化工学报 ›› 2024, Vol. 75 ›› Issue (6): 2243-2251.DOI: 10.11949/0438-1157.20240083
张广宇1( ), 付然飞2, 孙冰1, 袁俊聪2, 冯翔2(
), 付然飞2, 孙冰1, 袁俊聪2, 冯翔2( ), 杨朝合2, 徐伟1(
), 杨朝合2, 徐伟1( )
)
收稿日期:2024-01-17
修回日期:2024-02-27
出版日期:2024-06-25
发布日期:2024-07-03
通讯作者:
冯翔,徐伟
作者简介:张广宇(1990—),男,博士,副研究员,zhanggy.qday@sinopec.com
基金资助:
Guangyu ZHANG1( ), Ranfei FU2, Bing SUN1, Juncong YUAN2, Xiang FENG2(
), Ranfei FU2, Bing SUN1, Juncong YUAN2, Xiang FENG2( ), Chaohe YANG2, Wei XU1(
), Chaohe YANG2, Wei XU1( )
)
Received:2024-01-17
Revised:2024-02-27
Online:2024-06-25
Published:2024-07-03
Contact:
Xiang FENG, Wei XU
摘要:
“双碳”背景下,CO2的综合利用迎来了挑战与机遇,将CO2转化为高附加值化学品对于节能减排和碳循环利用具有重要意义。其中,将CO2与环氧化合物经过环加成反应转化为环状碳酸酯是碳资源循环利用的重要方式之一。研究了环氧丙烷(PO)在以四丁基溴化铵(TBABr)为催化剂的均相体系中氢键供体效应对环加成反应的影响,探究了不同碳数醇类以及酚类极性对反应的影响。研究发现,在温和条件(100℃、2 h、1.5 MPa)下,以邻苯二酚为氢键供体时,PO的转化率可以达到99.8%,碳酸丙烯酯(PC)选择性可以达到99.51%,性能远远高于无氢键供体体系。此外,通过建立线性溶剂化能关系式以及Kamlet-Taft表达式,定量描述了氢键供体(醇类、酚类)极性参数对反应性能的影响,阐明了氢键供体对提高CO2-PO环加成反应效率的影响。
中图分类号:
张广宇, 付然飞, 孙冰, 袁俊聪, 冯翔, 杨朝合, 徐伟. CO2-环氧丙烷合成碳酸丙烯酯:氢键供体效应研究[J]. 化工学报, 2024, 75(6): 2243-2251.
Guangyu ZHANG, Ranfei FU, Bing SUN, Juncong YUAN, Xiang FENG, Chaohe YANG, Wei XU. Synthesis of propylene carbonate from CO2 and propylene oxide: hydrogen bond activation strategy[J]. CIESC Journal, 2024, 75(6): 2243-2251.
| 催化剂名称 | 氢键供体类型 | 环氧丙烷 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸 丙烯酯 | 1,2- 丙二醇 | |||
| TBABr | — | 49.82 | 98.68 | 1.32 |
| CH3CH2OH | 53.63 | 100.00 | 0 | |
| CH3CH2CH2OH | 53.89 | 100.00 | 0 | |
| CH3(CH2)3OH | 57.46 | 100.00 | 0 | |
| CH3(CH2)5OH | 97.10 | 98.32 | 1.68 | |
| CH3(CH2)9OH | 94.12 | 98.83 | 1.17 | |
表1 不同氢键供体对环氧丙烷环加成反应的影响
Table 1 Effect of different hydrogen bond donors on propylene oxide
| 催化剂名称 | 氢键供体类型 | 环氧丙烷 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸 丙烯酯 | 1,2- 丙二醇 | |||
| TBABr | — | 49.82 | 98.68 | 1.32 |
| CH3CH2OH | 53.63 | 100.00 | 0 | |
| CH3CH2CH2OH | 53.89 | 100.00 | 0 | |
| CH3(CH2)3OH | 57.46 | 100.00 | 0 | |
| CH3(CH2)5OH | 97.10 | 98.32 | 1.68 | |
