化工学报 ›› 2024, Vol. 75 ›› Issue (4): 1543-1551.DOI: 10.11949/0438-1157.20231271
收稿日期:2023-11-30
修回日期:2024-01-12
出版日期:2024-04-25
发布日期:2024-06-06
通讯作者:
段学志,张晶
作者简介:蒋方涛(1999—), 男, 硕士研究生, jft990809@163.com
基金资助:
Fangtao JIANG( ), Gang QIAN, Xinggui ZHOU, Xuezhi DUAN(
), Gang QIAN, Xinggui ZHOU, Xuezhi DUAN( ), Jing ZHANG(
), Jing ZHANG( )
)
Received:2023-11-30
Revised:2024-01-12
Online:2024-04-25
Published:2024-06-06
Contact:
Xuezhi DUAN, Jing ZHANG
摘要:
氟代碳酸乙烯酯(FEC)是锂电池电解液添加剂的重要组分之一,其工业制备方法主要为卤素交换法,即氯代碳酸乙烯酯(CEC)与氟化钾(KF)通过取代反应制备FEC。该工艺中,取代反应速率受限于KF相际传质速率,且CEC易发生消去反应生成碳酸亚乙烯酯副产物。针对上述问题,研究了相转移催化剂(PTC)结构对KF相际传质速率和CEC制FEC主副反应能垒的影响规律和调控机制。优化条件下,PTC为[bmim][BF4](1-丁 基-3-甲基咪唑四氟硼酸盐),溶剂为乙腈,反应温度为乙腈回流温度(81.6℃),n(KF)∶n(CEC)=2.5∶1,此时FEC收率高达91.94%(摩尔分数)。密度泛函理论计算表明,在乙腈中[bmim][BF4]能与KF形成配合物,增加K+和F-的核间距并降低KF的溶解自由能,从而强化相际传质并降低取代反应能垒,实现CEC经KF取代高效制备FEC。
中图分类号:
蒋方涛, 钱刚, 周兴贵, 段学志, 张晶. 基于[bmim][BF4]相转移催化的氟代碳酸乙烯酯高效合成[J]. 化工学报, 2024, 75(4): 1543-1551.
Fangtao JIANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN, Jing ZHANG. Efficient synthesis of fluoroethylene carbonate via phase transfer catalysis using [bmim][BF4][J]. CIESC Journal, 2024, 75(4): 1543-1551.
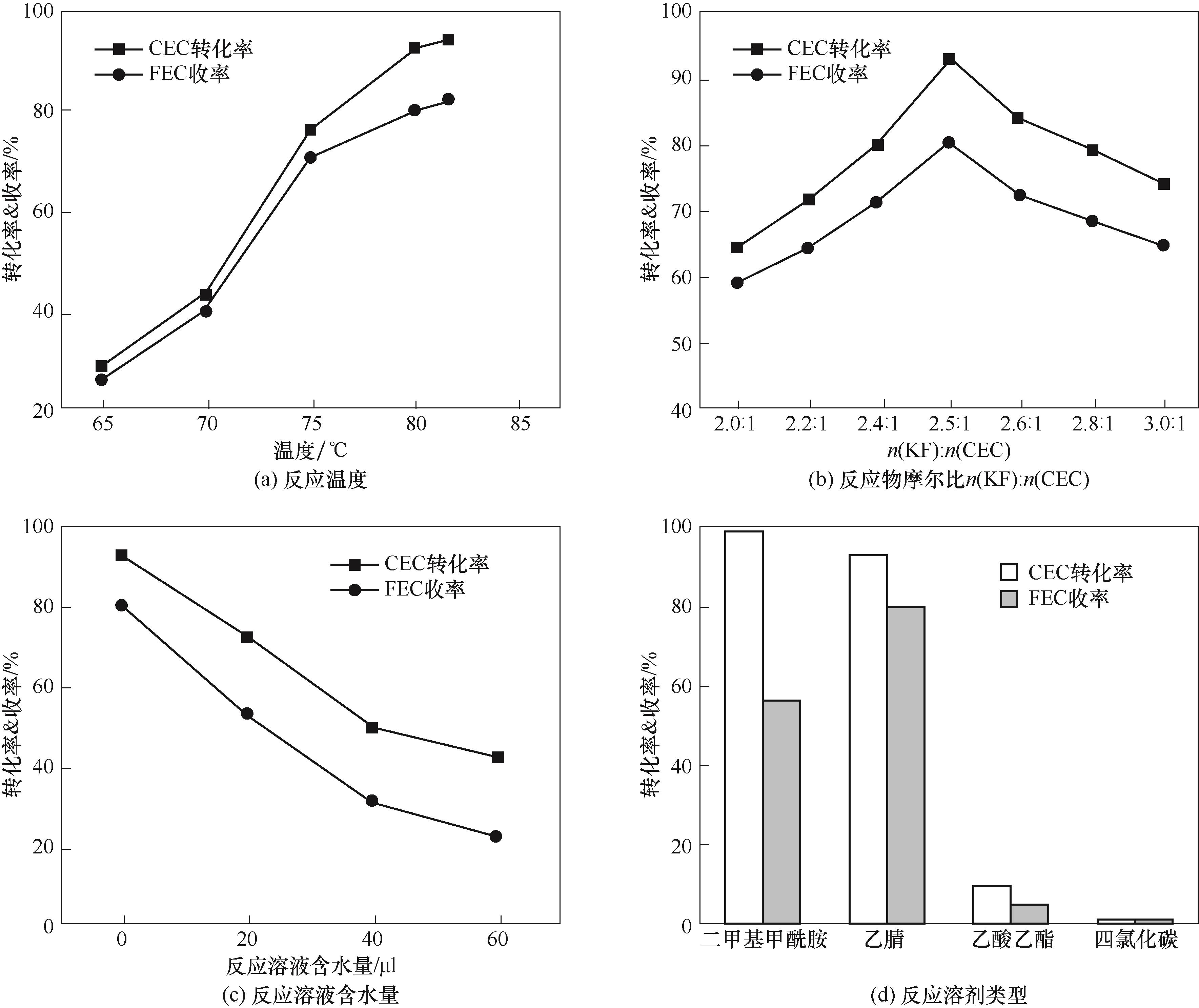
图3 反应温度、反应物摩尔比n(KF)∶n(CEC)、反应溶液含水量、反应溶剂类型对反应结果的影响
Fig.3 Effects of reaction temperature, molar ratio of n(KF)∶n(CEC), water content, and solvent type on reaction results
| 氟源 | 时间/h | 收率/% | 核间距/Å | SN2/ (kcal/mol) | E2/ (kcal/mol) |
|---|---|---|---|---|---|
| F- | — | — | — | 14.72 | 10.74 |
| CsF | 0.5 | 74.77 | 2.7230 | 20.30 | 18.93 |
| KF | 8.0 | 79.97 | 2.4243 | 24.15 | 24.52 |
| NaF | 8.0 | — | 2.0754 | 28.55 | 29.66 |
表1 不同氟源对反应结果的影响
Table 1 Effect of fluorine source on the reaction result
| 氟源 | 时间/h | 收率/% | 核间距/Å | SN2/ (kcal/mol) | E2/ (kcal/mol) |
