化工学报 ›› 2023, Vol. 74 ›› Issue (3): 1247-1259.DOI: 10.11949/0438-1157.20221413
收稿日期:2022-10-26
修回日期:2023-01-11
出版日期:2023-03-05
发布日期:2023-04-19
通讯作者:
马丽
作者简介:刘瑞琪(1997—),女,硕士研究生,LIU_ruiqi5@163.com
基金资助:
Ruiqi LIU( ), Xitong ZHOU, Yue ZHANG, Ying HE, Jing GAO, Li MA(
), Xitong ZHOU, Yue ZHANG, Ying HE, Jing GAO, Li MA( )
)
Received:2022-10-26
Revised:2023-01-11
Online:2023-03-05
Published:2023-04-19
Contact:
Li MA
摘要:
利用褶皱状二氧化硅纳米花(SiO2 NFs),通过原位还原法制备分散的金纳米颗粒修饰的氨基二氧化硅复合材料(Au@NH2-SiO2 NFs)。利用扫描电镜(SEM)、透射电镜(TEM)、X射线光电子能谱(XPS)、X射线衍射(XRD)、傅里叶红外光谱仪(FT-IR)和元素分析(elemental mapping)对Au@NH2-SiO2 NFs纳米材料进行表征。基于Au@NH2-SiO2 NFs构建AChE/Au@NH2-SiO2 NFs/GCE生物传感器。选择马拉硫磷和毒死蜱两种有机磷农药为代表,考察生物传感器的检测性能,其中马拉硫磷的检测范围是(1.00×10-11)~(1.00×10-5) mol/L,检测限为2.92×10-12 mol/L;毒死蜱的检测范围是(1.00×10-13)~(1.00×10-7) mol/L,检测限为5.60×10-14 mol/L。AChE/Au@NH2-SiO2 NFs/GCE电极在实际样品检测中的回收率在90.7%~107.0%之间,并展现出良好的抗干扰性、储藏稳定性和重现性。
中图分类号:
刘瑞琪, 周栖桐, 张悦, 贺莹, 高静, 马丽. 基于金纳米颗粒修饰二氧化硅纳米花的生物传感器构建及应用[J]. 化工学报, 2023, 74(3): 1247-1259.
Ruiqi LIU, Xitong ZHOU, Yue ZHANG, Ying HE, Jing GAO, Li MA. The construction and application of biosensor based on gold nanoparticles loaded SiO2-nanoflowers[J]. CIESC Journal, 2023, 74(3): 1247-1259.
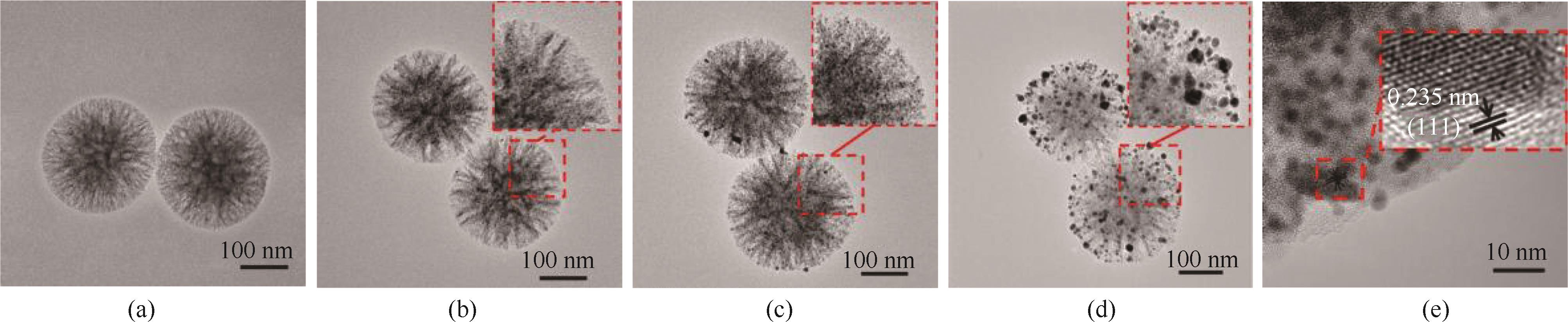
图1 SiO2 NF (a)、Au-15@NH2-SiO2 NFs (b)、Au-30@NH2-SiO2 NFs (c)和Au-45@NH2-SiO2 NFs (d)的透射电镜图;Au-30@NH2-SiO2 NFs的高倍透射电镜图(e)
Fig.1 TEM images of SiO2 NF (a), Au-15@NH2-SiO2 NFs (b), Au-30@NH2-SiO2 NFs (c) , and Au-45@NH2-SiO2 NFs (d); HRTEM image of Au-30@NH2-SiO2 NFs (e)
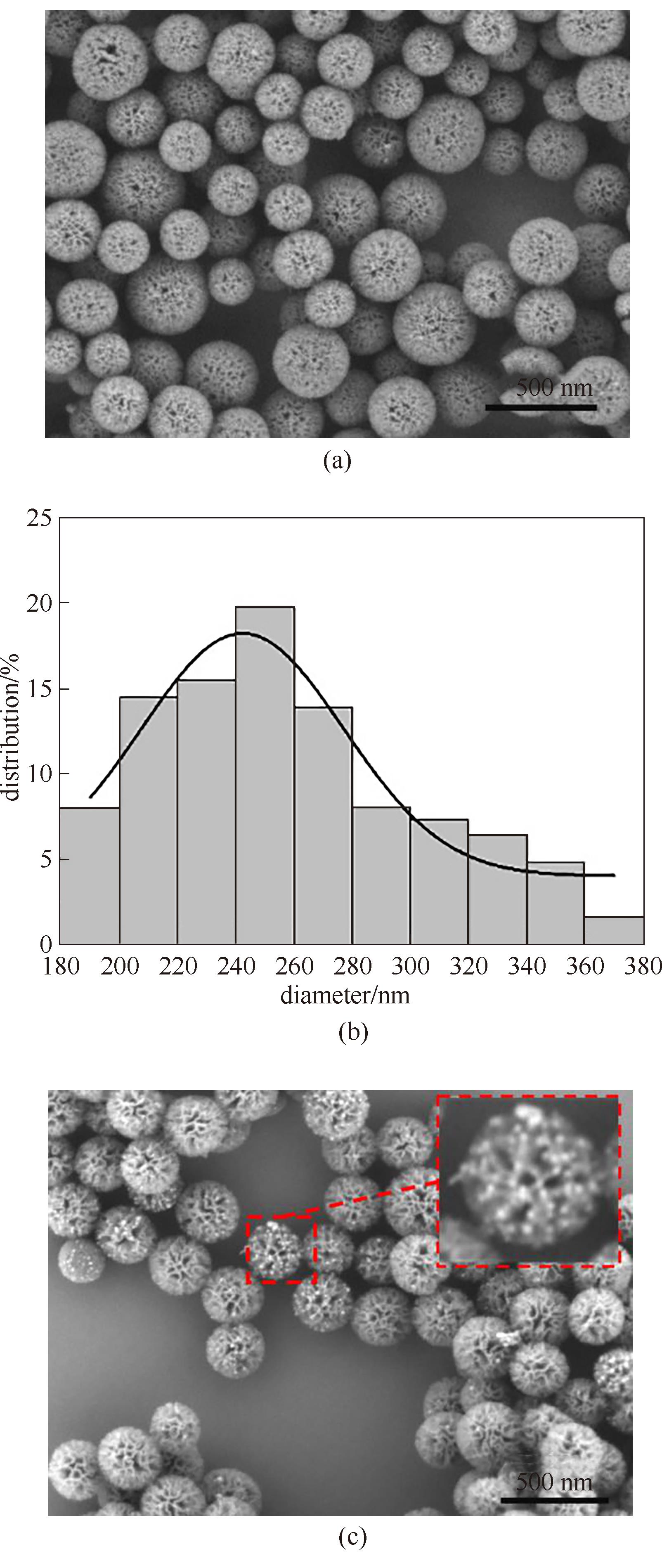
图2 SiO2 NFs的扫描电镜图(a)、粒径分布(b);Au-30@NH2-SiO2 NFs的扫描电镜图(c)
Fig.2 SEM image (a) and particle size distribution (b) of SiO2 NFs; SEM image of Au-30@NH2-SiO2 NFs (c)
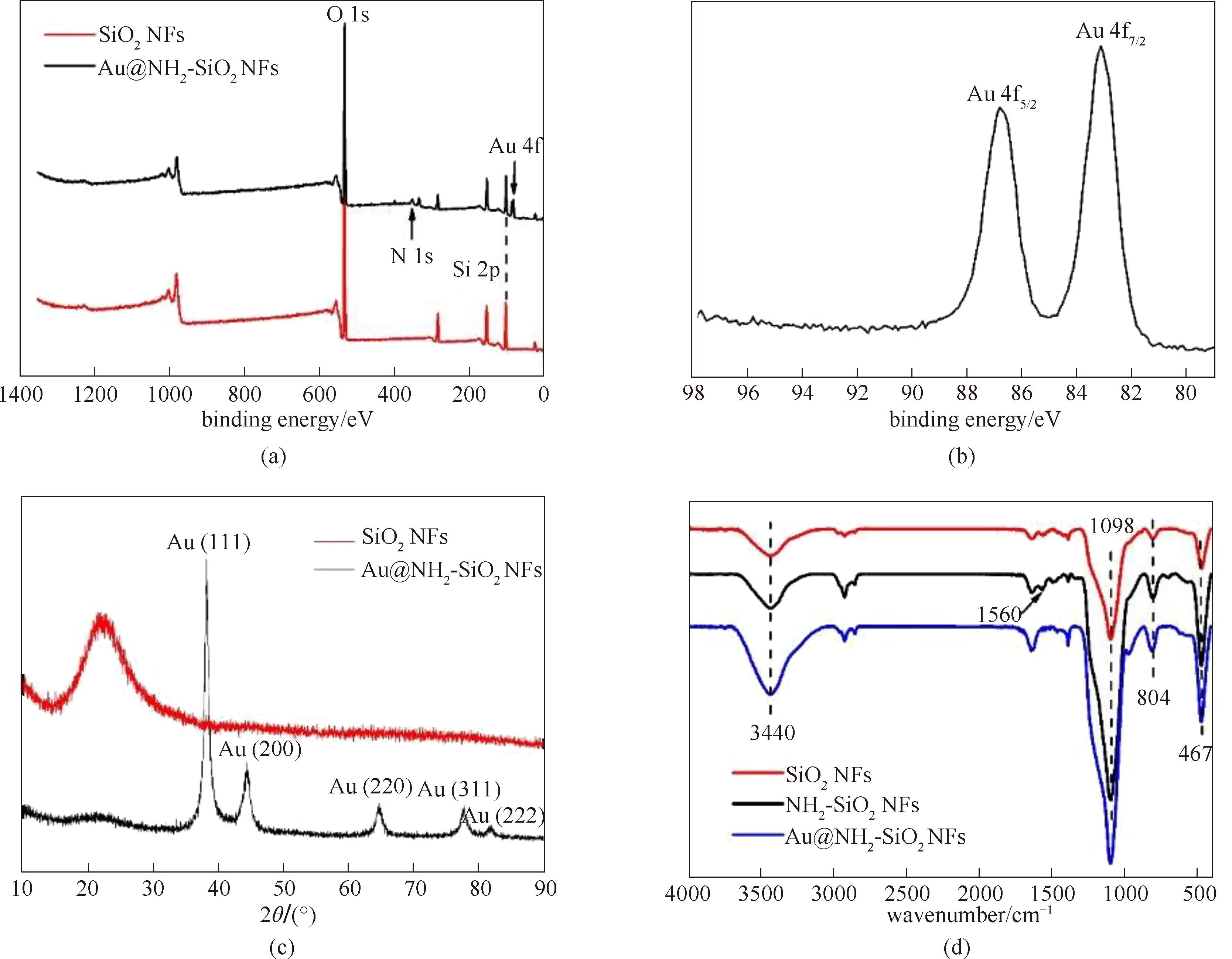
图5 SiO2 NFs和Au@NH2-SiO2 NFs的XPS全波长谱图(a);Au 4f的高分辨谱图(b);SiO2 NFs和Au@NH2-SiO2 NFs的XRD衍射谱图(c);SiO2 NFs、NH2-SiO2 NFs和Au@NH2-SiO2 NFs的红外谱图(d)
Fig.5 XPS spectra of the SiO2 NFs and Au@NH2-SiO2 NFs (a); the Au 4f core-level peaks of the Au@NH2-SiO2 NFs (b); wide-angle XRD patterns of the SiO2 NFs and Au@NH2-SiO2 NFs (c); FT-IR spectra of SiO2 NFs, NH2-SiO2 NFs, and Au@NH2-SiO2 NFs (d)
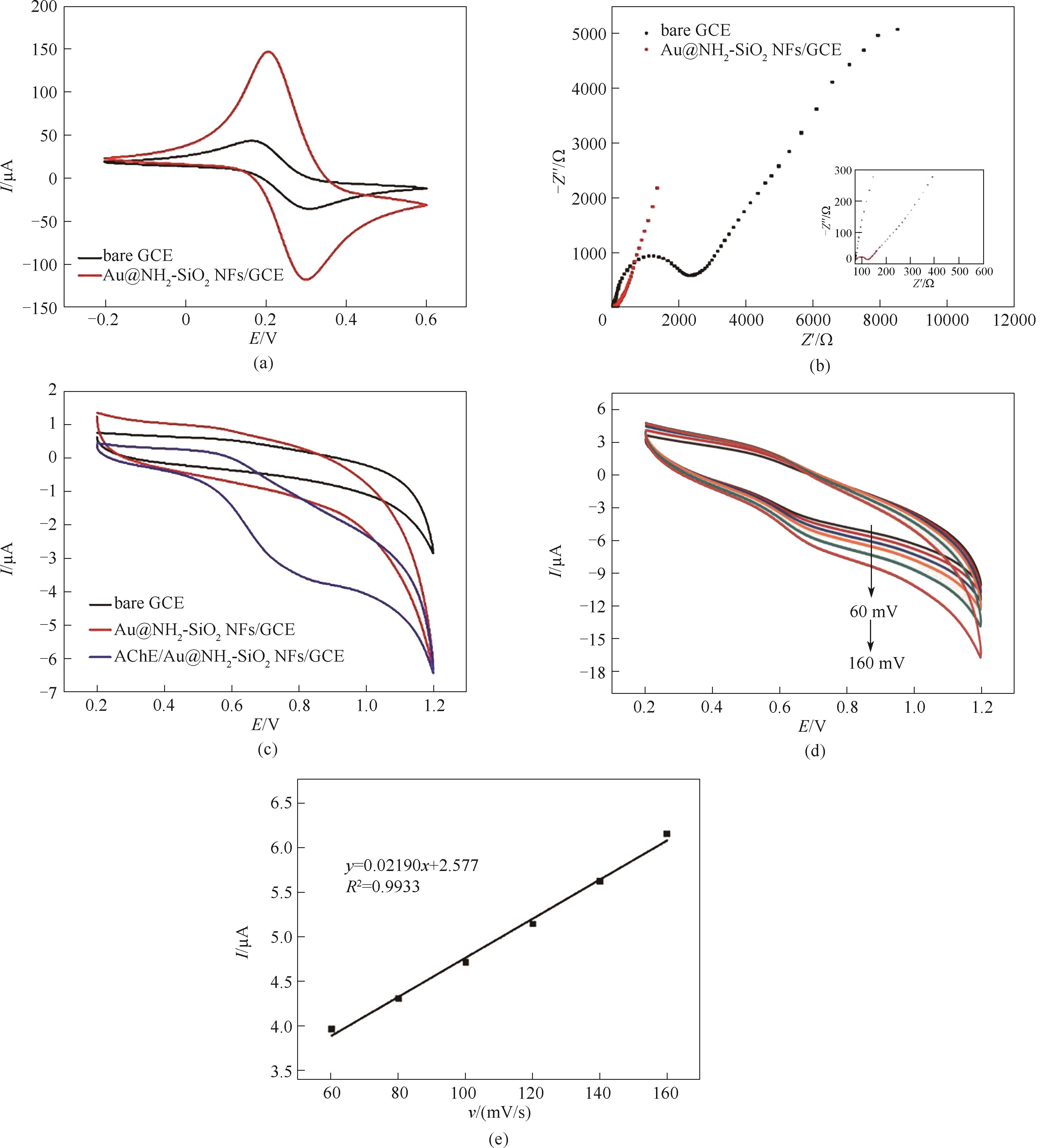
