化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3681-3696.DOI: 10.11949/0438-1157.20230660
收稿日期:2023-06-30
修回日期:2023-09-04
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
花儿
作者简介:米泽豪(1998—),男,硕士研究生,875501689@qq.com
基金资助:Received:2023-06-30
Revised:2023-09-04
Online:2023-09-25
Published:2023-11-20
Contact:
Er HUA
摘要:
开展以质子化的正己胺([HHexam]+)、己基乙二胺([HHexen]+)及己基二亚乙基三胺([HHexdien]+)为阳离子,TFSA [(CF3SO2)2N-]为阴离子的质子化离子液体(PILs),即[HHexam][TFSA]、[HHexen][TFSA]及[HHexdien][TFSA]型PILs吸收SO2的研究。首先,选择密度泛函理论,在M06-2X/6-311G(d,p)水平下,优化得到 PILs-nSO2 (n=1,2,3,4,5,6)的若干个最优构象,PILs阳离子极性头部N—H与SO2的O原子间形成N—H…O型氢键。N—H…O部位的振动频率、二阶微扰能、电子密度的结果显示,[HHexam][TFSA]、[HHexen][TFSA]及[HHexdien][TFSA]分别与3、4、5分子SO2结合时,其氢键能达到最大值:57、67、85 kJ/mol,并不再与更多的SO2形成氢键网络,说明PILs对SO2的吸收达到饱和。可以看出,随着PILs结构中氨基数目的增多,其对SO2的吸收能力随之增强。同时,采用COSMOtherm软件,计算了SO2气体在1 mol PILs中的溶解度分别为5.0、5.3、6.2 mol,即随着阳离子结构中氨基数目的增多,使得其对SO2的吸收能力增强,这与密度泛函理论计算得到的结论基本相一致。
中图分类号:
米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696.
Zehao MI, Er HUA. DFT and COSMO-RS theoretical analysis of SO2 absorption by polyamines type ionic liquids[J]. CIESC Journal, 2023, 74(9): 3681-3696.
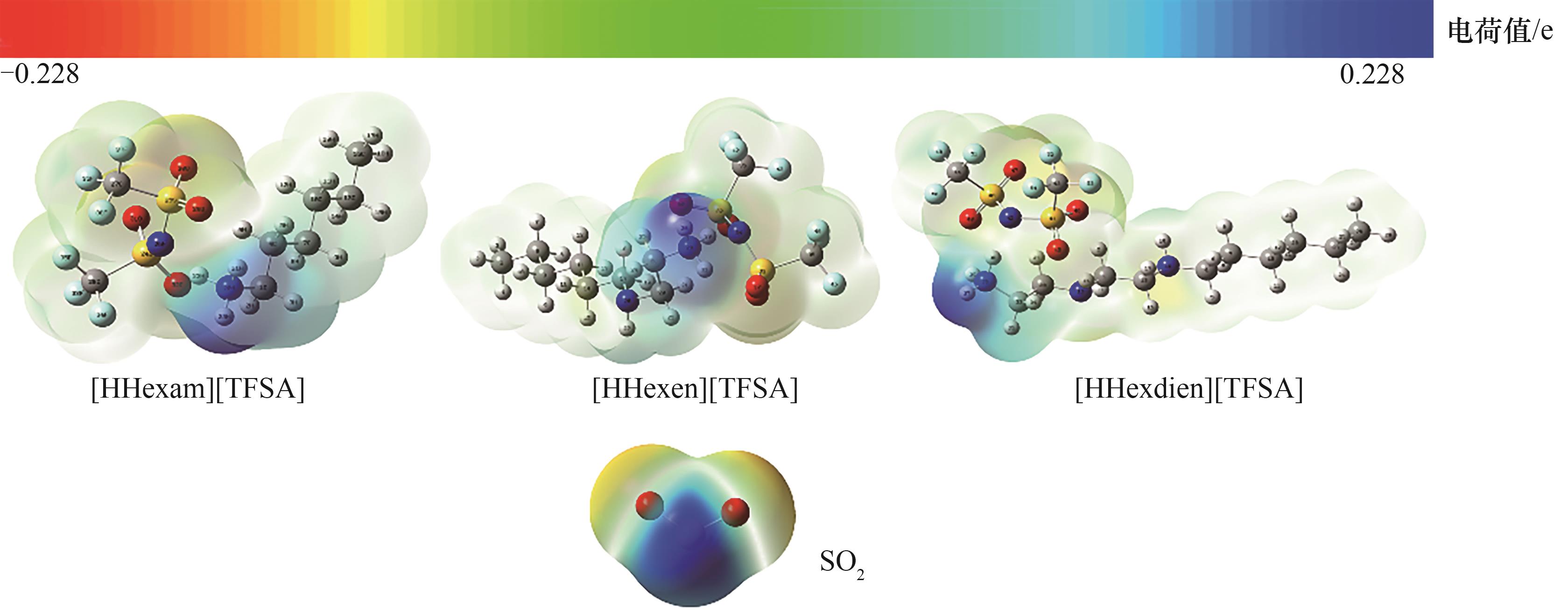
图2 [HHexam][TFSA]、[HHexen][TFSA]、 [HHexdien][TFSA]及SO2单体的静电势能面图(红色:O;蓝色:N;深灰:C;浅灰:H;黄色:S;绿色:F)
Fig.2 The electrostatic potential surface distribution for the monomers of [HHexam] [TFSA], [HHxen] [TFSA], [HHedien] [TFSA] and SO2 (red: O; blue: N; dark gray: C; light gray: H; yellow: S; green: F)
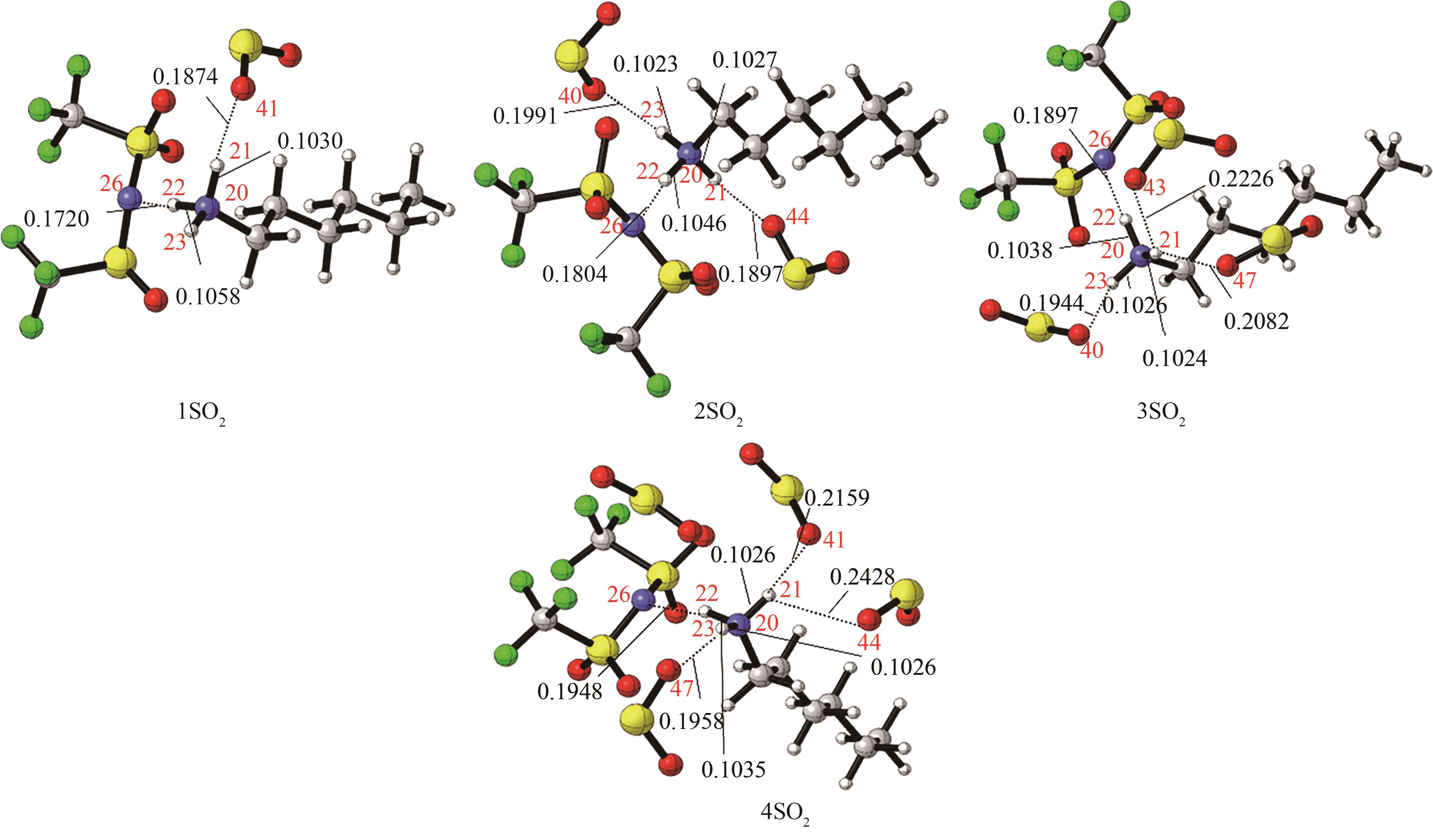
图4 [HHexam][TFSA]-nSO2(n=1,2,3,4)的最稳定构象[主要氢键部位的键长(nm)已标注。红色:O;蓝色:N;深灰:C;浅灰:H;黄色:S;绿色:F]
Fig.4 The most stable conformation of [HHexam][TFSA]-nSO2 (n=1,2,3,4) [the bond length (nm) of the main hydrogen bonding site has been marked, red: O; blue: N; dark gray: C; light gray: H; yellow: S; green: F]
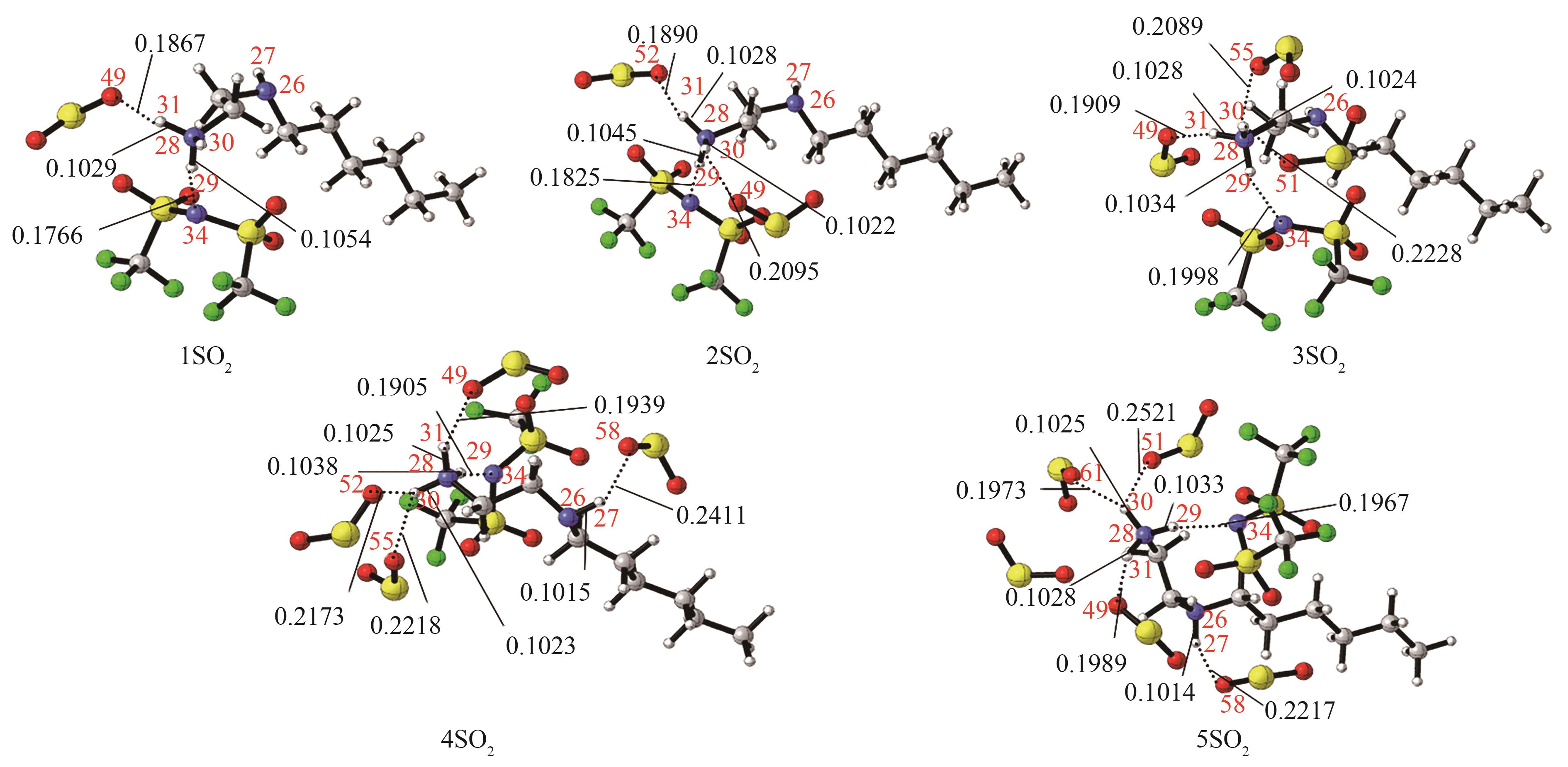
图5 [HHexen][TFSA]-nSO2(n=1,2,3,4,5)的最稳定构象[主要氢键部位的键长(nm)已标注。红色:O;蓝色:N;深灰:C;浅灰:H;黄色:S;绿色:F]
Fig.5 The most stable conformation of [HHexen][TFSA]-nSO2 (n=1,2,3,4,5) [the bond length (nm) of the main hydrogen bonding site has been marked, red: O; blue: N; dark gray: C; light gray: H; yellow: S; green: F]
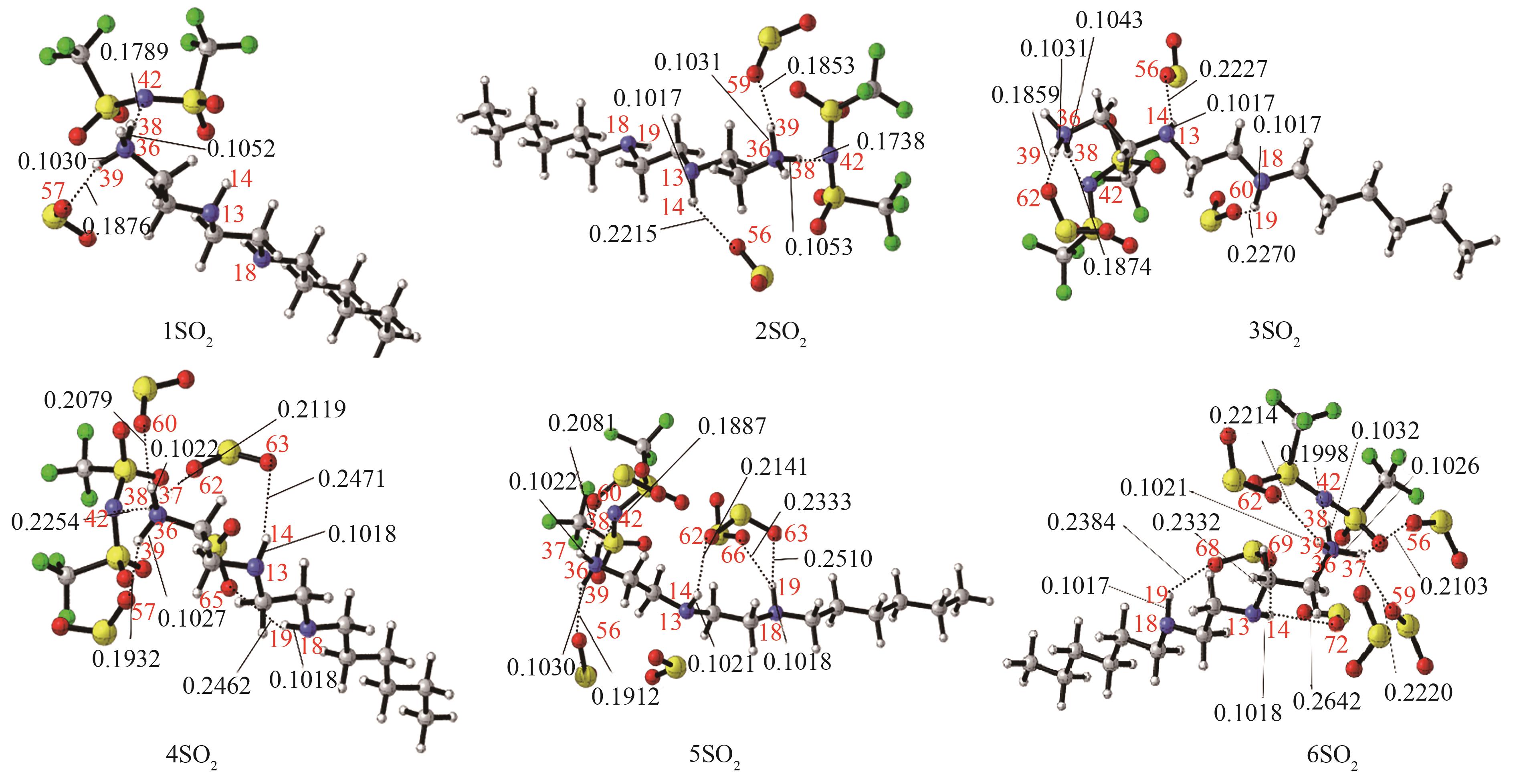
图6 [HHexdien][TFSA]-nSO2(n=1,2,3,4,5,6)的最稳定构象[主要氢键部位的键长(nm)已标注。红色:O; 蓝色:N; 深灰:C; 浅灰:H; 黄色:S; 绿色: F]
Fig.6 The most stable conformation of [HHexdien] [TFSA]-nSO2 (n=1,2,3,4,5,6) [the bond length (nm) of the main hydrogen bonding site has been marked, red: O; blue: N; dark gray: C; light gray: H; yellow: S; green: F]
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 | 键长r/nm | 键长变化值Δr/nm |
|---|---|---|---|---|---|
| [HHexam][TFSA] | 20N—21H | 3500 | ― | 0.1025 | ― |
| 20N—22H | 2591 | ― | 0.1073 | ― | |
| 20N—23H | 3563 | ― | 0.1018 | ― | |
| 1SO2 | 20N—21H | 3372 | +78 | 0.1030 | 0.0005 |
| 20N—22H | 2833 | -243 | 0.1058 | -0.0015 | |
| 2SO2 | 20N—21H | 3398 | +52 | 0.1027 | 0.0002 |
| 20N—22H | 3055 | -464 | 0.1046 | -0.0027 | |
| 20N—23H | 3472 | +91 | 0.1023 | 0.0005 | |
| 3SO2 | 20N—21H | 3422 | +28 | 0.1024 | -0.0001 |
