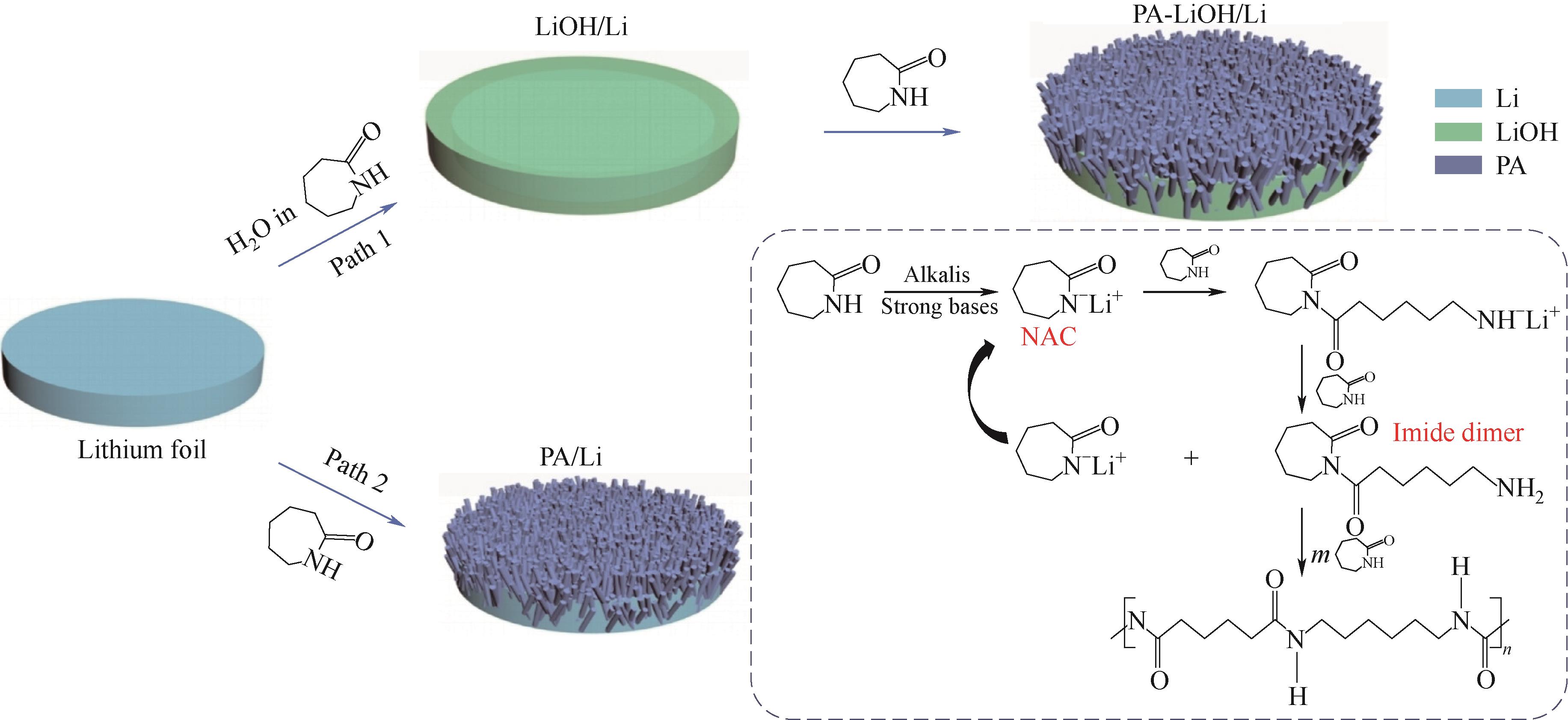
图6 使用催化化学方法设计的锂金属负极人工SEI膜的示意图及合成机理[79]
Fig.6 Schematic diagram and the corresponding synthesis mechanism of designing artificial SEI for lithium metal anode via catalytic chemistry[79]
| 5 | Wu W L, Xu Y T, Ke X, et al. Superorganophilic MAF-6/PP composite separator boosts lithium metal anode performance[J]. Energy Storage Materials, 2021, 37: 387-395. |
| 6 | Huang J Q, Shen Z C, Robertson S J, et al. Fluorine grafted gel polymer electrolyte by in situ construction for high-voltage lithium metal batteries[J]. Chemical Engineering Journal, 2023, 475: 145802. |
| 7 | Chen J B, Li D D, Lin K J, et al. Building a stable artificial solid electrolyte interphase on lithium metal anodes toward long-life Li-O2 batteries[J]. Journal of Power Sources, 2022, 540: 231603. |
| 8 | Pu J, Xue P, Li T T, et al. In situ regulation of dendrite-free lithium anode by improved solid electrolyte interface with defect-rich boron nitride quantum dots[J]. Journal of Materials Chemistry A, 2022, 10(38): 20265-20272. |
| 9 | Xie X T, Chen J B, Chen X Y, et al. Exploring the effect of lithium halide artificial SEI on the electrochemical performance of lithium metal batteries[J]. Journal of Electroanalytical Chemistry, 2023, 949: 117862. |
| 10 | Li D D, Chen J B, Chen Y T, et al. Superior oxygen electrocatalyst derived from metal organic coordination polymers by instantaneous nucleation and epitaxial growth for rechargeable Li-O2 battery[J]. Journal of Energy Chemistry, 2023, 78: 169-177. |
| 11 | Meda U S, Lal L, Sushantha M, et al. Solid electrolyte interphase (SEI), a boon or a bane for lithium batteries: a review on the recent advances[J]. Journal of Energy Storage, 2022, 47: 103564. |
| 12 | Cheng Y F, Chen J B, Chen Y M, et al. Lithium host: advanced architecture components for lithium metal anode[J]. Energy Storage Materials, 2021, 38: 276-298. |
| 13 | Wu Y, Wang C, Wang C J, et al. Recent progress in SEI engineering for boosting Li metal anodes[J]. Materials Horizons, 2023. DOI: 10.1039/d3mh01434g . |
| 14 | Zhao Q, Stalin S, Archer L A. Stabilizing metal battery anodes through the design of solid electrolyte interphases[J]. Joule, 2021, 5(5): 1119-1142. |
| 15 | Ding J F, Xu R, Yan C, et al. A review on the failure and regulation of solid electrolyte interphase in lithium batteries[J]. Journal of Energy Chemistry, 2021, 59: 306-319. |
| 16 | Tan J, Ma L L, Li Z H, et al. Structural insights into solid electrolyte interphase (SEI) on lithium metal anode: from design strategies to the stability evaluation[J]. Materials Today, 2023, 69: 287-332. |
| 17 | Shadike Z, Lee H, Borodin O, et al. Identification of LiH and nanocrystalline LiF in the solid-electrolyte interphase of lithium metal anodes[J]. Nature Nanotechnology, 2021, 16: 549-554. |
| 18 | Zheng J H, Ju Z J, Zhang B L, et al. Lithium ion diffusion mechanism on the inorganic components of the solid-electrolyte interphase[J]. Journal of Materials Chemistry A, 2021, 9(16): 10251-10259. |
| 19 | Heiskanen S K, Kim J, Lucht B L. Generation and evolution of the solid electrolyte interphase of lithium-ion batteries[J]. Joule, 2019, 3(10): 2322-2333. |
| 20 | Ye H J, Gui S W, Wang Z F, et al. In situ measurements of the mechanical properties of electrochemically deposited Li2CO3 and Li2O nanorods[J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44479-44487. |