图6 空白电极和Au@NH2-SiO2 NFs/GCE的循环伏安图(扫速:50 mV/s) (a)和交流阻抗图(b);空白电极、Au@NH2-SiO2 NFs/GCE和AChE/Au@NH2-SiO2 NFs/GCE的循环伏安图(扫速:50 mV/s) (c);AChE/Au@NH2-SiO2 NFs/GCE不同扫速下(60~160 mV/s)的循环伏安图(d);氧化峰电流值和扫速的线性关系(e)
Fig.6 CV curves at a scan rate of 50 mV/s (a) and Nyquist plots (b) of the bare GCE and Au@NH2-SiO2 NFs/GCE; CV responses from the bare GCE, Au@NH2-SiO2 NFs/GCE, and AChE/Au@NH2-SiO2 NFs/GCE at a scan rate of 50 mV/s (c); CV curves of AChE/Au@NH2-SiO2 NFs/GCE at 60—160 mV/s (d); plots of oxidation peak current vs scan rate (e)
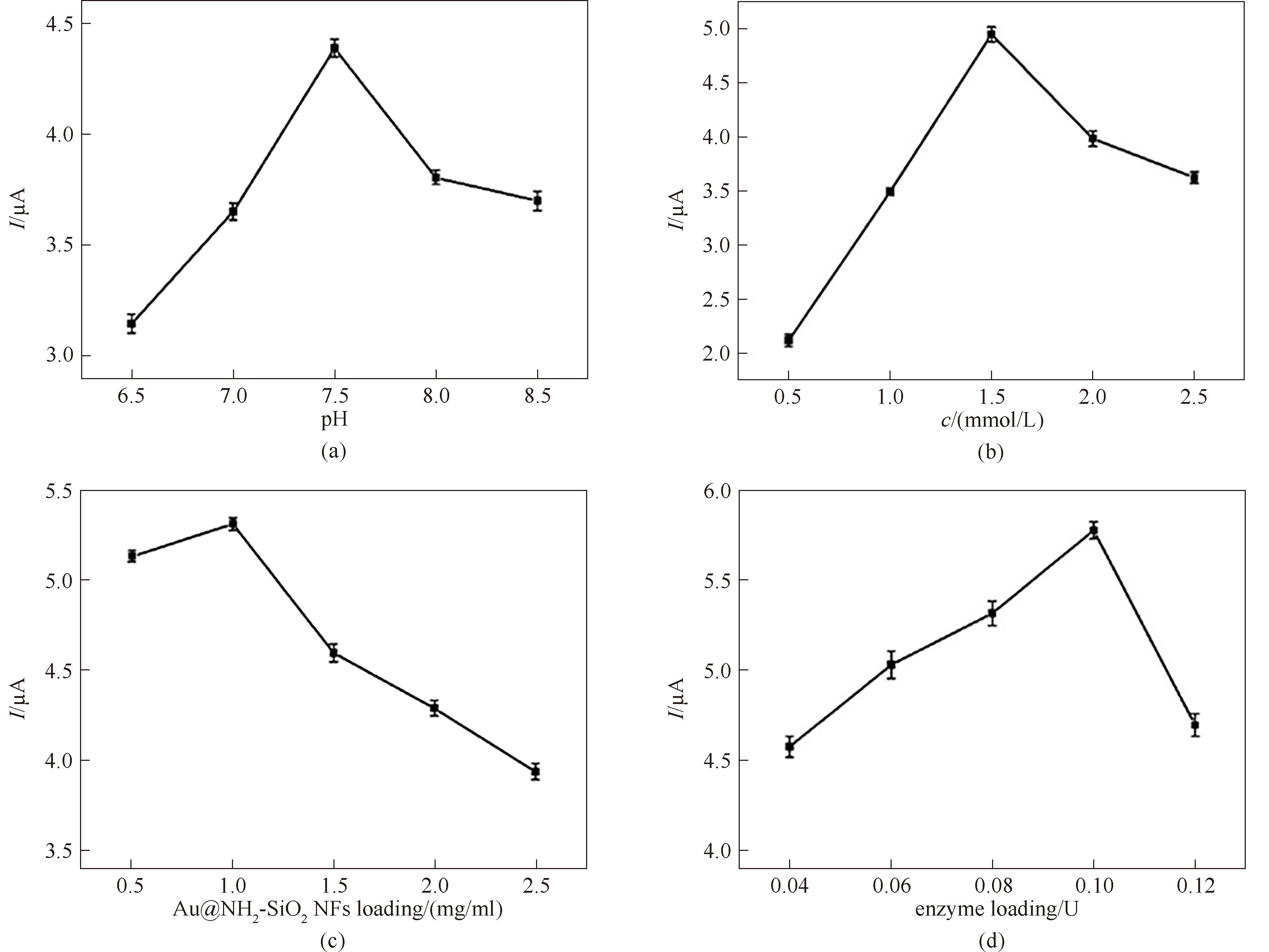
图7 溶液pH(a)、ATCl浓度(b)、Au@NH2-SiO2 NFs修饰量(c)和AChE负载量(d)对氧化峰电流值的影响
Fig.7 The effects of pH of PBS (a), concentration of ATCl (b), amount of Au@NH2-SiO2 NFs (c), and amount of AChE (d) on oxidation peak currents of ATCl
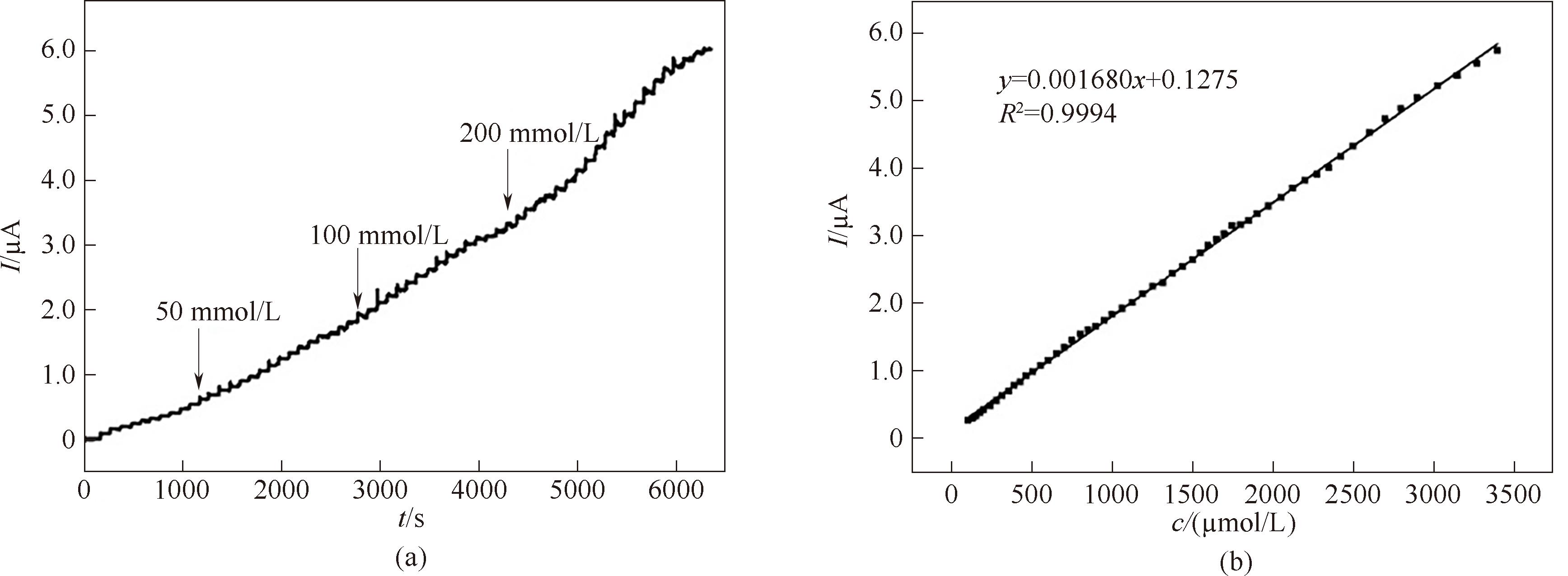
图8 AChE/Au@NH2-SiO2 NFs/GCE的时间-电流曲线(a)和ATCl浓度与电流的工作曲线(b)
Fig.8 Current-time plot of AChE/Au@NH2-SiO2 NFs/GCE (a) and calibration curves for ATCl determination (b)

图9 马拉硫磷(a)和毒死蜱(b)溶液中抑制不同时间对抑制率的影响;AChE/Au@NH2-SiO2 NFs/GCE 在不同浓度的马拉硫磷(c)和毒死蜱(d)溶液中的DPV曲线;马拉硫磷(e)和毒死蜱(f)溶液浓度与抑制率关系
Fig.9 Effect of incubation time on inhibition of AChE/Au@NH2-SiO2 NFs/GCE biosensor after inhibition with malathion (a) and chlopyrifos (b); DPV of AChE/Au@NH2-SiO2 NFs/GCE after inhibition with various concentrations of malathion for 500 s (c) and chlopyrifos for 300 s (d); the relationship between inhibition and concentration of malathion (e) and chlopyrifos (f) malathion concentration (0)—(7): 0, 1.00×10-11, 1.00×10-10, 1.00×10-9, 1.00×10-8, 1.00×10-7, 1.00×10-6, and 1.00×10-5 mol/L (c); chlopyrifos concentration (0)—(7): 0, 1.00×10-13, 1.00×10-12, 1.00×10-11, 1.00×10-10, 1.00×10-9, 1.00×10-8, and 1.00×10-7 mol/L (d)
| 检测目标 | 电极 | 检测范围/(mol/L) | 检测限/(mol/L) | 文献 |
|---|---|---|---|---|
| 马拉硫磷 | AChE-CS/3DG-CuO NFs/GCE | (4.67×10-12)~(3.00×10-8) | 9.20×10-11 | [ |
| poly(FBThF)/Ag-rGO-NH2/AChE/GCE | (3.00×10-10)~(3.00×10-8) | 9.69×10-11 | [ | |
| AChE-CS/rGO-TEPA-CuO NWs/GCE | (3.00×10-12)~(6.00×10-8) | 1.20×10-12 | [ | |
| AChE/COF@MWCNTs/ GCE | (1.00×10-9)~(1.00×10-5) | 5.00×10-10 | [ | |
| AChE/Au@NH2-SiO2 NFs/GCE | (1.00×10-11)~(1.00×10-5) | 2.92×10-12 | 本文 | |
| 毒死蜱 | AChE/CHIT-SnS2/GCE | (2.00×10-11)~(1.00×10-5) | 2.00×10-11 | [ |