|---|---|---|---|---|---|
| F- | — | — | — | 14.72 | 10.74 |
| CsF | 0.5 | 74.77 | 2.7230 | 20.30 | 18.93 |
| KF | 8.0 | 79.97 | 2.4243 | 24.15 | 24.52 |
| NaF | 8.0 | — | 2.0754 | 28.55 | 29.66 |
| 序号 | PTC | n(PTC)∶n(CEC) | 时间/ h | 转化率/ % | 收率/% (摩尔分数) |
|---|---|---|---|---|---|
| 1 | — | — | 8.0 | 93.89 | 82.21 |
| 2 | [bmim][BF4] | 0.15 | 6.0 | 99.33 | 89.80 |
| 3 | [bmim][BF4] | 0.2 | 6.0 | 99.26 | 91.94 |
| 4 | [bmim][BF4] | 0.25 | 6.0 | 99.59 | 89.02 |
| 5 | [bmim][SbF6] | 0.2 | 6.0 | 72.53 | 51.39 |
| 6 | [bmim][OMS] | 0.2 | 6.0 | 72.20 | 27.24 |
| 7 | [C4MPr][BF4] | 0.2 | 6.0 | 79.34 | 73.12 |
| 8 | [C4PyM][BF4] | 0.2 | 6.0 | 83.21 | 65.27 |
| 9 | 四丁基氯化铵 | 0.2 | 8.0 | 46.71 | 4.94 |
| 10 | 四丁基氯化磷 | 0.2 | 8.0 | 28.07 | 0.72 |
| 11 | PEG800 | 0.2 | 8.0 | 35.66 | 19.36 |
| 12 | 18-冠醚-6 | 0.2 | 8.0 | 41.78 | 9.80 |
| 13 | β-环糊精 | 0.2 | 8.0 | 82.13 | 66.37 |
表2 不同PTC对反应结果的影响
Table 2 Effect of different PTC on the reaction result
| 序号 | PTC | n(PTC)∶n(CEC) | 时间/ h | 转化率/ % | 收率/% (摩尔分数) |
|---|---|---|---|---|---|
| 1 | — | — | 8.0 | 93.89 | 82.21 |
| 2 | [bmim][BF4] | 0.15 | 6.0 | 99.33 | 89.80 |
| 3 | [bmim][BF4] | 0.2 | 6.0 | 99.26 | 91.94 |
| 4 | [bmim][BF4] | 0.25 | 6.0 | 99.59 | 89.02 |
| 5 | [bmim][SbF6] | 0.2 | 6.0 | 72.53 | 51.39 |
| 6 | [bmim][OMS] | 0.2 | 6.0 | 72.20 | 27.24 |
| 7 | [C4MPr][BF4] | 0.2 | 6.0 | 79.34 | 73.12 |
| 8 | [C4PyM][BF4] | 0.2 | 6.0 | 83.21 | 65.27 |
| 9 | 四丁基氯化铵 | 0.2 | 8.0 | 46.71 | 4.94 |
| 10 | 四丁基氯化磷 | 0.2 | 8.0 | 28.07 | 0.72 |
| 11 | PEG800 | 0.2 | 8.0 | 35.66 | 19.36 |
| 12 | 18-冠醚-6 | 0.2 | 8.0 | 41.78 | 9.80 |
| 13 | β-环糊精 | 0.2 | 8.0 | 82.13 | 66.37 |
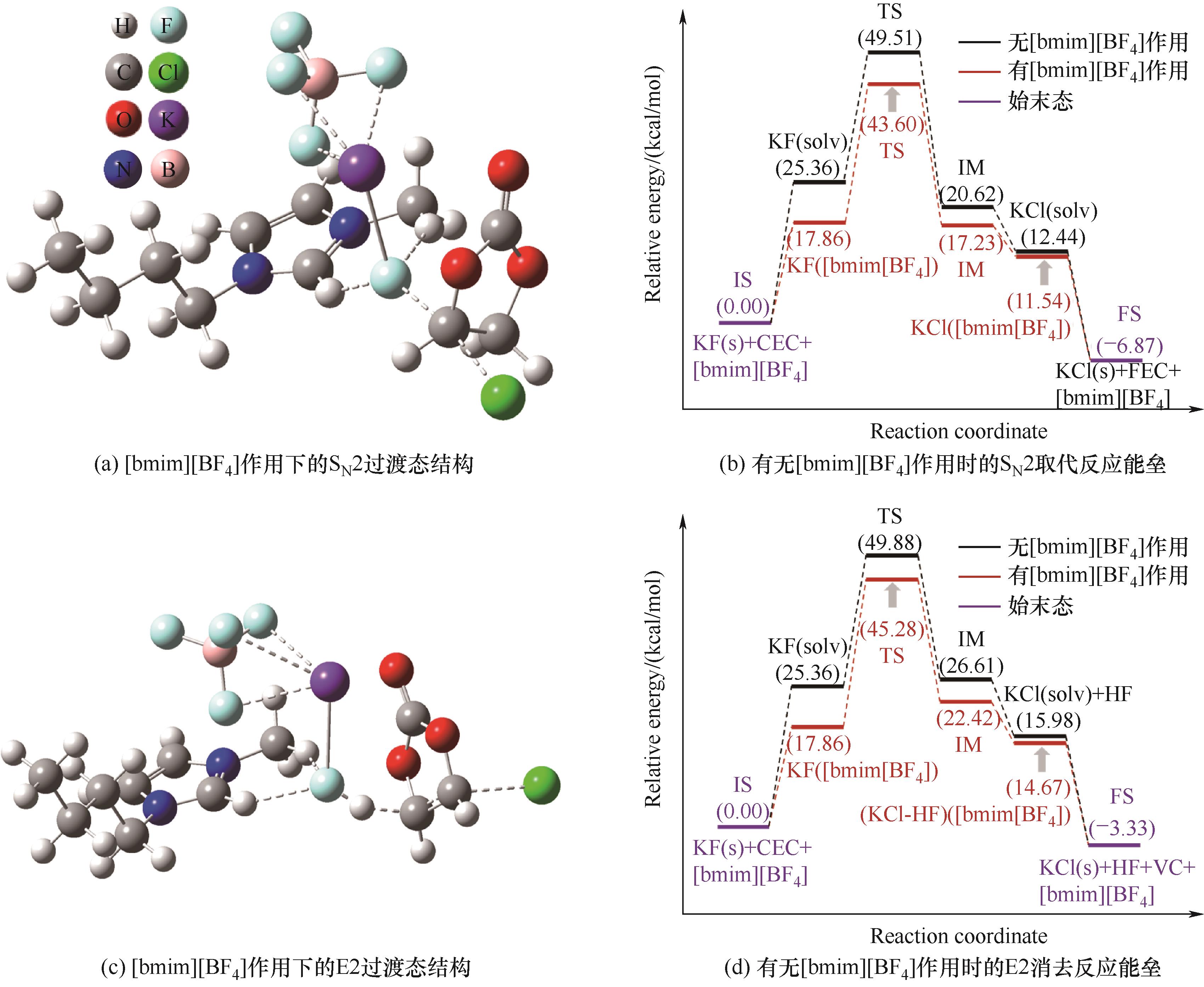
图5 [bmim][BF4]作用下SN2、E2过渡态结构以及有无[bmim][BF4]时SN2取代、E2消去反应能垒