| 20N—22H | 3202 | -611 | 0.1038 | -0.0035 | |
| 20N—23H | 3422 | +141 | 0.1026 | 0.0008 | |
| 4SO2 | 20N—22H | 3269 | -678 | 0.1035 | -0.0038 |
| 20N—23H | 3418 | +145 | 0.1026 | 0.0008 | |
| [HHexen][TFSA] | 28N—30H | 3554.78 | ― | 0.1018 | ― |
| 28N—31H | 3428.56 | ― | 0.1026 | ― | |
| 28N—29H | 2651.64 | ― | 0.1070 | ― | |
| 1SO2 | 28N—31H | 3361 | +68 | 0.1029 | 0.0003 |
| 28N—29H | 2923 | -271 | 0.1054 | -0.0016 | |
| 2SO2 | 28N—30H | 3499 | +56 | 0.1022 | 0.0004 |
| 28N—31H | 3397 | +32 | 0.1028 | 0.0002 | |
| 28N—29H | 3071 | -419 | 0.1045 | -0.0025 | |
| 3SO2 | 28N—30H | 3485.16 | +70 | 0.1023 | 0.0005 |
| 28N—31H | 3386.27 | +42 | 0.1027 | 0.0001 | |
| 28N—29H | 3206.82 | -555 | 0.1039 | -0.0031 | |
| 4SO2 | 28N—30H | 3512 | +42 | 0.1023 | 0.0005 |
| 28N—29H | 3182 | -531 | 0.1039 | -0.0031 | |
| 5SO2 | 28N—30H | 3471 | +84 | 0.1025 | 0.0007 |
| 28N—29H | 3305 | -653 | 0.1032 | -0.0038 | |
| [HHexdien][TFSA] | 36N—39H | 3450 | ― | 0.1025 | ― |
| 36N—38H | 2949 | ― | 0.1066 | ― | |
| 1SO2 | 36N—39H | 3351 | +99 | 0.1030 | 0.0005 |
| 36N—38H | 2949 | -244 | 0.1052 | -0.0014 | |
| 2SO2 | 36N—39H | 3318 | +132 | 0.1033 | 0.0008 |
| 36N—38H | 3049 | -343 | 0.1046 | -0.0020 | |
| 3SO2 | 36N—39H | 3344 | +107 | 0.1027 | 0.0002 |
| 36N—38H | 3104 | -399 | 0.1053 | -0.0013 | |
| 4SO2 | 36N—39H | 3365 | +85 | 0.1025 | 0.0000 |
| 36N—38H | 3365 | -660 | 0.1043 | -0.0023 | |
| 5SO2 | 36N—39H | 3383 | +67 | 0.1029 | 0.0004 |
| 36N—38H | 3185 | -480 | 0.1038 | -0.0028 | |
| 6SO2 | 36N—38H | 3321 | -616 | 0.1032 | -0.0034 |
表1 PILs及PILs-nSO2的最稳定构象氢键作用显著的BCP处的振动频率、键长及其变化值
Table 1 Bond lengths, vibration frequencies and their variation values, at the BCP of the most stable conformation for PILs and PILs-nSO2
| 构型 | 化学键 | 振动频率ν/cm-1 | 频率变化值Δν/cm-1 | 键长r/nm | 键长变化值Δr/nm |
|---|---|---|---|---|---|
| [HHexam][TFSA] | 20N—21H | 3500 | ― | 0.1025 | ― |
| 20N—22H | 2591 | ― | 0.1073 | ― | |
| 20N—23H | 3563 | ― | 0.1018 | ― | |
| 1SO2 | 20N—21H | 3372 | +78 | 0.1030 | 0.0005 |
| 20N—22H | 2833 | -243 | 0.1058 | -0.0015 | |
| 2SO2 | 20N—21H | 3398 | +52 | 0.1027 | 0.0002 |
| 20N—22H | 3055 | -464 | 0.1046 | -0.0027 | |
| 20N—23H | 3472 | +91 | 0.1023 | 0.0005 | |
| 3SO2 | 20N—21H | 3422 | +28 | 0.1024 | -0.0001 |
| 20N—22H | 3202 | -611 | 0.1038 | -0.0035 | |
| 20N—23H | 3422 | +141 | 0.1026 | 0.0008 | |
| 4SO2 | 20N—22H | 3269 | -678 | 0.1035 | -0.0038 |
| 20N—23H | 3418 | +145 | 0.1026 | 0.0008 | |
| [HHexen][TFSA] | 28N—30H | 3554.78 | ― | 0.1018 | ― |
| 28N—31H | 3428.56 | ― | 0.1026 | ― | |
| 28N—29H | 2651.64 | ― | 0.1070 | ― | |
| 1SO2 | 28N—31H | 3361 | +68 | 0.1029 | 0.0003 |
| 28N—29H | 2923 | -271 | 0.1054 | -0.0016 | |
| 2SO2 | 28N—30H | 3499 | +56 | 0.1022 | 0.0004 |
| 28N—31H | 3397 | +32 | 0.1028 | 0.0002 | |
| 28N—29H | 3071 | -419 | 0.1045 | -0.0025 | |
| 3SO2 | 28N—30H | 3485.16 | +70 | 0.1023 | 0.0005 |
| 28N—31H | 3386.27 | +42 | 0.1027 | 0.0001 | |
| 28N—29H | 3206.82 | -555 | 0.1039 | -0.0031 | |
| 4SO2 | 28N—30H | 3512 | +42 | 0.1023 | 0.0005 |