| AChE/OMC-CS/ATO-CS/SPCE | (2.85×10-11)~(2.85×10-6) | 2.85×10-11 | [ | |
| AChE-CS/GP-AuNP-PEDOT:PSS/SPCE | (1.00×10-10)~(1.00×10-8) | 7.00×10-11 | [ | |
| CD-AChE/GO | (7.10×10-9)~(2.85×10-7) | 4.10×10-10 | [ | |
| BSA/AChE/GA/CIS/rGO-9/SPCE | (1.42×10-9)~(1.34×10-6) | 6.56×10-11 | [ | |
| AChE/Au@NH2-SiO2 NFs/GCE | (1.00×10-13)~(1.00×10-7) | 5.60×10-14 | 本文 |
表1 本文中生物传感器对有机磷农药的检测性能与相关文献的比较
Table 1 Comparison of the analytical characteristics of the AChE electrochemical biosensors for OPs
| 检测目标 | 电极 | 检测范围/(mol/L) | 检测限/(mol/L) | 文献 |
|---|---|---|---|---|
| 马拉硫磷 | AChE-CS/3DG-CuO NFs/GCE | (4.67×10-12)~(3.00×10-8) | 9.20×10-11 | [ |
| poly(FBThF)/Ag-rGO-NH2/AChE/GCE | (3.00×10-10)~(3.00×10-8) | 9.69×10-11 | [ | |
| AChE-CS/rGO-TEPA-CuO NWs/GCE | (3.00×10-12)~(6.00×10-8) | 1.20×10-12 | [ | |
| AChE/COF@MWCNTs/ GCE | (1.00×10-9)~(1.00×10-5) | 5.00×10-10 | [ | |
| AChE/Au@NH2-SiO2 NFs/GCE | (1.00×10-11)~(1.00×10-5) | 2.92×10-12 | 本文 | |
| 毒死蜱 | AChE/CHIT-SnS2/GCE | (2.00×10-11)~(1.00×10-5) | 2.00×10-11 | [ |
| AChE/OMC-CS/ATO-CS/SPCE | (2.85×10-11)~(2.85×10-6) | 2.85×10-11 | [ | |
| AChE-CS/GP-AuNP-PEDOT:PSS/SPCE | (1.00×10-10)~(1.00×10-8) | 7.00×10-11 | [ | |
| CD-AChE/GO | (7.10×10-9)~(2.85×10-7) | 4.10×10-10 | [ | |
| BSA/AChE/GA/CIS/rGO-9/SPCE | (1.42×10-9)~(1.34×10-6) | 6.56×10-11 | [ | |
| AChE/Au@NH2-SiO2 NFs/GCE | (1.00×10-13)~(1.00×10-7) | 5.60×10-14 | 本文 |
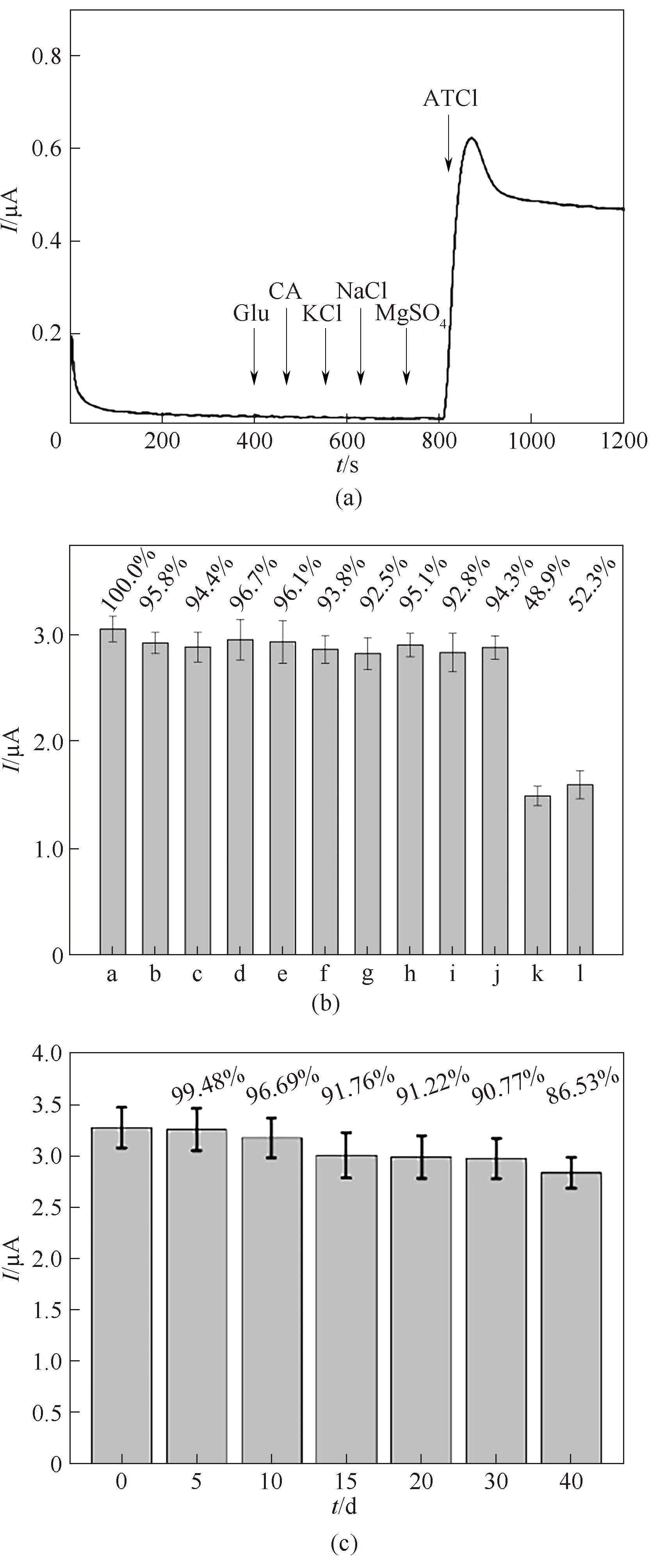
图10 不同干扰物对底物ATCl电流值的影响(a);不同干扰物对测定毒死蜱氧化峰电流值的影响(b);AChE/Au@NH2-SiO2 NFs/GCE储存稳定性测定(c)a—the absence of substances; b—1 mmol/L of glucose; c—1 mmol/L of citric acid; d—1 mmol/L of Cl-; e—1 mmol/L of PO43-; f—1 mmol/L of SO42-; g—1 mmol/L of Na+; h—1 mmol/L of Mg2+;i—1 mmol/L of K+; j—1×10-9 mmol/L kelthane; k—1×10-9 mmol/L parathion methyl; l—1×10-9 mmol/L carbaryl
Fig.10 Influence of different interfering substances on the amperometric response to ATCl (a); Influence of different interfering substances on oxidation peak current of chlorpyrifos (b); the storage stability of AChE/Au@NH2-SiO2 NFs/GCE (c)
| 样品编号 | 加入浓度/(mol/L) | 检出浓度/(mol/L) | 回收率/% | 相对标准偏差 (n=3) |
|---|---|---|---|---|
| 1# | 1.0×10-10 | 0.91×10-10 | 90.7 | 2.8 |
| 2# | 1.0×10-8 | 0.98×10-8 | 98.1 | 3.1 |