| CH3(CH2)9OH | 94.12 | 98.83 | 1.17 | |
催化剂 名称 | 氢键供体类型 | 环氧丙烷转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸丙烯酯 | 1,2-丙二醇 | |||
| TBABr | 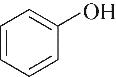 | 99.30 | 97.93 | 2.07 |
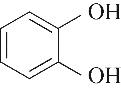 | 99.80 | 99.51 | 0.49 | |
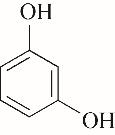 | 96.68 | 99.52 | 0.48 | |
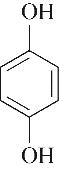 | 98.19 | 97.00 | 3.00 | |
 | 95.86 | 97.72 | 2.28 | |
表2 不同氢键供体对环氧丙烷环加成反应的影响
Table 2 Effect of different hydrogen bond donors on propylene oxide
催化剂 名称 | 氢键供体类型 | 环氧丙烷转化率/% | 选择性/% | |
|---|---|---|---|---|
| 碳酸丙烯酯 | 1,2-丙二醇 | |||
| TBABr |  | 99.30 | 97.93 | 2.07 |
 | 99.80 | 99.51 | 0.49 | |
 | 96.68 | 99.52 | 0.48 | |
 | 98.19 | 97.00 | 3.00 | |
 | 95.86 | 97.72 | 2.28 | |
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
| CH3CH2OH | 51.90 | 0.66 | 0.86 | 0.75 | 0.54 | 53.63 |
| CH3CH2CH2OH | 50.70 | 0.62 | 0.84 | 0.90 | 0.52 | 53.89 |
| CH3(CH2)3OH | 50.20 | 0.60 | 0.84 | 0.84 | 0.47 | 57.46 |
| CH3(CH2)5OH | 48.80 | 0.56 | 0.80 | 0.84 | 0.40 | 97.10 |
| CH3(CH2)9OH | 47.60 | 0.53 | 0.70 | 0.82 | 0.45 | 94.12 |
表3 醇类Kamlet-Taft参数[28-30]
Table 3 Kamlet-Taft parameters of alcohol[28-30]
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
| CH3CH2OH | 51.90 | 0.66 | 0.86 | 0.75 | 0.54 | 53.63 |
| CH3CH2CH2OH | 50.70 | 0.62 | 0.84 | 0.90 | 0.52 | 53.89 |
| CH3(CH2)3OH | 50.20 | 0.60 | 0.84 | 0.84 | 0.47 | 57.46 |
| CH3(CH2)5OH | 48.80 | 0.56 | 0.80 | 0.84 | 0.40 | 97.10 |
| CH3(CH2)9OH | 47.60 | 0.53 | 0.70 | 0.82 | 0.45 | 94.12 |
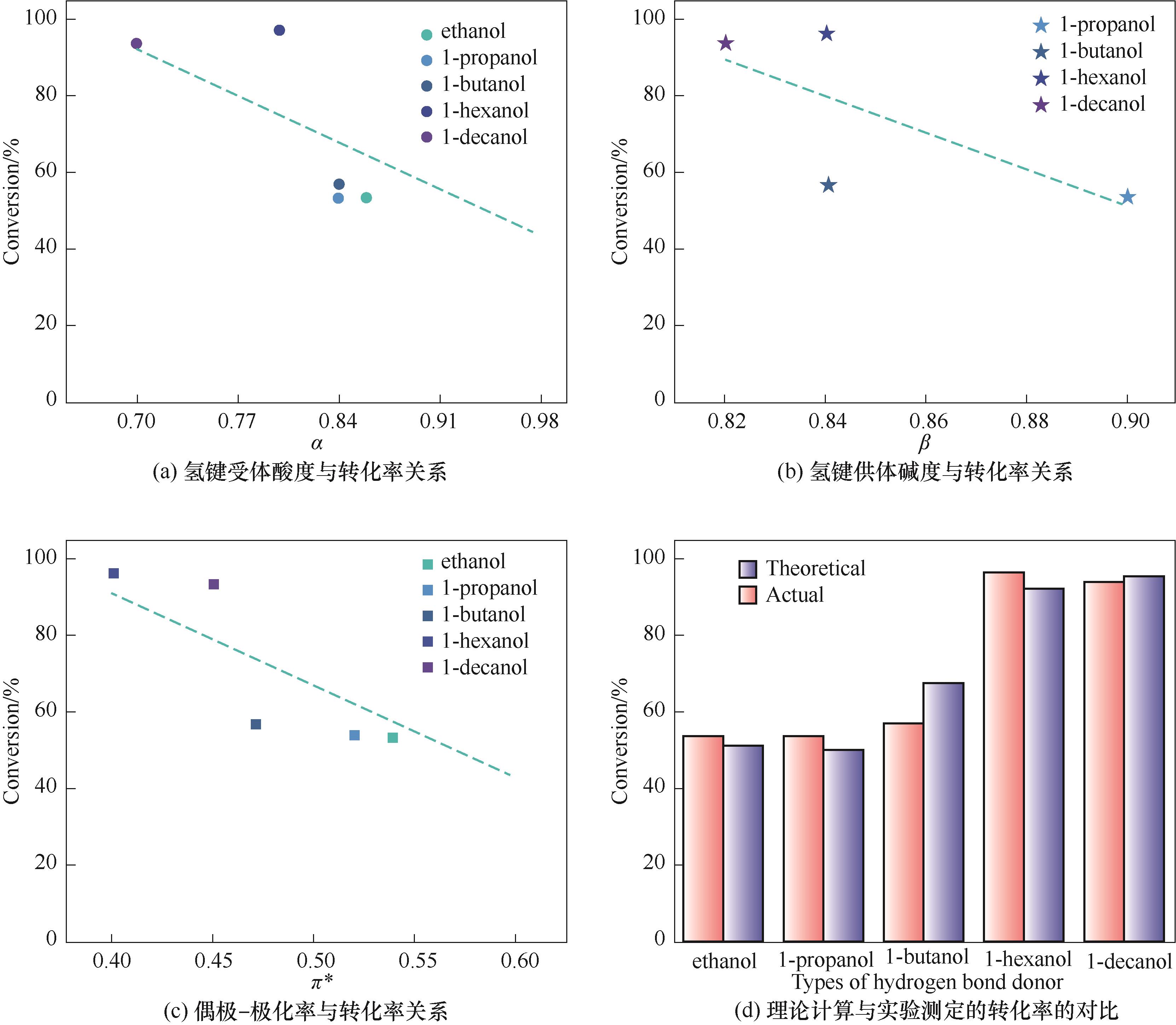
图2 醇类Kamlet-Taft参数与转化率关系以及理论计算与实验测定的转化率的对比
Fig.2 The relationship between Kamlet-Taft parameters of alcohols and conversion of PO and the comparison between theoretical calculation and experimental conversion
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
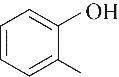
|---|---|---|---|---|---|---|
 | 53.40 | 0.70 | 1.10 | 0.30 | 0.67 | 99.30 |