| 21 | Guo R, Gallant B M. Li2O solid electrolyte interphase: probing transport properties at the chemical potential of lithium[J]. Chemistry of Materials, 2020, 32(13): 5525-5533. |
| 22 | Kim M S, Zhang Z W, Rudnicki P E, et al. Suspension electrolyte with modified Li+ solvation environment for lithium metal batteries[J]. Nature Materials, 2022, 21: 445-454. |
| 23 | Wen Y C, Ding J Y, Yang Y, et al. Introducing N O 3 - into carbonate-based electrolytes via covalent organic framework to incubate stable interface for Li-metal batteries[J]. Advanced Functional Materials, 2022, 32(15): 2109377. |
| 24 | Tan J, Ye M X, Shen J F. Deciphering the role of LiNO3 additives in Li-S batteries[J]. Materials Horizons, 2022, 9(9): 2325-2334. |
| 25 | Li X, Zhao R X, Fu Y Z, et al. Nitrate additives for lithium batteries: mechanisms, applications, and prospects[J]. eScience, 2021, 1(2): 108-123. |
| 26 | Ma X Y, Yu J T, Zou X Y, et al. Single additive to regulate lithium-ion solvation structure in carbonate electrolytes for high-performance lithium-metal batteries[J]. Cell Reports Physical Science, 2023, 4: 101379. |
| 27 | Zhang Q K, Sun S Y, Zhou M Y, et al. Reforming the uniformity of solid electrolyte interphase by nanoscale structure regulation for stable lithium metal batteries[J]. Angewandte Chemie (International Ed. in English), 2023, 62(42): e202306889. |
| 28 | Shan X Y, Zhong Y, Zhang L J, et al. A brief review on solid electrolyte interphase composition characterization technology for lithium metal batteries: challenges and perspectives[J]. The Journal of Physical Chemistry C, 2021, 125(35): 19060-19080. |
| 29 | Liu F F, Wang L F, Zhang Z W, et al. A mixed lithium-ion conductive Li2S/Li2Se protection layer for stable lithium metal anode[J]. Advanced Functional Materials, 2020, 30(23): 2001607. |
| 30 | Jiang Z P, Guo H J, Zeng Z Q, et al. Reconfiguring organosulfur cathode by over-lithiation to enable ultrathick lithium metal anode toward practical lithium-sulfur batteries[J]. ACS Nano, 2020, 14(10): 13784-13793. |
| 31 | Zhang R, Chen B, Shi C S, et al. Decreasing interfacial pitfalls with self-grown sheet-like Li2S artificial solid-electrolyte interphase for enhanced cycling performance of lithium metal anode[J]. Small, 2023, 19(27): e2208095. |
| 32 | Feng G X, Jia H, Shi Y P, et al. Imaging solid-electrolyte interphase dynamics using operando reflection interference microscopy[J]. Nature Nanotechnology, 2023, 18: 780-789. |
| 33 | Liu Y J, Tao X Y, Wang Y, et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries[J]. Science, 2022, 375(6582): 739-745. |
| 34 | Huang K, Song S P, Xue Z Y, et al. In-situ formation of LiF-rich solid-electrolyte interphases on 3D lithiophilic skeleton for stable lithium metal anode[J]. Energy Storage Materials, 2023, 55: 301-311. |
| 35 | Yu K C, Chen J B, Xie X T, et al. Constructing LiF-rich artificial SEI at a two-dimensional copper net current collector in anode-free lithium metal batteries[J]. Surfaces and Interfaces, 2022, 34: 102326. |
| 36 | Li Z D, Huai L Y, Li S, et al. Insight into bulk charge transfer of lithium metal anodes by synergism of nickel seeding and LiF-Li3N-Li2S co-doped interphase[J]. Energy Storage Materials, 2021, 37: 491-500. |
| 37 | Yang J, Hou J M, Fang Z X, et al. Simultaneously in situ fabrication of lithium fluoride and sulfide enriched artificial solid electrolyte interface facilitates high stable lithium metal anode[J]. Chemical Engineering Journal, 2022, 433: 133193. |