| 28N—29H | 3182 | -531 | 0.1039 | -0.0031 | |
| 5SO2 | 28N—30H | 3471 | +84 | 0.1025 | 0.0007 |
| 28N—29H | 3305 | -653 | 0.1032 | -0.0038 | |
| [HHexdien][TFSA] | 36N—39H | 3450 | ― | 0.1025 | ― |
| 36N—38H | 2949 | ― | 0.1066 | ― | |
| 1SO2 | 36N—39H | 3351 | +99 | 0.1030 | 0.0005 |
| 36N—38H | 2949 | -244 | 0.1052 | -0.0014 | |
| 2SO2 | 36N—39H | 3318 | +132 | 0.1033 | 0.0008 |
| 36N—38H | 3049 | -343 | 0.1046 | -0.0020 | |
| 3SO2 | 36N—39H | 3344 | +107 | 0.1027 | 0.0002 |
| 36N—38H | 3104 | -399 | 0.1053 | -0.0013 | |
| 4SO2 | 36N—39H | 3365 | +85 | 0.1025 | 0.0000 |
| 36N—38H | 3365 | -660 | 0.1043 | -0.0023 | |
| 5SO2 | 36N—39H | 3383 | +67 | 0.1029 | 0.0004 |
| 36N—38H | 3185 | -480 | 0.1038 | -0.0028 | |
| 6SO2 | 36N—38H | 3321 | -616 | 0.1032 | -0.0034 |
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 36N | 37H | 38H | 39H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexdien][TFSA] | 36N—37H | -0.738 | 0.415 | — | — | — |
| 36N—38H | — | — | 0.487 | — | — | |
| 36N—39H | — | — | — | 0.444 | — | |
| 1SO2 | 36N—39H…57O | -0.741(-0.003) | — | — | 0.459(+0.015) | -0.893(-0.107) |
| 2SO2 | 36N—39H…59O | -0.743(-0.005) | — | — | 0.468(+0.024) | -0.895(-0.109) |
| 3SO2 | 36N—39H…62O | -0.735(+0.003) | — | — | 0.459(+0.015) | -0.892(-0.106) |
| 4SO2 | 36N—39H…57O | -0.733(+0.005) | — | — | 0.456(+0.012) | -0.878(-0.092) |
| 36N—38H…62O | — | — | 0.475(-0.012) | — | -0.902(-0.116) | |
| 5SO2 | 36N—39H…56O | -0.733(+0.005) | — | — | 0.469(+0.025) | -0.915(-0.129) |
| 36N—37H…60O | — | 0.445(+0.030) | — | — | -0.878(-0.092) | |
| 6SO2 | 36N—39H…62O | -0.728(+0.010) | — | — | 0.442(-0.002) | — |
| 36N—37H…56O | — | 0.470 (+0.055) | — | — | -0.827(-0.041) | |
| 36N—37H…59O | — | — | — | — | -0.895(-0.109) | |
表4 [HHexdien][TFSA]-nSO2主要氢键部位原子上的NPA电荷分布值
Table 4 Selected partial charges from NPA on each atom of the main hydrogen bond site of [HHexdien][TFSA]-nSO2
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 36N | 37H | 38H | 39H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexdien][TFSA] | 36N—37H | -0.738 | 0.415 | — | — | — |
| 36N—38H | — | — | 0.487 | — | — | |
| 36N—39H | — | — | — | 0.444 | — | |
| 1SO2 | 36N—39H…57O | -0.741(-0.003) | — | — | 0.459(+0.015) | -0.893(-0.107) |
| 2SO2 | 36N—39H…59O | -0.743(-0.005) | — | — | 0.468(+0.024) | -0.895(-0.109) |
| 3SO2 | 36N—39H…62O | -0.735(+0.003) | — | — | 0.459(+0.015) | -0.892(-0.106) |
| 4SO2 | 36N—39H…57O | -0.733(+0.005) | — | — | 0.456(+0.012) | -0.878(-0.092) |
| 36N—38H…62O | — | — | 0.475(-0.012) | — | -0.902(-0.116) | |
| 5SO2 | 36N—39H…56O | -0.733(+0.005) | — | — | 0.469(+0.025) | -0.915(-0.129) |
| 36N—37H…60O | — | 0.445(+0.030) | — | — | -0.878(-0.092) | |
| 6SO2 | 36N—39H…62O | -0.728(+0.010) | — | — | 0.442(-0.002) | — |
| 36N—37H…56O | — | 0.470 (+0.055) | — | — | -0.827(-0.041) | |
| 36N—37H…59O | — | — | — | — | -0.895(-0.109) | |
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexam][TFSA] | 20N—21H | -0.737 | 0.441 | — | — | — |
| 20N—22H | — | — | 0.485 | — | — | |