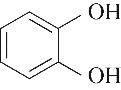 | 52.10 | 0.66 | 0.85 | 0.58 | 1.07 | 99.80 |
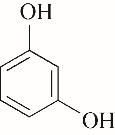 | 53.60 | 0.71 | 0.61 | 0.60 | 1.00 | 96.68 |
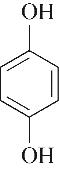 | 54.40 | 0.73 | 1.16 | 0.52 | 1.00 | 98.19 |
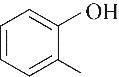 | 51.90 | 0.66 | 0.52 | 0.34 | 0.69 | 95.86 |
表4 酚类Kamlet-Taft参数与转化率[31-33]
Table 4 Kamlet-Taft parameters and conversion of phenol[31-33]
| 氢键供体 | α | β | π* | 环氧丙烷 转化率/% | ||
|---|---|---|---|---|---|---|
 | 53.40 | 0.70 | 1.10 | 0.30 | 0.67 | 99.30 |
 | 52.10 | 0.66 | 0.85 | 0.58 | 1.07 | 99.80 |
 | 53.60 | 0.71 | 0.61 | 0.60 | 1.00 | 96.68 |
 | 54.40 | 0.73 | 1.16 | 0.52 | 1.00 | 98.19 |
 | 51.90 | 0.66 | 0.52 | 0.34 | 0.69 | 95.86 |
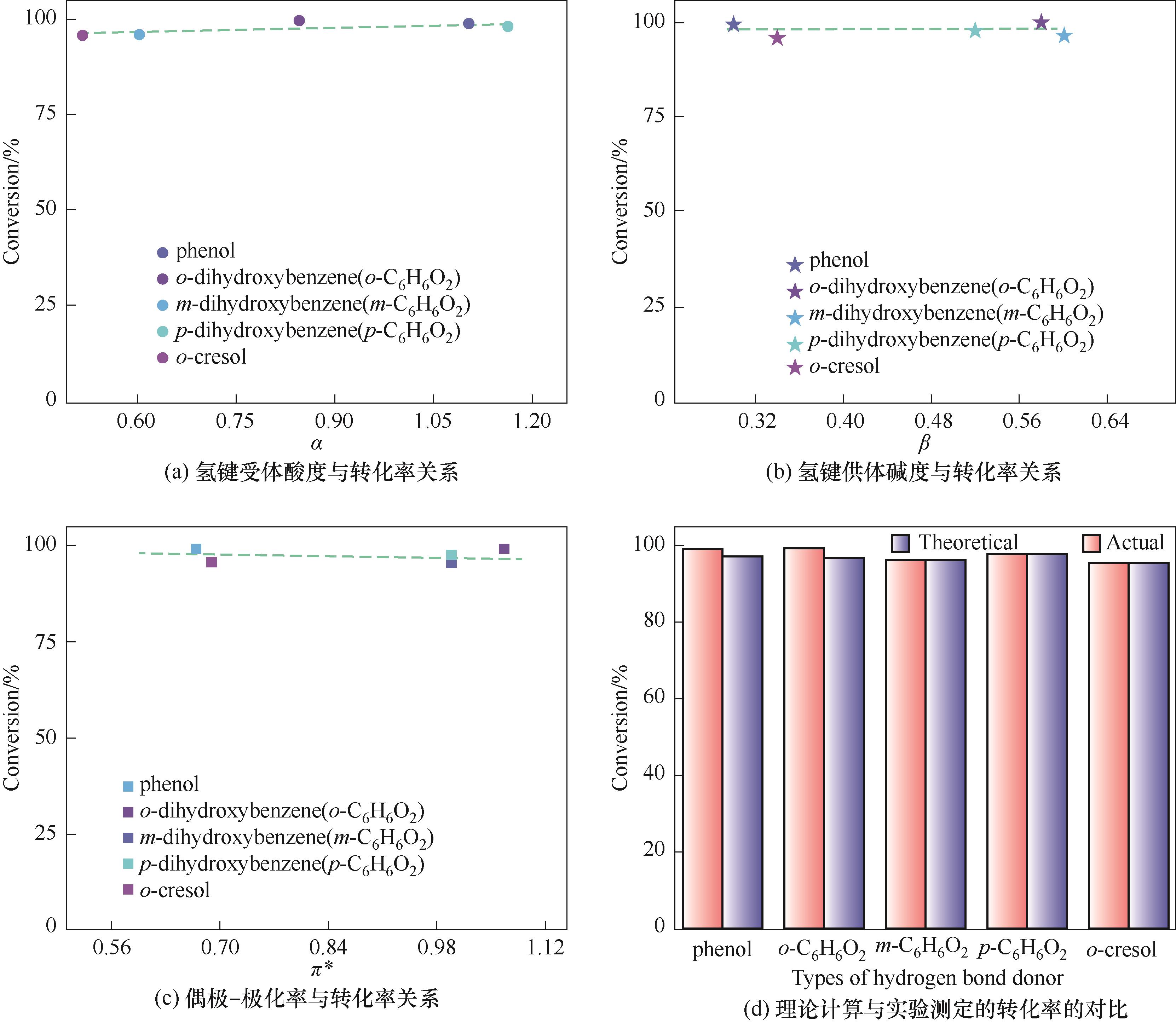
图3 酚类Kamlet-Taft参数与转化率关系以及理论计算与实验测定的转化率对比
Fig.3 The relationship between Kamlet-Taft parameters of phenols and conversion of PO and the comparison between theoretical calculation and experimental conversion
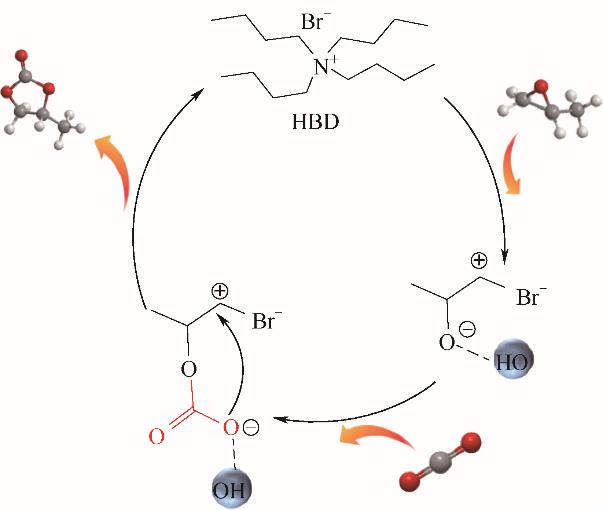
图4 氢键供体存在下TBABr催化PO环加成反应机理
Fig.4 Plausible reaction mechanism of the cycloaddition reaction for the synthesis of propylene carbonate over the TBABr in the presence of HBD
| 1 | Nemirowsky J. Ueber die einwirkung von chlorkohlenoxyd auf aethylenglycol; vorläufige mittheilung[J]. Journal Für Praktische Chemie, 1883, 28(1): 439-440. |
| 2 | Khokarale S G, Mikkola J P. Metal free synthesis of ethylene and propylene carbonate from alkylene halohydrin and CO2 at room temperature[J]. RSC Advances, 2019, 9(58): 34023-34031. |
| 3 | Deng L L, Sun W Z, Shi Z J, et al. Highly synergistic effect of ionic liquids and Zn-based catalysts for synthesis of cyclic carbonates from urea and diols[J]. Journal of Molecular Liquids, 2020, 316: 113883. |
| 4 | Zhang Z F, Liu Z W, Lu J, et al. Synthesis of dimethyl carbonate from carbon dioxide and methanol over Ce x Zr1- x O2 and [EMIM]Br/Ce0.5Zr0.5O2 [J]. Industrial & Engineering Chemistry Research, 2011, 50(4): 1981-1988. |