| 3# | 1.0×10-6 | 1.1×10-6 | 107.0 | 4.2 |
表2 实际样品中毒死蜱的检测
Table 2 Detection of chlopyrifos in spiked samples using AChE/Au@NH2-SiO2 NFs/GCE
| 样品编号 | 加入浓度/(mol/L) | 检出浓度/(mol/L) | 回收率/% | 相对标准偏差 (n=3) |
|---|---|---|---|---|
| 1# | 1.0×10-10 | 0.91×10-10 | 90.7 | 2.8 |
| 2# | 1.0×10-8 | 0.98×10-8 | 98.1 | 3.1 |
| 3# | 1.0×10-6 | 1.1×10-6 | 107.0 | 4.2 |
| 1 | Huang Y Z, Luo X F, Li Z L. Substitution or complementarity: why do rice farmers use a mix of biopesticides and chemical pesticides in China? [J]. Pest Management Science, 2022, 78(4): 1630-1639. |
| 2 | Chio E H, Li Q X. Pesticide research and development: general discussion and spinosad case[J]. Journal of Agricultural and Food Chemistry, 2022, 70(29): 8913-8919. |
| 3 | Cao J, Wang M, Yu H, et al. An overview on the mechanisms and applications of enzyme inhibition-based methods for determination of organophosphate and carbamate pesticides[J]. Journal of Agricultural and Food Chemistry, 2020, 68(28): 7298-7315. |
| 4 | Sette K N, Alugubelly N, Glenn L B, et al. The mechanistic basis for the toxicity difference between juvenile rats and mice following exposure to the agricultural insecticide chlorpyrifos[J]. Toxicology, 2022, 480: 153317. |
| 5 | Zhu J B, Wang J, Ding Y, et al. A systems-level approach for investigating organophosphorus pesticide toxicity[J]. Ecotoxicology and Environmental Safety, 2018, 149: 26-35. |
| 6 | Kou J, Li X, Zhang M Y, et al. Accumulative levels, temporal and spatial distribution of common chemical pollutants in the blood of Chinese adults[J]. Environmental Pollution, 2022, 311: 119980. |
| 7 | Sinha S N, Kumpati R K, Ramavath P N, et al. Investigation of acute organophosphate poisoning in humans based on sociodemographic and role of neurotransmitters with survival study in South India[J]. Scientific Reports, 2022, 12: 16513. |
| 8 | Alex A V, Deosarkar T, Chandrasekaran N, et al. An ultra-sensitive and selective AChE based colorimetric detection of malathion using silver nanoparticle-graphene oxide (Ag-GO) nanocomposite[J]. Analytica Chimica Acta, 2021, 1142: 73-83. |