| 20N—23H | — | — | — | 0.413 | — | |
| 1SO2 | 20N—21H…41O | -0.742(-0.005) | 0.454(+0.013) | — | — | -0.891(-0.105) |
| 2SO2 | 20N—21H…44O | -0.749(-0.012) | 0.450(+0.009) | — | — | -0.885(-0.099) |
| 20N—23H…40O | — | — | — | 0.446(+0.033) | -0.881(-0.095) | |
| 3SO2 | 20N—21H…43O | -0.746 (-0.009) | 0.453(+0.012) | — | — | -0.851(-0.065) |
| 20N—21H…47O | — | — | — | — | -0.870(-0.084) | |
| 20N—23H…40O | — | — | — | 0.450(+0.037) | -0.879(-0.093) | |
| 4SO2 | 20N—21H…41O | -0.747 (-0.010) | 0.450(+0.009) | — | — | -0.899(-0.113) |
| 20N—21H…44O | — | — | — | — | -0.835(-0.049) | |
| 20N—23H…47O | — | 0.458(+0.017) | — | — | -0.866(-0.080) | |
表2 [HHexam][TFSA]-nSO2主要氢键部位原子上的NPA电荷分布
Table 2 Selected partial charges from NPA on each atom of the main hydrogen bond site of [HHexam][TFSA]-nSO2
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 20N | 21H | 22H | 23H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexam][TFSA] | 20N—21H | -0.737 | 0.441 | — | — | — |
| 20N—22H | — | — | 0.485 | — | — | |
| 20N—23H | — | — | — | 0.413 | — | |
| 1SO2 | 20N—21H…41O | -0.742(-0.005) | 0.454(+0.013) | — | — | -0.891(-0.105) |
| 2SO2 | 20N—21H…44O | -0.749(-0.012) | 0.450(+0.009) | — | — | -0.885(-0.099) |
| 20N—23H…40O | — | — | — | 0.446(+0.033) | -0.881(-0.095) | |
| 3SO2 | 20N—21H…43O | -0.746 (-0.009) | 0.453(+0.012) | — | — | -0.851(-0.065) |
| 20N—21H…47O | — | — | — | — | -0.870(-0.084) | |
| 20N—23H…40O | — | — | — | 0.450(+0.037) | -0.879(-0.093) | |
| 4SO2 | 20N—21H…41O | -0.747 (-0.010) | 0.450(+0.009) | — | — | -0.899(-0.113) |
| 20N—21H…44O | — | — | — | — | -0.835(-0.049) | |
| 20N—23H…47O | — | 0.458(+0.017) | — | — | -0.866(-0.080) | |
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 28N | 27H | 30H | 31H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexen][TFSA] | 28N—29H | -0.740 | — | — | — | — |
| 28N—30H | — | — | 0.415 | — | — | |
| 28N—31H | — | — | — | 0.441 | — | |
| 1SO2 | 28N—31H…49O | -0.743(-0.003) | — | — | 0.452(+0.011) | -0.902(-0.116) |
| 2SO2 | 28N—30H…49O | -0.747(-0.007) | — | 0.443(+0.028) | — | -0.875(-0.089) |
| 28N—31H…52O | — | — | — | 0.451(+0.010) | -0.896(-0.110) | |
| 3SO2 | 28N—30H…51O | -0.745(-0.005) | — | 0.447(+0.032) | — | -0.884(-0.098) |
| 28N—31H…49O | — | — | — | 0.451(+0.010) | -0.885(-0.099) | |
| 4SO2 | 28N—30H…52O | -0.739(+0.001) | — | 0.451(+0.036) | — | -0.914(-0.128) |
| 28N—30H…55O | — | — | — | — | -0.847(-0.061) | |
| 28N—31H…49O | — | — | — | 0.449(+0.008) | -0.877(-0.091) | |
| 5SO2 | 28N—30H…51O | -0.744(-0.004) | — | 0.452(+0.037) | — | -0.900(-0.114) |
| 28N—30H…61O | — | — | — | — | -0.832(-0.046) | |
| 28N—31H…49O | — | — | — | 0.457(+0.016) | -0.867(-0.081) | |
表3 [HHexen][TFSA]-nSO2主要氢键部位原子上的NPA电荷分布
Table 3 Selected partial charges from NPA on each atom of the main hydrogen bond site of [HHexen][TFSA]-nSO2
| 构型 | 化学键 | 电荷分布/e | ||||
|---|---|---|---|---|---|---|
| 28N | 27H | 30H | 31H | O | ||
| SO2 | S—O | — | — | — | — | -0.786 |
| [HHexen][TFSA] | 28N—29H | -0.740 | — | — | — | — |