| 38 | Zhang D C, Liu Z B, Wu Y W, et al. In situ construction a stable protective layer in polymer electrolyte for ultralong lifespan solid-state lithium metal batteries[J]. Advanced Science, 2022, 9(12): e2104277. |
| 39 | Guan M R, Huang Y X, Meng Q Q, et al. Stabilization of lithium metal interfaces by constructing composite artificial solid electrolyte interface with mesoporous TiO2 and perfluoropolymers[J]. Small, 2022, 18(40): e2202981. |
| 40 | Vilá R A, Boyle D T, Dai A L, et al. LiH formation and its impact on Li batteries revealed by cryogenic electron microscopy[J]. Science Advances, 2023, 9(12): eadf3609. |
| 41 | Tan S, Kim J M, Corrao A, et al. Unravelling the convoluted and dynamic interphasial mechanisms on Li metal anodes[J]. Nature Nanotechnology, 2023, 18: 243-249. |
| 42 | Wu H P, Jia H, Wang C M, et al. Recent progress in understanding solid electrolyte interphase on lithium metal anodes[J]. Advanced Energy Materials, 2021, 11(5): 2003092. |
| 43 | Zhang S M, Yang G J, Liu S, et al. Understanding the dropping of lithium plating potential in carbonate electrolyte[J]. Nano Energy, 2020, 70: 104486. |
| 44 | Tian J X, Hu T P, Xu S Z, et al. Molecular dynamics simulations of the Li-ion diffusion in the amorphous solid electrolyte interphase[J]. Chinese Chemical Letters, 2023, 34(11): 108242. |
| 45 | Jin T, Chen J S, Chen X C, et al. Artificial interphase layers for Li metal anode, what's next?[J]. Next Energy, 2023, 1(3): 100040. |
| 46 | Gao S L, Li Z X, Zhang Z, et al. Constructing a multi-functional polymer network for ultra-stable and safe Li-metal batteries[J]. Energy Storage Materials, 2023, 55: 214-224. |
| 47 | Shen Z C, Cheng Y F, Sun S H, et al. The critical role of inorganic nanofillers in solid polymer composite electrolyte for Li+ transportation[J]. Carbon Energy, 2021, 3(3): 482-508. |
| 48 | Lin Y H, Shen Z C, Huang J Q, et al. In situ construction of fluorine-containing modified gel polymer electrolyte with high interfacial stability for high-rate lithium metal battery[J]. Journal of Power Sources, 2023, 584: 233612. |
| 49 | Sun S, Myeong S, Kim J, et al. Design of inorganic/organic bi-layered Li protection layer enabled dendrite-free practical Li metal battery[J]. Chemical Engineering Journal, 2022, 450: 137993. |
| 50 | Sun X R, Yang S H, Zhang T, et al. Regulating Li-ion flux with a high-dielectric hybrid artificial SEI for stable Li metal anodes[J]. Nanoscale, 2022, 14(13): 5033-5043. |
| 51 | Cao W Z, Lu J Z, Zhou K, et al. Organic-inorganic composite SEI for a stable Li metal anode by in situ polymerization[J]. Nano Energy, 2022, 95: 106983. |
| 52 | Peled E, Golodnitsky D, Ardel G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes[J]. Journal of the Electrochemical Society, 1997, 144(8): L208. |
| 53 | Aurbach D, Markovsky B, Levi M D, et al. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries[J]. Journal of Power Sources, 1999, 81: 95-111. |
| 1 | Shen Z C, Zhong J W, Chen J H, et al. SiO2 nanofiber composite gel polymer electrolyte by in situ polymerization for stable Li metal batteries[J]. Chinese Chemical Letters, 2023, 34(3): 107370. |
| 2 | Lin Y H, Chen J H, Zhu J L, et al. In-situ construction of tetraethylene glycol diacrylate based gel polymer electrolyte for long lifespan lithium metal batteries[J]. Surfaces and Interfaces, 2023, 37: 102737. |