| 5 | Han F, Li H, Zhuang H F, et al. Direct synthesis of cyclic carbonates from olefins and CO2: single- or multi-component catalytic systems via epoxide or halohydrin intermediate[J]. Journal of CO2 Utilization, 2021, 53: 101742. |
| 6 | Fierro F, Lamparelli D H, Genga A, et al. I-LDH as a heterogeneous bifunctional catalyst for the conversion of CO2 into cyclic organic carbonates[J]. Molecular Catalysis, 2023, 538: 112994. |
| 7 | Zhang F, Wang Y Y, Zhang X C, et al. Recent advances in the coupling of CO2 and epoxides into cyclic carbonates under halogen-free condition[J]. Green Chemical Engineering, 2020, 1(2): 82-93. |
| 8 | Wang J L, Wang J Q, He L N, et al. A CO2/H2O2-tunable reaction: direct conversion of styrene into styrene carbonate catalyzed by sodium phosphotungstate/n-Bu4NBr[J]. Green Chemistry, 2008, 10(11): 1218-1223. |
| 9 | Li J X, Yue C G, Ji W H, et al. Recent advances in cycloaddition of CO2 with epoxides: halogen-free catalysis and mechanistic insights[J]. Frontiers of Chemical Science and Engineering, 2023, 17(12): 1879-1894. |
| 10 | Chen Y L, Xu P, Arai M, et al. Cycloaddition of carbon dioxide to epoxides for the synthesis of cyclic carbonates with a mixed catalyst of layered double hydroxide and tetrabutylammonium bromide at ambient temperature[J]. Advanced Synthesis & Catalysis, 2019, 361(2): 335-344. |
| 11 | Isaeva V I, Timofeeva M N, Lukoyanov I A, et al. Novel MOF catalysts based on calix[4]arene for the synthesis of propylene carbonate from propylene oxide and CO2 [J]. Journal of CO2 Utilization, 2022, 66: 102262. |
| 12 | Alassmy Y A, Pescarmona P P. The role of water revisited and enhanced: a sustainable catalytic system for the conversion of CO2 into cyclic carbonates under mild conditions[J]. ChemSusChem, 2019, 12(16): 3856-3863. |
| 13 | Roy S, Das K, Halder S. Development of suitable hydrogen bond donor (HBD) catalysts for the synthesis of cyclic carbonates and dithiocarbonates from epoxide[J]. Catalysis Letters, 2023, 154: 2243-2254. |
