化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5114-5127.DOI: 10.11949/0438-1157.20250252
杜宇鹏1( ), 葛春亮2, 丁垒琳1, 张力2, 胡家俊1, 俞峰苹2, 林益1, 王峰1, 蒋师1, 郭燏1(
), 葛春亮2, 丁垒琳1, 张力2, 胡家俊1, 俞峰苹2, 林益1, 王峰1, 蒋师1, 郭燏1( )
)
收稿日期:2025-03-14
修回日期:2025-06-05
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
郭燏
作者简介:杜宇鹏(2000—),男,硕士研究生,202261104180@njtech.edu.cn
基金资助:
Yupeng DU1( ), Chunliang GE2, Leilin DING1, Li ZHANG2, Jiajun HU1, Fengping YU2, Yi LIN1, Feng WANG1, Shi JIANG1, Yu GUO1(
), Chunliang GE2, Leilin DING1, Li ZHANG2, Jiajun HU1, Fengping YU2, Yi LIN1, Feng WANG1, Shi JIANG1, Yu GUO1( )
)
Received:2025-03-14
Revised:2025-06-05
Online:2025-10-25
Published:2025-11-25
Contact:
Yu GUO
摘要:
选取氨水、碳酸铵、碳酸氢铵和尿素为沉淀剂,以高比表面积Al2O3作为非溶解性铝源,Ce(NO3)3·6H2O作为可溶性铈源,通过沉积沉淀法合成CeO2-Al2O3载体,经Pt浸渍负载制备了Pt/CeO2-Al2O3催化剂,考察了沉淀剂类型对CO催化氧化性能的影响。表征结果表明,尿素均匀沉淀法制备的催化剂展现出最优活性,被归因于增强的Pt-CeO2界面相互作用。沉淀剂类型通过调控结晶动力学和前体种类,影响了CeO2的分散度、分布均匀性、形貌特征及其与Pt的相互作用。在Al2O3存在的尿素均匀沉淀中,限制孔道内的缓慢沉淀机制促进了纳米棒状CeO2粒子在Al2O3孔道内的均匀分布且高度分散,避免了氨水沉淀剂因快速沉淀而引起的纳米球状CeO2在Al2O3外表面上的富集团聚,从而强化了Pt与CeO2间的相互作用,最终实现催化性能的提升。
中图分类号:
杜宇鹏, 葛春亮, 丁垒琳, 张力, 胡家俊, 俞峰苹, 林益, 王峰, 蒋师, 郭燏. 尿素均匀沉淀法合成CeO2-Al2O3载体负载Pt催化剂的CO氧化性能[J]. 化工学报, 2025, 76(10): 5114-5127.
Yupeng DU, Chunliang GE, Leilin DING, Li ZHANG, Jiajun HU, Fengping YU, Yi LIN, Feng WANG, Shi JIANG, Yu GUO. CO oxidation performance of Pt catalysts supported on CeO2-Al2O3 supports synthesized via urea homogeneous precipitation[J]. CIESC Journal, 2025, 76(10): 5114-5127.