| 28N—30H | — | — | 0.415 | — | — | |
| 28N—31H | — | — | — | 0.441 | — | |
| 1SO2 | 28N—31H…49O | -0.743(-0.003) | — | — | 0.452(+0.011) | -0.902(-0.116) |
| 2SO2 | 28N—30H…49O | -0.747(-0.007) | — | 0.443(+0.028) | — | -0.875(-0.089) |
| 28N—31H…52O | — | — | — | 0.451(+0.010) | -0.896(-0.110) | |
| 3SO2 | 28N—30H…51O | -0.745(-0.005) | — | 0.447(+0.032) | — | -0.884(-0.098) |
| 28N—31H…49O | — | — | — | 0.451(+0.010) | -0.885(-0.099) | |
| 4SO2 | 28N—30H…52O | -0.739(+0.001) | — | 0.451(+0.036) | — | -0.914(-0.128) |
| 28N—30H…55O | — | — | — | — | -0.847(-0.061) | |
| 28N—31H…49O | — | — | — | 0.449(+0.008) | -0.877(-0.091) | |
| 5SO2 | 28N—30H…51O | -0.744(-0.004) | — | 0.452(+0.037) | — | -0.900(-0.114) |
| 28N—30H…61O | — | — | — | — | -0.832(-0.046) | |
| 28N—31H…49O | — | — | — | 0.457(+0.016) | -0.867(-0.081) | |
| 项目 | 弱 | 中 | 强 | PILs-nSO2构型 | 强度 |
|---|---|---|---|---|---|
| H∙∙∙Y/nm | >0.22 | 0.15~0.22 | 0.12~0.15 | 0.10~0.26 | 中等 |
| X—H vs H…Y | X—H≪H…Y | X—H˂H…Y | X—H≈H…Y | N—H˂H…O | 中等 |
| 电子密度值ρc | 0.02~0.002 | 0.02~0.05 | >0.05 | 0.027~0.084 | 中等 |
| 二阶微扰能 E(2)/(kJ/mol) | <29 | 29~150 | >150 | 29~71 | 中等 |
| 氢键能 EHB/(kJ/mol) | <16 | 16~62 | 62~167 | 30~85 | 中等 |
表5 氢键强度分类[40]
Table 5 Classification of hydrogen bond strength[40]
| 项目 | 弱 | 中 | 强 | PILs-nSO2构型 | 强度 |
|---|---|---|---|---|---|
| H∙∙∙Y/nm | >0.22 | 0.15~0.22 | 0.12~0.15 | 0.10~0.26 | 中等 |
| X—H vs H…Y | X—H≪H…Y | X—H˂H…Y | X—H≈H…Y | N—H˂H…O | 中等 |
| 电子密度值ρc | 0.02~0.002 | 0.02~0.05 | >0.05 | 0.027~0.084 | 中等 |
| 二阶微扰能 E(2)/(kJ/mol) | <29 | 29~150 | >150 | 29~71 | 中等 |
| 氢键能 EHB/(kJ/mol) | <16 | 16~62 | 62~167 | 30~85 | 中等 |
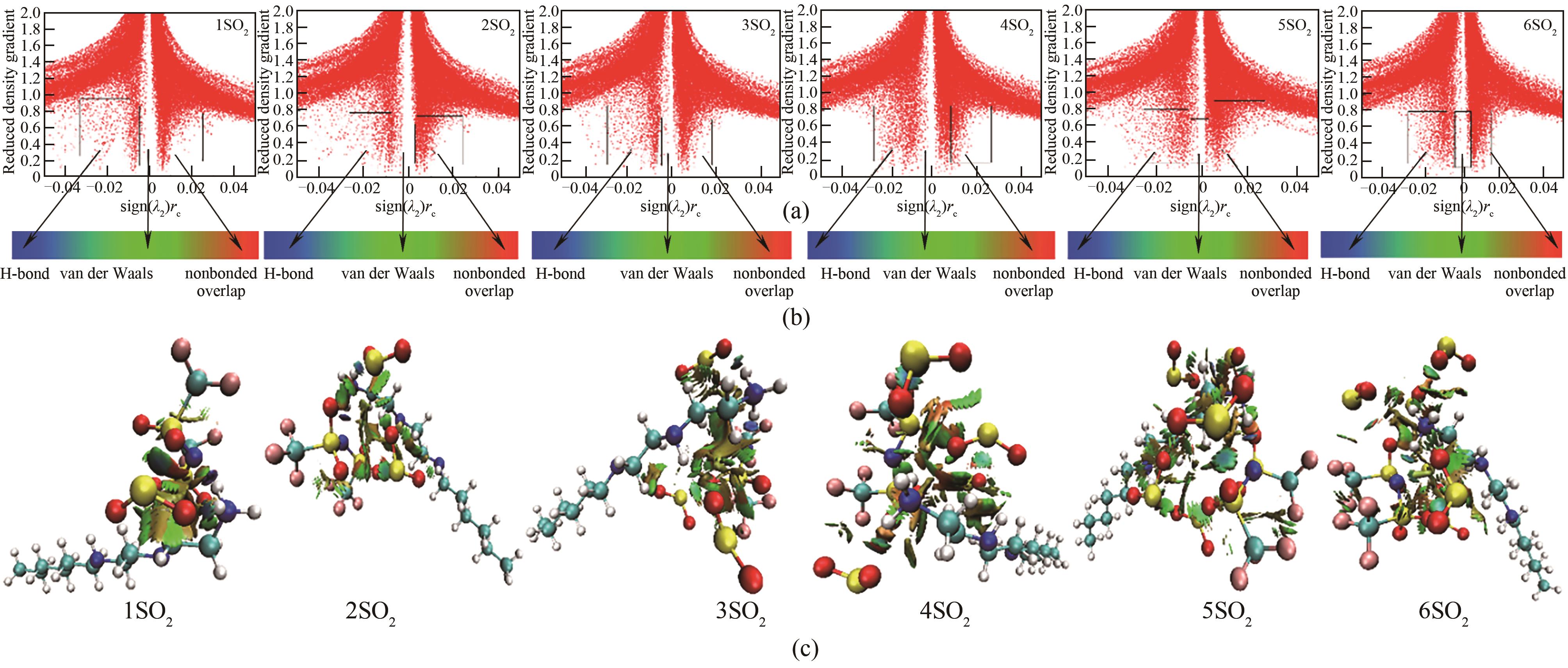
图13 [HHexdien][TFSA]-nCO2(n=1,2,3,4,5,6)的散点图和RDG等值填色面图
Fig.13 Scatter diagram and color-filled RDG isosurface map of [HHexdien][TFSA]-nSO2 (n=1,2,3,4,5,6)
| 14 | Ma J, Zhu M X, Yang X Q, et al. Different cation-anion interaction mechanisms of diamino protic ionic liquids: a density functional theory study[J]. Chemical Physics Letters, 2021, 774: 138615. |
| 15 | Patil K R, Barge S S, Bhosale B D, et al. Influence of protic ionic liquids on hydration of glycine based peptides[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2022, 265: 120378. |
| 16 | Kondratenko Y A, Antuganov D O, Kadnikova O Y, et al. Synthesis, crystal structure and properties of tris(2-hydroxypropyl)ammonium based protic ionic liquids and protic molten salts[J]. Journal of Molecular Liquids, 2021, 324: 114717. |
| 17 | Rasmus F, Anders R, Susanne M, et al. Selective gas absorption by ionic liquids[J]. Abstracts of Papers of the American Chemical Society, 2015, 250. |
| 18 | Xu Q, Jiang W, Xiao J B, et al. Solubility of sulfur dioxide in tetraglyme-NH4SCN ionic liquid: high absorption efficiency[J]. RSC Advances, 2018, 8(73): 42116-42122. |
| 19 | Yang J W, Chen P B, Pan Y M, et al. Fixation of carbon dioxide with functionalized ionic liquids[J]. Asian Journal of Organic Chemistry, 2022, 11(11): e202200527. |