| 14 | Liu X J, Yan P, Han Y. H2O-polyaluminium chloride-TBAB as synergistic catalysts for the synthesis of cyclic carbonate[J]. IOP Conference Series: Materials Science and Engineering, 2018, 292: 012118. |
| 15 | Yue S, Qu H L, Song X X, et al. Novel hydroxyl-functionalized ionic liquids as efficient catalysts for the conversion of CO2 into cyclic carbonates under metal/halogen/cocatalyst/solvent-free conditions[J]. New Journal of Chemistry, 2022, 46(12): 5881-5888. |
| 16 | Wen Q, Yuan X X, Zhou Q Q, et al. Functionalized β-cyclodextrins catalyzed environment-friendly cycloaddition of carbon dioxide and epoxides[J]. Materials, 2022, 16(1): 53. |
| 17 | Liang S G, Liu H Z, Jiang T, et al. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI[J]. Chemical Communications, 2011, 47(7): 2131-2133. |
| 18 | Emenike B U, Sevimler A, Farshadmand A, et al. Rationalizing hydrogen bond solvation with Kamlet-Taft LSER and molecular torsion balances[J]. Physical Chemistry Chemical Physics: PCCP, 2023, 25(27): 17808-17814. |
| 19 | Kim Y, Oh H. Comparison between multiple regression analysis, polynomial regression analysis, and an artificial neural network for tensile strength prediction of BFRP and GFRP[J]. Materials, 2021, 14(17): 4861. |
| 20 | Wang X Y, Rinaldi R. Solvent effects on the hydrogenolysis of diphenyl ether with Raney nickel and their implications for the conversion of lignin[J]. ChemSusChem, 2012, 5(8): 1455-1466. |
| 21 | Soares B, Cunha F, Silva I, et al. Sodium hexanoate and dodecanoate salt-based eutectic solvents: density, viscosity, and Kamlet-Taft parameters[J]. Journal of Chemical & Engineering Data, 2021, 66(7): 2793-2802. |
| 22 | Taft R W, Kamlet M J. The solvatochromic comparison method(2): The α-scale of solvent hydrogen-bond donor (HBD) acidities[J]. Journal of the American Chemical Society, 1976, 98(10): 2886-2894. |