| Sample | Specific surface area/(m2/g) | Total pore volume/(cm3/g) | Average pore diameter/nm | CeO2 grain size①/nm | Pt dispersion②/% | T90/℃ |
|---|---|---|---|---|---|---|
| Pt/CeAl-PAH | 133 | 0.463 | 14.0 | 5.6 | 29 | 165 |
| Pt/CeAl-PAC | 130 | 0.459 | 14.1 | 11.8 | 49 | 161 |
| Pt/CeAl-PAB | 133 | 0.461 | 13.9 | 13.0 | 63 | 159 |
| Pt/CeAl-HPU | 133 | 0.449 | 13.6 | 9.3 | 69 | 149 |
| Pt/CeAl-I | 112 | 0.425 | 15.2 | 8.9 | 57 | 162 |
| Pt/Al-I | 140 | 0.476 | 13.3 | — | 41 | 174 |
| Al2O3 | 142 | 0.478 | 13.5 | — | — | — |
表1 不同催化剂上的比表面积、孔容、孔径、CeO2晶粒尺寸、Pt分散度和T90
Table 1 Specific surface area, pore volume, pore size, CeO2 grain size, Pt dispersion, and T90 on different catalysts
| Sample | Specific surface area/(m2/g) | Total pore volume/(cm3/g) | Average pore diameter/nm | CeO2 grain size①/nm | Pt dispersion②/% | T90/℃ |
|---|---|---|---|---|---|---|
| Pt/CeAl-PAH | 133 | 0.463 | 14.0 | 5.6 | 29 | 165 |
| Pt/CeAl-PAC | 130 | 0.459 | 14.1 | 11.8 | 49 | 161 |
| Pt/CeAl-PAB | 133 | 0.461 | 13.9 | 13.0 | 63 | 159 |
| Pt/CeAl-HPU | 133 | 0.449 | 13.6 | 9.3 | 69 | 149 |
| Pt/CeAl-I | 112 | 0.425 | 15.2 | 8.9 | 57 | 162 |
| Pt/Al-I | 140 | 0.476 | 13.3 | — | 41 | 174 |
| Al2O3 | 142 | 0.478 | 13.5 | — | — | — |