| 3 | Jagger B, Pasta M. Solid electrolyte interphases in lithium metal batteries[J]. Joule, 2023, 7(10): 2228-2244. |
| 4 | Shen Z C, Zhong J W, Jiang S Y, et al. Polyacrylonitrile porous membrane-based gel polymer electrolyte by in situ free-radical polymerization for stable Li metal batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(36): 41022-41036. |
| 54 | Zhou Y F, Su M, Yu X F, et al. Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery[J]. Nature Nanotechnology, 2020, 15: 224-230. |
| 55 | Rikka V R, Sahu S R, Chatterjee A, et al. In situ/ex situ investigations on the formation of the mosaic solid electrolyte interface layer on graphite anode for lithium-ion batteries[J]. The Journal of Physical Chemistry C, 2018, 122(50): 28717-28726. |
| 56 | Li Y Z, Li Y B, Pei A, et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy[J]. Science, 2017, 358(6362): 506-510. |
| 57 | Li Y Z, Huang W, Li Y B, et al. Correlating structure and function of battery interphases at atomic resolution using cryoelectron microscopy[J]. Joule, 2018, 2(10): 2167-2177. |
| 58 | Xu Y B, Wu H P, He Y, et al. Atomic to nanoscale origin of vinylene carbonate enhanced cycling stability of lithium metal anode revealed by cryo-transmission electron microscopy[J]. Nano Letters, 2020, 20(1): 418-425. |
| 59 | Li M H, Zhang Q, Yang X M, et al. Deciphering the mechanism of concentrated electrolyte for lithium metal anode via cryogenic electron microscopy[J]. Journal of Power Sources, 2023, 557: 232543. |
| 60 | Cheng D Y, Wynn T A, Wang X F, et al. Unveiling the stable nature of the solid electrolyte interphase between lithium metal and LiPON via cryogenic electron microscopy[J]. Joule, 2020, 4(11): 2484-2500. |
| 61 | Han B, Li X Y, Bai S, et al. Conformal three-dimensional interphase of Li metal anode revealed by low-dose cryoelectron microscopy[J]. Matter, 2021, 4(11): 3741-3752. |
| 62 | Zhang E, Mecklenburg M, Yuan X T, et al. Expanding the cryogenic electron microscopy toolbox to reveal diverse classes of battery solid electrolyte interphase[J]. iScience, 2022, 25(12): 105689. |
| 63 | Wu L S, Hu J P, Chen S J, et al. Lithium nitrate mediated dynamic formation of solid electrolyte interphase revealed by in situ Fourier transform infrared spectroscopy[J]. Electrochimica Acta, 2023, 466: 142973. |
| 64 | Sun S Y, Yao N, Jin C B, et al. The crucial role of electrode potential of a working anode in dictating the structural evolution of solid electrolyte interphase[J]. Angewandte Chemie International Edition, 2022, 61(42): e202208743. |
| 65 | Weng S T, Zhang X, Yang G J, et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries[J]. Nature Communications, 2023, 14: 4474. |
| 66 | Wang J Y, Huang W, Pei A, et al. Improving cyclability of Li metal batteries at elevated temperatures and its origin revealed by cryo-electron microscopy[J]. Nature Energy, 2019, 4: 664-670. |
| 67 | Xu Y B, Wu H P, Jia H, et al. Current density regulated atomic to nanoscale process on Li deposition and solid electrolyte interphase revealed by cryogenic transmission electron microscopy[J]. ACS Nano, 2020, 14(7): 8766-8775. |
| 68 | Wang M S, Liang H M, Wang C Y, et al. Can we see SEI directly by naked eyes?[J]. Advanced Materials, 2023, 35(51): e2306683. |