| 23 | Weiß N, Schmidt C H, Thielemann G, et al. The physical significance of the Kamlet-Taft π* parameter of ionic liquids[J]. Physical Chemistry Chemical Physics, 2021, 23(2): 1616-1626. |
| 24 | Duereh A, Guo H X, Honma T, et al. Solvent polarity of cyclic ketone (cyclopentanone, cyclohexanone): alcohol (methanol, ethanol) renewable mixed-solvent systems for applications in pharmaceutical and chemical processing[J]. Industrial & Engineering Chemistry Research, 2018, 57(22): 7331-7344. |
| 25 | Rosés M, Ortega J, Bosch E. Variation of E T 30 polarity and the Kamlet-Taft solvatochromic parameters with composition in alcohol-alcohol mixtures[J]. Journal of Solution Chemistry, 1995, 24(1): 51-63. |
| 26 | Kamlet M J, Taft R W. The solvatochromic comparison method(Ⅰ): The β-scale of solvent hydrogen-bond acceptor (HBA) basicities[J]. Journal of the American Chemical Society, 1976, 98(2): 377-383. |
| 27 | Marcus Y. The properties of organic liquids that are relevant to their use as solvating solvents[J]. Chemical Society Reviews, 1993, 22(6): 409-416. |
| 28 | 邹建卫, 俞庆森, 商志才. 醇类溶剂溶剂化显色极性的理论分析[J]. 化学学报, 2000, 58(10): 1247-1253. |
| Zou J W, Yu Q S, Shang Z C. Theoretical analysis of solvatochromic polarity scales on alcoholic solvents[J]. Acta Chimica Sinica, 2000, 58(10): 1247-1253. | |
| 29 | 刘汉文, 胡彩玲. 醇类溶剂的溶剂化显色参数 E T N 的相关分析[J]. 湘潭大学自然科学学报, 2005, 27(3): 67-70. |
| Liu H W, Hu C L. Relevant analysis of solvatochromic parameter on alcoholic solvents[J]. Natural Science Journal of Xiangtan University, 2005, 27(3): 67-70. | |
| 30 | Laurence C, Mansour S, Vuluga D, et al. Theoretical, semiempirical, and experimental solvatochromic comparison methods for the construction of the α1 scale of hydrogen-bond donation of solvents[J]. The Journal of Organic Chemistry, 2022, 87(9): 6273-6287. |
| 31 | 冯流, 韩朔睽, 王连生, 等. 苯系物Lewis酸碱性定量及其应用[J]. 环境化学, 1995, 14(5): 417-424. |
| Feng L, Han S K, Wang L S, et al. A new quantiative parameter of the Lewis acidity and basicity of benzene and its derivatives[J]. Environmental Chemistry, 1995, 14(5): 417-424. | |