| Sample | Ce atomic concentration/% | [Pt0/(Pt δ++Pt0)]/% | [Ce3+/(Ce3++Ce4+)]/% | [Osur/(Osur+Olat)]/% |
|---|---|---|---|---|
| Pt/CeAl-PAH | 7.8 | 20.7 | 13.6 | 36.4 |
| Pt/CeAl-PAC | 9.9 | 27.2 | 15.3 | 38.0 |
| Pt/CeAl-PAB | 8.6 | 21.6 | 14.5 | 38.2 |
| Pt/CeAl-HPU | 1.7 | 51.2 | 20.8 | 43.0 |
| Pt/CeAl-I | 4.0 | 18.7 | 15.3 | 38.3 |
| Pt/Al-I | — | 30.6 | — | 32.6 |
表2 不同催化剂上的表面Ce原子浓度、Pt0比例、Ce3+比例和Osur比例
Table 2 Surface Ce atomic concentration, Pt0 ratio, Ce3+ ratio, and Osur ratio on different catalysts
| Sample | Ce atomic concentration/% | [Pt0/(Pt δ++Pt0)]/% | [Ce3+/(Ce3++Ce4+)]/% | [Osur/(Osur+Olat)]/% |
|---|---|---|---|---|
| Pt/CeAl-PAH | 7.8 | 20.7 | 13.6 | 36.4 |
| Pt/CeAl-PAC | 9.9 | 27.2 | 15.3 | 38.0 |
| Pt/CeAl-PAB | 8.6 | 21.6 | 14.5 | 38.2 |
| Pt/CeAl-HPU | 1.7 | 51.2 | 20.8 | 43.0 |
| Pt/CeAl-I | 4.0 | 18.7 | 15.3 | 38.3 |
| Pt/Al-I | — | 30.6 | — | 32.6 |
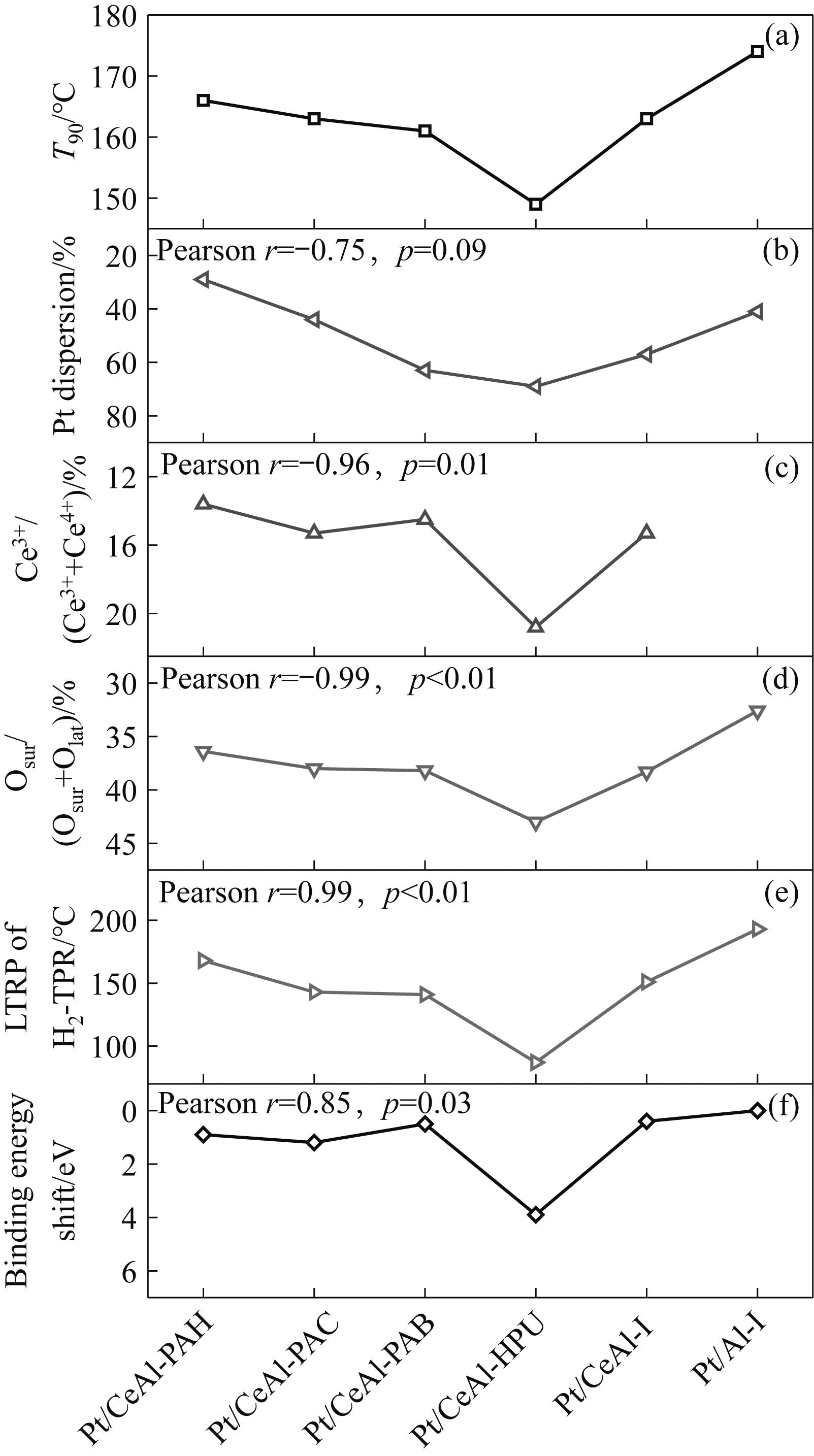
图5 不同催化剂上T90(a)、Pt分散度(b)、Ce3+比例(c)、Osur比例(d)、H2-TPR的LTRP (e)及Pt 4f结合能偏移量(f)之间的关系
Fig.5 Relationships between T90 (a), Pt dispersion (b), Ce3+ ratio (c), Osur ratio (d), low-temperature peak temperature of H₂-TPR (e), and binding energy shift of Pt 4f (f) on different catalysts
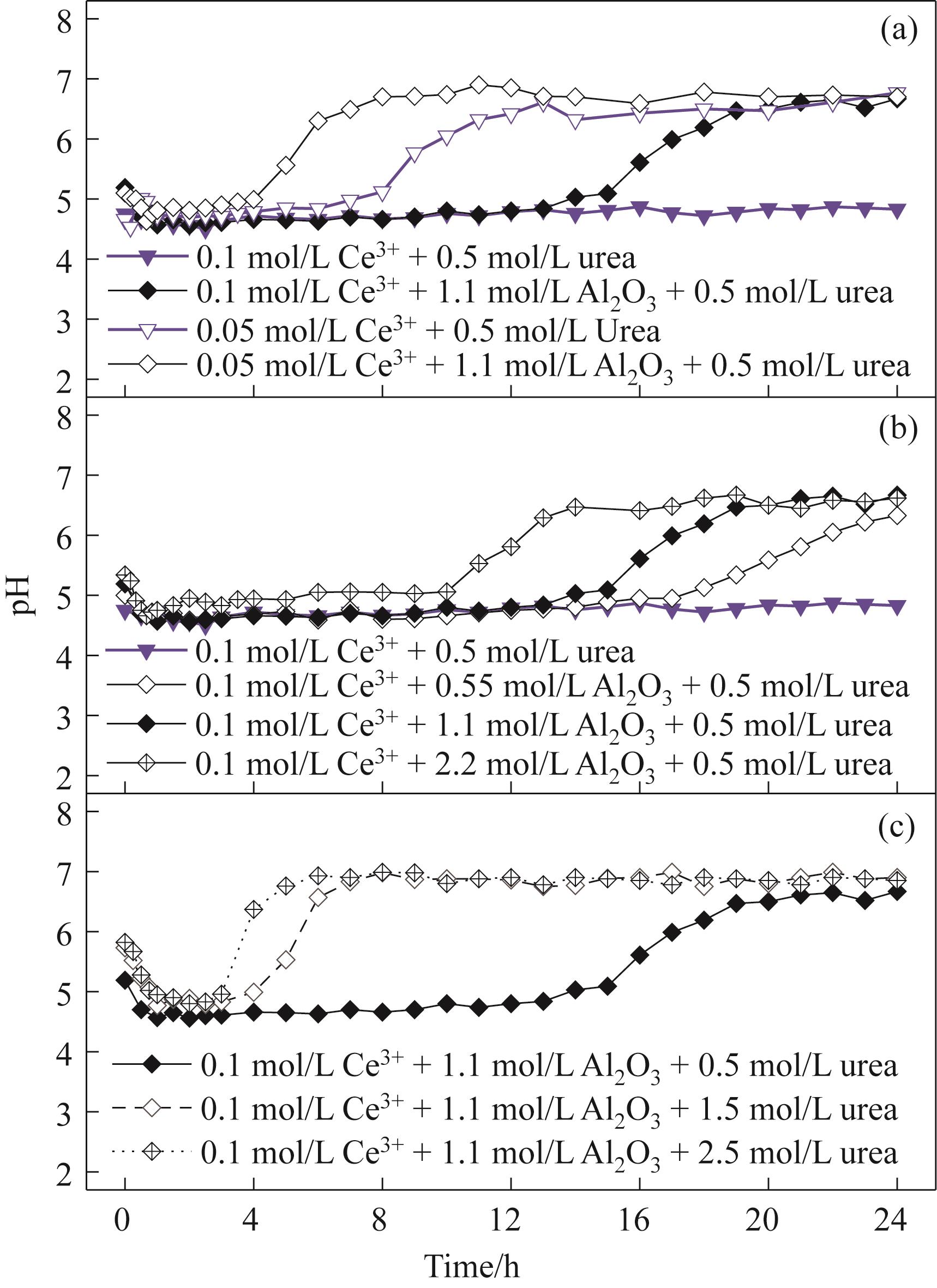
图7 Ce3+(a)、Al2O3(b)和尿素(c)的含量对尿素均匀沉淀过程中pH经时变化趋势的影响