Fig.5 Transition state structures of SN2 and E2 with [bmim][BF4] and the reaction energy barriers of SN2 and E2 with or without [bmim][BF4]
| 序号 | 过程 | ΔG/(kcal/mol) |
|---|---|---|
| 1 | KF(s)  KF(g)① KF(g)① | 46.40 |
| 2 | KF(g)  KF(solv) KF(solv) | -21.04 |
| 3 | KF(solv)+[bmim][BF4]  KF([bmim][BF4]) KF([bmim][BF4]) | -7.50 |
| 4 | KCl(s)  KCl(g)① KCl(g)① | 41.91 |
| 5 | KCl(g)  KCl(solv) KCl(solv) | -22.60 |
| 6 | KCl(solv)+[bmim][BF4]  KCl([bmim][BF4]) KCl([bmim][BF4]) | -0.90 |
| 7 | KCl(solv)+HF+[bmim][BF4]  (KCl-HF)([bmim][BF4]) (KCl-HF)([bmim][BF4]) | -1.31 |
| 8 | TS-KF(solv)-SN2 | 24.15 |
| 9 | TS-KF([bmim][BF4])-SN2 | 25.74 |
| 10 | TS-KF(solv)-E2 | 24.52 |
| 11 | TS-KF([bmim][BF4])-E2 | 27.42 |
表3 [bmim][BF4]和KF在乙腈溶剂中活化及氟化反应数据
Table 3 Activation and fluorination reaction data of [bmim][BF4] and KF in acetonitrile solvent
| 序号 | 过程 | ΔG/(kcal/mol) |
|---|---|---|
| 1 | KF(s)  KF(g)① KF(g)① | 46.40 |
| 2 | KF(g)  KF(solv) KF(solv) | -21.04 |
| 3 | KF(solv)+[bmim][BF4]  KF([bmim][BF4]) KF([bmim][BF4]) | -7.50 |
| 4 | KCl(s)  KCl(g)① KCl(g)① | 41.91 |
| 5 | KCl(g)  KCl(solv) KCl(solv) | -22.60 |
| 6 | KCl(solv)+[bmim][BF4]  KCl([bmim][BF4]) KCl([bmim][BF4]) | -0.90 |
| 7 | KCl(solv)+HF+[bmim][BF4]  (KCl-HF)([bmim][BF4]) (KCl-HF)([bmim][BF4]) | -1.31 |
| 8 | TS-KF(solv)-SN2 | 24.15 |
| 9 | TS-KF([bmim][BF4])-SN2 | 25.74 |
| 10 | TS-KF(solv)-E2 | 24.52 |
| 11 | TS-KF([bmim][BF4])-E2 | 27.42 |
| 1 | Horstmann B, Shi J Y, Amine R, et al. Strategies towards enabling lithium metal in batteries: interphases and electrodes[J]. Energy & Environmental Science, 2021, 14(10): 5289-5314. |
| 2 | Zhang X Q, Cheng X B, Chen X, et al. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries[J]. Advanced Functional Materials, 2017, 27(10): 1605989. |