| 9 | Jin R, Xing Z H, Kong D S, et al. Sensitive colorimetric sensor for point-of-care detection of acetylcholinesterase using cobalt oxyhydroxide nanoflakes[J]. Journal of Materials Chemistry. B, 2019, 7(8): 1230-1237. |
| 10 | Ray S, Biswas R, Banerjee R, et al. A gold nanoparticle-intercalated mesoporous silica-based nanozyme for the selective colorimetric detection of dopamine[J]. Nanoscale Advances, 2019, 2(2): 734-745. |
| 11 | Lu Q, Lin N, Cheng X M, et al. Simultaneous determination of 16 urinary metabolites of organophosphate flame retardants and organophosphate pesticides by solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry[J]. Chemosphere, 2022, 300: 134585. |
| 12 | 毛金竹, 肖淑玲, 杨智淳, 等. 合成生物学在农残检测领域的应用[J]. 化工学报, 2021, 72(5): 2413-2425. |
| Mao J Z, Xiao S L, Yang Z C, et al. Application of synthetic biology in pesticides residues detection[J]. CIESC Journal, 2021, 72(5): 2413-2425. | |
| 13 | 阮云飞. 果蔬中18种有机磷农药残留检测方法的探索与研究[J]. 中国食品, 2021(4): 108-109. |
| Ruan Y F. Exploration and study on detection methods of 18 organophosphorus pesticide residues in fruits and vegetables[J]. China Food, 2021(4): 108-109. | |
| 14 | Hernández F, Pozo O J, Sancho J V, et al. Multiresidue liquid chromatography tandem mass spectrometry determination of 52 non gas chromatography-amenable pesticides and metabolites in different food commodities[J]. Journal of Chromatography A, 2006, 1109(2): 242-252. |
| 15 | Vyas T, Singh V, Kodgire P, et al. Insights in detection and analysis of organophosphates using organophosphorus acid anhydrolases (OPAA) enzyme-based biosensors[J]. Critical Reviews in Biotechnology, 2022, DOI: 10.1080/07388551.2022.2052012 . |
| 16 | Yang Y J, Wang S Q, Wen H M, et al. Nanoporous gold embedded ZIF composite for enhanced electrochemical nitrogen fixation[J]. Angewandte Chemie (International Ed. in English), 2019, 58(43): 15362-15366. |
| 17 | Liu C P, Chen K C, Su C F, et al. Revealing the active site of gold nanoparticles for the peroxidase-like activity: the determination of surface accessibility[J]. Catalysts, 2019, 9(6): 517. |
| 18 | Yang X, Yang M X, Pang B, et al. Gold nanomaterials at work in biomedicine[J]. Chemical Reviews, 2015, 115(19): 10410-10488. |