| 20 | 米泽豪, 花儿. 多元胺-TFSA型质子化离子液体吸收CO2的理论分析[J]. 化工进展, 2023. DOI: 10.16085/j.issn.1000-6613.2022-2319 . |
| Mi Z H, Hua E. Theoretical analysis of CO2 absorption by polyamines-TFSA type protic ionic liquids[J]. Chemical Industry and Engineering Progress, 2023. DOI: 10.16085/j.issn.1000-6613.2022-2319 . | |
| 21 | Hua E, Liu Z, Qin L L. Effects of water content on physicochemical properties of a protic ionic liquid with monoprotic N-hexylethylenediaminium as cation and bis(trifluoromethylsulfonyl) imide as anion[J]. Journal of Solution Chemistry, 2021, 50(4): 503-516. |
| 22 | Paolone A, Haddad B, Villemin D, et al. Thermal decomposition, low temperature phase transitions and vapor pressure of less common ionic liquids based on the bis(trifuoromethanesulfonyl)imide anion[J]. Materials, 2022, 15(15): 5255. |
| 23 | Xue Z M, Qin L, Jiang J Y, et al. Thermal, electrochemical and radiolytic stabilities of ionic liquids[J]. Physical Chemistry Chemical Physics, 2018, 20(13): 8382-8402. |
| 24 | Dudko V S, Proskurnin M A. Russian studies on microfluidic systems for chemical analysis[J]. Journal of Analytical Chemistry, 2011, 66(11): 1035-1041. |
| 25 | Carolina C, Alina C. Phase transitions and electrochemical properties of ionic liquids and ionic liquid-solvent mixtures[J]. Molecules, 2021, 26(12): 3668. |
| 26 | Jaime-Leal J E, Bonilla-Petriciolet A, Bhargava V, et al. Nonlinear parameter estimation of e-NRTL model for quaternary ammonium ionic liquids using Cuckoo search[J]. Chemical Engineering Research and Design, 2015, 93: 464-472. |
| 27 | Philippi F, Rauber D, Zapp J, et al. Multiple ether-functionalized phosphonium ionic liquids as highly fluid electrolytes[J]. ChemPhysChem, 2019, 20(3): 443-455. |
| 28 | Rout A, Mishra S, Venkatesan K A, et al. Physicochemical and radiolytic degradation properties of dihexyloctanmide-imidazolium ionic liquid[J]. Journal of Molecular Liquids, 2017, 247: 93-99. |
| 29 | Zhang B, Sudre G, Quintard G, et al. Imidazolium-based poly(ionic liquid)/ionic liquid solutions: rheology, structuration and ionic transport properties[J]. Polymer, 2021, 237: 124305. |
| 30 | Xu Y, Hua E, Haghani A. Structure and hydrogen bonding of mono-protic ionic liquids composed of N-alkylethylenediaminium cations and trifluoromethanesulfonate anion[J]. Materials Today Communications, 2021, 28: 102633. |
| 31 | Er H, Xu Y, Zhao H. Properties of mono-protic ionic liquids composed of hexylammonium and hexylethylenediaminium cations with trifluoroacetate and bis(trifluoromethylsulfonyl) imide anions[J]. Journal of Molecular Liquids, 2019, 276: 379-384. |
| 32 | Hua E, Masayasu I. Protonation Properties, Applications and Effects: Properties of Protic Ionic Liquids Comprising N-alkyl Polyamines[M]. New York: Nova Science Publishers, 2019: 237-247. |
| 33 | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian09 Rev. D.01[CP]. Wallingford: Gaussian Inc., CT. , 2009. |
| 34 | Klamt A, Schüürmann G. COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient[J]. J. Chem. Soc., Perkin Trans 2, 1993(5): 799-805. |