| 3 | Markevich E, Salitra G, Aurbach D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries[J]. ACS Energy Letters, 2017, 2(6): 1337-1345. |
| 4 | Liu Q C, Xu J J, Yuan S, et al. Artificial protection film on lithium metal anode toward long-cycle-life lithium-oxygen batteries[J]. Advanced Materials, 2015, 27(35): 5241-5247. |
| 5 | 胡华坤, 薛文东, 霍思达, 等. 锂离子电池电解液SEI成膜添加剂的研究进展[J]. 化工学报, 2022, 73(4): 1436-1454. |
| Hu H K, Xue W D, Huo S D, et al. Review of SEI film forming additives for electrolyte of lithium ion battery[J]. CIESC Journal, 2022, 73(4): 1436-1454. | |
| 6 | 任章顺, 朱志峰, 袁胜芳, 等. 氟代碳酸乙烯酯的合成与精制进展[J]. 化学推进剂与高分子材料, 2015, 13(6): 39-44. |
| Ren Z S, Zhu Z F, Yuan S F, et al. Progress in synthesis and purification of fluoroethylene carbonate[J]. Chemical Propellants & Polymeric Materials, 2015, 13(6): 39-44. | |
| 7 | 吕俊奇, 阎子祯, 周羿凝, 等. 氟代碳酸乙烯酯的制备和精制工艺最新进展[J]. 化学推进剂与高分子材料, 2023, 21(2): 30-44. |
| Lyu J Q, Yan Z Z, Zhou Y N, et al. Recent advances in preparation and purification process of fluoroethylene carbonate[J]. Chemical Propellants & Polymeric Materials, 2023, 21(2): 30-44. | |
| 8 | Starks C M. Phase-transfer catalysis(Ⅰ): Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts[J]. Journal of the American Chemical Society, 1971, 93(1): 195-199. |
| 9 | Pozzi G, Quici S, Fish R H. Fluorous phase transfer catalysts: from onium salts to crown ethers[J]. Journal of Fluorine Chemistry, 2008, 129(10): 920-929. |
| 10 | Shirakawa S, Maruoka K. Recent developments in asymmetric phase-transfer reactions[J]. Angewandte Chemie (International Ed. in English), 2013, 52(16): 4312-4348. |
| 11 | Greer A J, Jacquemin J, Hardacre C. Industrial applications of ionic liquids[J]. Molecules, 2020, 25(21): 5207. |