| 32 | Brehmer T, Duong B, Boeker P, et al. Simulation of gas chromatographic separations and estimation of distribution-centric retention parameters using linear solvation energy relationships[J]. Journal of Chromatography A, 2024, 1717: 464665. |
| 33 | 韩长日. 溶剂极性的ET 经验参数及其应用[J]. 化学通报, 1985, 48(7): 40-43. |
| Han C R. ET empirical parameter of solvent polarity and its application[J]. Chemistry, 1985, 48(7): 40-43. | |
| 34 | Liu M S, Wang X, Jiang Y C, et al. Hydrogen bond activation strategy for cyclic carbonates synthesis from epoxides and CO2: current state-of-the art of catalyst development and reaction analysis[J]. Catalysis Reviews, 2019, 61(2): 214-269. |
| [1] | 李子扬, 郑楠, 方嘉宾, 魏进家. 再压缩S-CO2布雷顿循环性能分析及多目标优化[J]. 化工学报, 2024, 75(6): 2143-2156. |
| [2] | 常成功, 宋皓楠, 雷飞霞, 狄子琛, 程芳琴. 高炉喷吹重整焦炉气工艺分析及减碳潜力研究[J]. 化工学报, 2024, 75(6): 2344-2352. |
| [3] | 武颖韬, 费立涵, 孔祥东, 王帜, 汤成龙, 黄佐华. 咪唑二氰胺离子液体掺混糠醇的自燃及推进性能[J]. 化工学报, 2024, 75(5): 2017-2025. |
| [4] | 马旭, 滕亚栋, 刘杰, 王宇璐, 张鹏, 张莲海, 姚万龙, 展静, 吴青柏. 喷雾法水合物法捕集分离烟道气中CO2[J]. 化工学报, 2024, 75(5): 2001-2016. |
| [5] | 王瑞瑞, 金颖, 刘玉梅, 李梦悦, 朱胜文, 闫瑞一, 刘瑞霞. 聚合离子液体设计及催化环己烷选择性氧化性能研究[J]. 化工学报, 2024, 75(4): 1552-1564. |
| [6] | 蒋方涛, 钱刚, 周兴贵, 段学志, 张晶. 基于[bmim][BF4]相转移催化的氟代碳酸乙烯酯高效合成[J]. 化工学报, 2024, 75(4): 1543-1551. |
| [7] | 肖拥君, 时兆翀, 万仁, 宋璠, 彭昌军, 刘洪来. 反向传播神经网络用于预测离子液体的自扩散系数[J]. 化工学报, 2024, 75(2): 429-438. |
| [8] | 孙瑞, 田华, 吴子睿, 孙孝存, 舒歌群. 二氧化碳混合工质临界参数计算模型对比研究[J]. 化工学报, 2024, 75(2): 439-449. |
| [9] | 朱芝, 许恒杰, 陈维, 毛文元, 邓强国, 孙雪剑. 超临界二氧化碳螺旋槽干气密封热流耦合润滑临界阻塞特性研究[J]. 化工学报, 2024, 75(2): 604-615. |
| [10] | 赵若晗, 黄蒙蒙, 朱春英, 付涛涛, 高习群, 马友光. 缩口T型微通道内纳米流体吸收CO2的流动与传质研究[J]. 化工学报, 2024, 75(1): 221-230. |
| [11] | 张泽欣, 郑伟中, 徐益升, 胡冬冬, 卓欣宇, 宗原, 孙伟振, 赵玲. 超临界二氧化碳介质中晶圆清洗与选择性刻蚀研究进展[J]. 化工学报, 2024, 75(1): 110-119. |
| [12] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [13] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [14] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [15] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号