| 35 | Parr R G. Density functional theory of atoms and molecules[C]//Fukui K, Pullman B. Horizons of Quantum Chemistry. Dordrecht: Springer, 1980: 5-15. |
| 36 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| 37 | Boys S F, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 1970, 19(4): 553-566. |
| 38 | Duarte L J, Silva A F, Richter W E, et al. Infrared intensification and hydrogen bond stabilization: beyond point charges[J]. The Journal of Physical Chemistry A, 2019, 123(30): 6482-6490. |
| 39 | Minch M J. An introduction to hydrogen bonding[J]. Journal of Chemical Education, 1999, 76(6): 759. |
| 40 | Hunt P A, Ashworth C R, Matthews R P. Hydrogen bonding in ionic liquids[J]. Chemical Society Reviews, 2015, 44(5): 1257-1288. |
| 41 | Lv R Q, Wu C C, Lin J, et al. Theoretical study on interactions between trifluoromethanesulfonate (triflate) based ionic liquid and thiophene[J]. Journal of Molecular Liquids, 2017, 237: 289-294. |
| 42 | Anbu V, Vijayalakshmi K A, Karunathan R, et al. Explosives properties of high energetic trinitrophenyl nitramide molecules: a DFT and AIM analysis[J]. Arabian Journal of Chemistry, 2019, 12(5): 621-632. |
| 43 | Klamt A. Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena[J]. The Journal of Physical Chemistry, 1995, 99(7): 2224-2235. |
| 44 | Salleh M Z M, Hadj-Kali M K, Hashim M A, et al. Ionic liquids for the separation of benzene and cyclohexane-COSMO-RS screening and experimental validation[J]. Journal of Molecular Liquids, 2018, 266: 51-61. |
| 45 | Cao Z X, Wu X J, Wei X H. Ionic liquid screening for desulfurization of coke oven gas based on COSMO-SAC model and process simulation[J]. Chemical Engineering Research and Design, 2021, 176: 146-161. |
| 46 | Sander R, Acree W E, De Visscher A, et al. Henry’s law constants (IUPAC recommendations 2021)[J]. Pure and Applied Chemistry, 2022, 94(1): 71-85. |
| 47 | Greer A J, Jacquemin J, Hardacre C. Industrial applications of ionic liquids[J]. Molecules, 2020, 25(21): 5207. |
| 48 | Neo C Y, Ouyang J Y. Functionalized carbon nanotube-induced viscosity reduction of an ionic liquid and performance improvement of dye-sensitized solar cells[J]. Electrochimica Acta, 2012, 85: 1-8. |
| 49 | Yang F X, Wang X P, Tan H Z, et al. Improvement the viscosity of imidazolium-based ionic liquid using organic solvents for biofuels[J]. Journal of Molecular Liquids, 2017, 248: 626-633. |
| 1 | Wang X B, Cui H, Liu X H, et al. Sulfur dioxide: foe or friend for life?[J]. Histology and Histopathology, 2017, 32(12): 1231-1238. |
| 2 | Cui G K, Zhao N, Wang J J, et al. Computer-assisted design of imidazolate-based ionic liquids for improving sulfur dioxide capture, carbon dioxide capture, and sulfur dioxide/carbon dioxide selectivity[J]. Chemistry—An Asian Journal, 2017, 12(21): 2863-2872. |
| 3 | Al-Enezi G, Ettouney, El-Dessouky H, et al. Solubility of sulfur dioxide in seawater[J]. Industrial & Engineering Chemistry Research, 2001, 40(5): 1434-1441. |