| 69 | Chen Y Y, Chen Y X, Wang R, et al. Generation of a highly conductive and stable solid electrolyte interphase at lithium anode under additional electric filed[J]. Chemical Engineering Journal, 2022, 446: 137435. |
| 70 | Qu J L, Liu J J, Leng G R, et al. Overcoming the obstacles of lithium-metal anodes for high-energy batteries[J]. Electrochemistry Communications, 2023, 153: 107537. |
| 71 | Xu R, Cheng X B, Yan C, et al. Artificial interphases for highly stable lithium metal anode[J]. Matter, 2019, 1(2): 317-344. |
| 72 | Yu Z A, Cui Y, Bao Z N. Design principles of artificial solid electrolyte interphases for lithium-metal anodes[J]. Cell Reports Physical Science, 2020, 1: 100119. |
| 73 | Fu X X, Duan H H, Zhang S W, et al. Hexachloro-1, 3-butadiene as a functional additive for constructing an efficient solid electrolyte interface layer for long-life stable Li anodes[J]. ACS Applied Materials & Interfaces, 2022, 14(50): 55709-55718. |
| 74 | Oyakhire S T, Liao S L, Shuchi S B, et al. Proximity matters: interfacial solvation dictates solid electrolyte interphase composition[J]. Nano Letters, 2023, 23(16): 7524-7531. |
| 75 | Zhang S H, Zhuang X C, Du X F, et al. A novel potassium salt regulated solvation chemistry enabling excellent Li-anode protection in carbonate electrolytes[J]. Advanced Materials, 2023, 35(25): e2301312. |
| 76 | Fang W Q, Wen Z X, Chen L, et al. Constructing inorganic-rich solid electrolyte interphase via abundant anionic solvation sheath in commercial carbonate electrolytes[J]. Nano Energy, 2022, 104: 107881. |
| 77 | Li X, Liu J D, He J, et al. Separator-wetted, acid- and water-scavenged electrolyte with optimized Li-ion solvation to form dual efficient electrode electrolyte interphases via hexa-functional additive[J]. Advanced Science, 2022, 9(20): e2201297. |
| 78 | Li C, Liang Z Y, Li Z Z, et al. Self-assembly monolayer inspired stable artificial solid electrolyte interphase design for next-generation lithium metal batteries[J]. Nano Letters, 2023, 23(9): 4014-4022. |
| 79 | Cheng Y F, Wang Z J, Chen J B, et al. Catalytic chemistry derived artificial solid electrolyte interphase for stable lithium metal anodes working at 20 mA·cm-2 and 20 mAh·cm-2 [J]. Angewandte Chemie International Edition, 2023, 62(30): e202305723. |
| 80 | Cheng Z Z, Chen Y, Shi L, et al. Long-lifespan lithium metal batteries enabled by a hybrid artificial solid electrolyte interface layer[J]. ACS Applied Materials & Interfaces, 2023, 15(8): 10585-10592. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [3] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [4] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [5] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [6] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [7] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [8] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [9] | 郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874. |
| [10] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [11] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [12] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [13] | 刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259. |
| [14] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [15] | 宋悦, 张启成, 彭文朝, 李阳, 张凤宝, 范晓彬. MoS2基单原子催化剂的合成及其在电催化中的应用[J]. 化工学报, 2023, 74(2): 535-545. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号