Fig.7 Effects of contents of Ce3+(a), Al2O3(b), and urea(c) on the time-dependent pH variation trend during the urea homogeneous precipitation
| Sample | Ce3+/Al2O3/urea | CeO2 grain size/nm | Specific surface area/(m2/g) | Pt dispersion | LTRP of H2-TPR/℃ | CO T90/℃ |
|---|---|---|---|---|---|---|
| Pt/CeAl-HPU5 | 0.1/1.1/0.5 | 9.3 | 132 | 0.69 | 87 | 149 |
| Pt/CeAl-HPU15 | 0.1/1.1/1.5 | 11.4 | 137 | 0.61 | 97 | 154 |
| Pt/CeAl-HPU25 | 0.1/1.1/2.5 | 13.7 | 135 | 0.51 | 112 | 160 |
表3 均匀沉淀过程中尿素含量对CeO2晶粒尺寸、比表面积、Pt分散度、H2-TPR的LTRP及T90的影响
Table 3 Effect of urea content in the homogeneous precipitation process on the grain size of CeO2, specific surface area, Pt dispersion, LTRP in H2-TPR, and T90
| Sample | Ce3+/Al2O3/urea | CeO2 grain size/nm | Specific surface area/(m2/g) | Pt dispersion | LTRP of H2-TPR/℃ | CO T90/℃ |
|---|---|---|---|---|---|---|
| Pt/CeAl-HPU5 | 0.1/1.1/0.5 | 9.3 | 132 | 0.69 | 87 | 149 |
| Pt/CeAl-HPU15 | 0.1/1.1/1.5 | 11.4 | 137 | 0.61 | 97 | 154 |
| Pt/CeAl-HPU25 | 0.1/1.1/2.5 | 13.7 | 135 | 0.51 | 112 | 160 |
| [1] | 贺克斌. 打赢蓝天保卫战需要加快钢铁行业超低排放改造[N]. 中国环境报, 2019-05-06. |
| He K B. Winning the battle to defend the blue sky requires accelerating the ultra-low emission transformation of the steel industry[N]. China Environment News, 2019-05-06. | |