| 4 | Ma X X, Kaneko T, Tashimo T, et al. Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder-particle spouted bed[J]. Chemical Engineering Science, 2000, 55(20): 4643-4652. |
| 5 | Córdoba P. Status of flue gas desulphurisation (FGD) systems from coal-fired power plants: overview of the physic-chemical control processes of wet limestone FGDs[J]. Fuel, 2015, 144: 274-286. |
| 6 | Fang D A, Liao X, Zhang X F, et al. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: as a reductant to remove chromium and vanadium from vanadium industrial wastewater[J]. Journal of Hazardous Materials, 2018, 342: 436-445. |
| 7 | 张雅婷, 熊文杰, 赵天翔, 等. 咪唑类离子液体混合物用于二氧化硫高效吸收[J]. 化工学报, 2020, 71(11): 5035-5042. |
| Zhang Y T, Xiong W J, Zhao T X, et al. High capacity absorption of SO2 using imidazole ionic liquid mixtures[J]. CIESC Journal, 2020, 71(11): 5035-5042. | |
| 8 | Lei Z G, Dai C N, Chen B H. Gas solubility in ionic liquids[J]. Chemical Reviews, 2014, 114(2): 1289-1326. |
| 9 | Cui G K, Wang J J, Zhang S J. Active chemisorption sites in functionalized ionic liquids for carbon capture[J]. Chemical Society Reviews, 2016, 45(15): 4307-4339. |
| 10 | Wu W Z, Han B X, Gao H X, et al. Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angewandte Chemie International Edition, 2004, 43(18): 2415-2417. |
| 11 | Zhang H, Wu J, Zhang J, et al. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose[J]. Macromolecules, 2005, 38(20): 8272-8277. |
| 12 | Huang J, Riisager A, Berg R W, et al. Tuning ionic liquids for high gas solubility and reversible gas sorption[J]. Journal of Molecular Catalysis A: Chemical, 2008, 279(2): 170-176. |
| 13 | 李学良, 席俊松, 罗梅, 等. 一种绿色高效可循环的SO2气体吸收剂及其制备方法: 200810025024.7[P]. 2008-04-23. |
| Li X L, Xi J S, Luo M, et al. Green and efficient recyclable SO2 gas absorbent and preparation method thereof: 200810025024.7[P]. 2008-04-23. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [3] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [4] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [5] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [6] | 陈宇豪, 陈晓平, 马吉亮, 梁财. 市政污泥回转窑焚烧气态污染物排放特性研究[J]. 化工学报, 2023, 74(5): 2170-2178. |
| [7] | 李木金, 胡松, 施德磐, 赵鹏, 高瑞, 李进龙. 环氧丁烷尾气溶剂吸收及精制工艺[J]. 化工学报, 2023, 74(4): 1607-1618. |
| [8] | 杨灿, 孙雪琦, 尚明华, 张建, 张香平, 曾少娟. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(4): 1419-1432. |
| [9] | 何万媛, 陈一宇, 朱春英, 付涛涛, 高习群, 马友光. 阵列凸起微通道内气液两相传质特性研究[J]. 化工学报, 2023, 74(2): 690-697. |
| [10] | 王煦清, 严圣林, 朱礼涛, 张希宝, 罗正鸿. 填料塔中有机胺吸收CO2气液传质的研究进展[J]. 化工学报, 2023, 74(1): 237-256. |
| [11] | 姜焱龙, 张妮, 李淡然, 朱冰冰, 蒋怡晨, 陈海军, 朱跃钊. 基于COSMO-RS方法筛选离子液体用于焦油脱除[J]. 化工学报, 2022, 73(4): 1704-1713. |
| [12] | 张逸伟, 唐海荣, 何勇, 朱燕群, 王智化. 臭氧低温氧化烟气脱硝过程中的氮平衡试验研究[J]. 化工学报, 2022, 73(4): 1732-1742. |
| [13] | 霍猛, 彭晓婉, 赵金, 马秋伟, 邓春, 刘蓓, 陈光进. 基于COSMO-RS的离子液体吸收CO的溶剂筛选及H2/CO分离实验[J]. 化工学报, 2022, 73(12): 5305-5313. |
| [14] | 安美燕, 王洁冰, 徐震原, 王如竹. 基于LiCl溶液太阳能界面蒸发的连续式空气取水[J]. 化工学报, 2021, 72(S1): 70-76. |
| [15] | 徐梦凯, 李舒宏, 金正浩. 氨-水-溴化锂三元工质氨吸收式制冷性能[J]. 化工学报, 2021, 72(S1): 127-133. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