| 12 | 姚桂, 段正康, 贺玉平, 等. 氟代碳酸乙烯酯的合成[J]. 精细化工, 2012, 29(4): 394-397. |
| Yao G, Duan Z K, He Y P, et al. Synthesis of fluoroethylene carbonate[J]. Fine Chemicals, 2012, 29(4): 394-397. | |
| 13 | 李云峰, 王永勤. 氟代碳酸乙烯酯的合成工艺研究[J]. 河南化工, 2018, 35(7): 29-31. |
| Li Y F, Wang Y Q. Study on the synthetic process of fluoroethylene carbonate[J]. Henan Chemical Industry, 2018, 35(7): 29-31. | |
| 14 | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09 Rev. E.01[CP]. Wallingford: Gaussian Inc., CT., 2009. |
| 15 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| 16 | Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy[J]. Physical Chemistry Chemical Physics: PCCP, 2005, 7(18): 3297-3305. |
| 17 | Hariharan P C, Pople J A. Accuracy of AH n equilibrium geometries by single determinant molecular orbital theory[J]. Molecular Physics, 1974, 27(1): 209-214. |
| 18 | Petersson G A, Bennett A, Tensfeldt T G, et al. A complete basis set model chemistry(Ⅰ): The total energies of closed-shell atoms and hydrides of the first-row elements[J]. The Journal of Chemical Physics, 1988, 89(4): 2193-2218. |
| 19 | Marenich A V, Cramer C J, Truhlar D G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions[J]. The Journal of Physical Chemistry B, 2009, 113(18): 6378-6396. |
| 20 | Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| 21 | Maeda S, Harabuchi Y, Ono Y, et al. Intrinsic reaction coordinate: calculation, bifurcation, and automated search[J]. International Journal of Quantum Chemistry, 2015, 115(5): 258-269. |
| 22 | Oh Y H, Yun W, Kim C H, et al. Inter- and intra-molecular organocatalysis of SN2 fluorination by crown ether: kinetics and quantum chemical analysis[J]. Molecules, 2021, 26(10): 2947. |