| 19 | Gao H X, Cao Y, Chen Y, et al. Au nanoparticle-decorated NiCo2O4 nanoflower with enhanced electrocatalytic activity toward methanol oxidation[J]. Journal of Alloys and Compounds, 2018, 732: 460-469. |
| 20 | Gu X, Xu Z X, Gu L P, et al. Preparation and antibacterial properties of gold nanoparticles: a review[J]. Environmental Chemistry Letters, 2021, 19(1): 167-187. |
| 21 | Baek S H, Roh J, Park C Y, et al. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection[J]. Materials Science & Engineering. C, Materials for Biological Applications, 2020, 107: 110273. |
| 22 | Zhao L R, Ren X L, Zhang J, et al. Dendritic silica with carbon dots and gold nanoclusters for dual nanozymes[J]. New Journal of Chemistry, 2020, 44(5): 1988-1992. |
| 23 | Lin Y H, Li Z H, Chen Z W, et al. Mesoporous silica-encapsulated gold nanoparticles as artificial enzymes for self-activated cascade catalysis[J]. Biomaterials, 2013, 34(11): 2600-2610. |
| 24 | Gao J, Kong W X, Zhou L Y, et al. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization[J]. Chemical Engineering Journal, 2017, 309: 70-79. |
| 25 | Li Y X, Cox J T, Zhang B. Electrochemical responses and electrocatalysis at single Au nanoparticles[J]. Journal of the American Chemical Society, 2010, 132(9): 3047-3054. |
| 26 | Sun J F, Xu Z Q, Li W F, et al. Effect of nano-SiO2 on the early hydration of alite-sulphoaluminate cement[J]. Nanomaterials, 2017, 7(5): 102. |
| 27 | Sun J Y, Gan T, Zhai R, et al. Sensitive and selective electrochemical sensor of diuron against indole-3-acetic acid based on core-shell structured SiO2@Au particles[J]. Ionics, 2018, 24(8): 2465-2472. |
| 28 | Fatimah I, Prakoso N I, Sahroni I, et al. Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves[J]. Heliyon, 2019, 5(11): e02766. |
| 29 | Sun W Z, Yang W Y, Gao S, et al. Elevated N2 selectivity in catalytic denitrification by amino group-assisted in-situ buffering effect of NH2-SiO2 supported PdCu bimetallic nanocatalyst[J]. Chemical Engineering Journal, 2020, 390: 124617. |
| 30 | Zhang T T, Song Y, Xing Y, et al. The synergistic effect of Au-COF nanosheets and artificial peroxidase Au@ZIF-8(NiPd) rhombic dodecahedra for signal amplification for biomarker detection[J]. Nanoscale, 2019, 11(42): 20221-20227. |