| 23 | Silva S L, Valle M S, Pliego J R. Nucleophilic fluorination with KF catalyzed by 18-crown-6 and bulky diols: a theoretical and experimental study[J]. The Journal of Organic Chemistry, 2020, 85(23): 15457-15465. |
| 24 | O'Hair R A J, Davico G E, Hacaloglu J, et al. Measurements of solvent and secondary kinetic isotope effects for the gas-phase SN2 reactions of fluoride with methyl halides[J]. Journal of the American Chemical Society, 1994, 116(8): 3609-3610. |
| 25 | Liu X, Yang L, Zhang J X, et al. Competition of F/OH-induced SN2 and proton-transfer reactions with increased solvation[J]. The Journal of Physical Chemistry A, 2018, 122(49): 9446-9453. |
| 26 | Anslyn E V, Dougherty D A. Modern Physical Organic Chemistry[M]. Sausalito, Calif.: University Science, 2006. |
| 27 | Tang W Q, Zhao J H, Jiang P, et al. Solvent effects on the symmetric and asymmetric SN2 reactions in the acetonitrile solution: a reaction density functional theory study[J]. The Journal of Physical Chemistry. B, 2020, 124(15): 3114-3122. |
| 28 | 杜治平, 姚洁, 曾毅, 等. 碳酸乙烯酯的酯交换反应研究进展[J]. 天然气化工, 2003, 28(4): 22-29. |
| Du Z P, Yao J, Zeng Y, et al. Progress in study of transesterification of ethylene carbonate[J]. Natural Gas Chemical Industry, 2003, 28(4): 22-29. | |
| 29 | Laloo J Z A, Rhyman L, Ramasami P, et al. Ion-pair SN2 substitution: activation strain analyses of counter-ion and solvent effects[J]. Chemistry-A European Journal, 2016, 22(13): 4431-4439. |
| 30 | Kim D W, Song C E, Chi D Y. New method of fluorination using potassium fluoride in ionic liquid: significantly enhanced reactivity of fluoride and improved selectivity[J]. Journal of the American Chemical Society, 2002, 124(35): 10278-10279. |
| 31 | Oh Y H, Choi H, Park C, et al. Harnessing ionic interactions and hydrogen bonding for nucleophilic fluorination[J]. Molecules, 2020, 25(3): 721. |