| 31 | Wang B, Li Y R, Hu H Y, et al. Acetylcholinesterase electrochemical biosensors with graphene-transition metal carbides nanocomposites modified for detection of organophosphate pesticides[J]. PLoS One, 2020, 15(4): e0231981. |
| 32 | Cheng J Y, Wang X D, Nie T Y, et al. A novel electrochemical sensing platform for detection of dopamine based on gold nanobipyramid/multi-walled carbon nanotube hybrids[J]. Analytical and Bioanalytical Chemistry, 2020, 412(11): 2433-2441. |
| 33 | Zhang Q Q, Xu Q C, Guo Y M, et al. Acetylcholinesterase biosensor based on the mesoporous carbon/ferroferric oxide modified electrode for detecting organophosphorus pesticides[J]. RSC Advances, 2016, 6(29): 24698-24703. |
| 34 | Fenoy G E, Marmisollé W A, Azzaroni O, et al. Acetylcholine biosensor based on the electrochemical functionalization of graphene field-effect transistors[J]. Biosensors and Bioelectronics, 2020, 148: 111796. |
| 35 | Pundir C S, Malik A, Preety. Bio-sensing of organophosphorus pesticides: a review[J]. Biosensors and Bioelectronics, 2019, 140: 111348. |
| 36 | Sharma K, Kaur M, Rattan G, et al. Effective biocatalyst developed via genipin mediated acetylcholinesterase immobilization on rice straw derived cellulose nanofibers for detection and bioremediation of organophosphorus pesticide[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 640: 128484. |
| 37 | Bao J, Huang T, Wang Z N, et al. 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides[J]. Sensors and Actuators B: Chemical, 2019, 279: 95-101. |
| 38 | Zhang P, Sun T T, Rong S Z, et al. A sensitive amperometric AChE-biosensor for organophosphate pesticides detection based on conjugated polymer and Ag-rGO-NH2 nanocomposite[J]. Bioelectrochemistry, 2019, 127: 163-170. |
| 39 | Li S, Qu L M, Wang J F. Acetylcholinesterase based rGO-TEPA-copper nanowires biosensor for detecting malathion[J]. International Journal of Electrochemical Science, 2020, 15: 505-514. |
| 40 | Wang X, Yang S, Shan J J, et al. Novel electrochemical acetylcholinesterase biosensor based on core-shell covalent organic framework@multi-walled carbon nanotubes (COF@MWCNTs) composite for detection of malathion[J]. International Journal of Electrochemical Science, 2022, 17(5): 220543. |
| 41 | Liu X K, Sakthivel R, Liu W C, et al. Ultra-highly sensitive organophosphorus biosensor based on chitosan/tin disulfide and British housefly acetylcholinesterase[J]. Food Chemistry, 2020, 324: 126889. |
| 42 | Hou W J, Zhang Q Q, Dong H W, et al. Acetylcholinesterase biosensor modified with ATO/OMC for detecting organophosphorus pesticides[J]. New Journal of Chemistry, 2019, 43(2): 946-952. |
| 43 | Theansun W, Sriprachuabwong C, Chuenchom L, et al. Acetylcholinesterase modified inkjet-printed graphene/gold nanoparticle/poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) hybrid electrode for ultrasensitive chlorpyrifos detection[J]. Bioelectrochemistry, 2023, 149: 108305. |
| 44 | Gaviria M I, Barrientos K, Arango J P, et al. Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots-graphene oxide quenching test for analytical and commercial organophosphate pesticide detection[J]. Frontiers in Environmental Science, 2022, 10: 825112. |
| 45 | Itsoponpan T, Thanachayanont C, Hasin P. Sponge-like CuInS2 microspheres on reduced graphene oxide as an electrocatalyst to construct an immobilized acetylcholinesterase electrochemical biosensor for chlorpyrifos detection in vegetables[J]. Sensors and Actuators B: Chemical, 2021, 337: 129775. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [3] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [4] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [5] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [6] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [7] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [8] | 张澳, 罗英武. 低模量、高弹性、高剥离强度丙烯酸酯压敏胶[J]. 化工学报, 2023, 74(7): 3079-3092. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| [11] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [12] | 崔张宁, 胡紫璇, 吴雷, 周军, 叶干, 刘田田, 张秋利, 宋永辉. 可降解纤维素基材料的耐水性能研究进展[J]. 化工学报, 2023, 74(6): 2296-2307. |
| [13] | 李振, 张博, 王丽伟. PEG-EG固-固相变材料的制备和性能研究[J]. 化工学报, 2023, 74(6): 2680-2688. |
| [14] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [15] | 代佳琳, 毕唯东, 雍玉梅, 陈文强, 莫晗旸, 孙兵, 杨超. 热物性对混合型CPCMs固液相变特性影响模拟研究[J]. 化工学报, 2023, 74(5): 1914-1927. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号