| 32 | Carvalho N F, Pliego J R. Theoretical design and calculation of a crown ether phase-transfer-catalyst scaffold for nucleophilic fluorination merging two catalytic concepts[J]. The Journal of Organic Chemistry, 2016, 81(18): 8455-8463. |
| 33 | NIST chemistry webbook, NIST standard reference database number 69 [DB/OL]. Gaithersburg MD, 20899: National Institute of Standards and Technology, 2024. . |
| [1] | 冯彬彬, 卢明佳, 黄志宏, 常译文, 崔志明. 碳载体在质子交换膜燃料电池中的应用及优化[J]. 化工学报, 2024, 75(4): 1469-1484. |
| [2] | 王瑞瑞, 金颖, 刘玉梅, 李梦悦, 朱胜文, 闫瑞一, 刘瑞霞. 聚合离子液体设计及催化环己烷选择性氧化性能研究[J]. 化工学报, 2024, 75(4): 1552-1564. |
| [3] | 程婷, 焦纬洲, 刘有智. 功能性填料在超重力旋转填料床中的应用和研究进展[J]. 化工学报, 2024, 75(4): 1414-1428. |
| [4] | 董霄, 白志山, 杨晓勇, 殷伟, 刘宁普, 于启凡. CHPPO工艺氧化液耦合除杂技术的研究与工业应用[J]. 化工学报, 2024, 75(4): 1630-1641. |
| [5] | 王娟, 李秀明, 邵炜涛, 丁续, 霍莹, 付连超, 白云宇, 李迪. 多孔板鼓泡塔流动与传质特性数值模拟[J]. 化工学报, 2024, 75(3): 801-814. |
| [6] | 王佳琪, 魏皓琦, 苟阿静, 刘佳兴, 周昕霖, 葛坤. 纳米粒子作用下CO2水合物生成机理研究[J]. 化工学报, 2024, 75(3): 956-966. |
| [7] | 李文俊, 赵中阳, 倪震, 周灿, 郑成航, 高翔. 基于气-液传质强化的湿法烟气脱硫CFD模拟研究[J]. 化工学报, 2024, 75(2): 505-519. |
| [8] | 肖拥君, 时兆翀, 万仁, 宋璠, 彭昌军, 刘洪来. 反向传播神经网络用于预测离子液体的自扩散系数[J]. 化工学报, 2024, 75(2): 429-438. |
| [9] | 张欣, 薛宇, 马懿星, 王学谦, 王郎郎, 谢妮霏, 陈怡, 周晓霞. 电晕放电与介质阻挡放电净化氰化氢的机理[J]. 化工学报, 2024, 75(2): 675-684. |
| [10] | 赵若晗, 黄蒙蒙, 朱春英, 付涛涛, 高习群, 马友光. 缩口T型微通道内纳米流体吸收CO2的流动与传质研究[J]. 化工学报, 2024, 75(1): 221-230. |
| [11] | 崔怡洲, 李成祥, 翟霖晓, 刘束玉, 石孝刚, 高金森, 蓝兴英. 亚毫米气泡和常规尺寸气泡气液两相流流动与传质特性对比[J]. 化工学报, 2024, 75(1): 197-210. |
| [12] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [13] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [14] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [15] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号