| [2] | 周昊, 成毅, 周明熙, 等. Pt涂层蜂窝金属和Ce改性Fe2O3催化CO的性能对比[J]. 工程科学学报, 2020, 42(1): 70-77. |
| Zhou H, Cheng Y, Zhou M X, et al. Analysis of CO catalytic oxidation by Pt-loading catalyst and Ce-doped Fe2O3 [J]. Chinese Journal of Engineering, 2020, 42(1): 70-77. | |
| [3] | Zhang T T, Xu J C, Sun Y, et al. Insight into the metal-support interaction of Pt and β-MnO2 in CO oxidation[J]. Molecules, 2023, 28(19): 6879. |
| [4] | Kim H, Kim J, Kwak J H. Origin of higher CO oxidation activity of Pt/rutile than that of Pt/anatase[J]. The Journal of Physical Chemistry C, 2023, 127(15): 7142-7150. |
| [5] | Liu J H, Ding T, Zhang H, et al. Engineering surface defects and metal-support interactions on Pt/TiO2(B) nanobelts to boost the catalytic oxidation of CO[J]. Catalysis Science & Technology, 2018, 8(19): 4934-4944. |
| [6] | Tomita A, Miki T, Tai Y. Effect of water treatment and Ce doping of Pt/Al2O3 catalysts on Pt sintering and propane oxidation[J]. Research on Chemical Intermediates, 2021, 47(7): 2935-2950. |
| [7] | Peng R S, Sun X B, Li S J, et al. Shape effect of Pt/CeO2 catalysts on the catalytic oxidation of toluene[J]. Chemical Engineering Journal, 2016, 306: 1234-1246. |
| [8] | Pastor-Pérez L, Ramos-Fernández E V, Sepúlveda-Escribano A. Effect of the CeO2 synthesis method on the behaviour of Pt/CeO2 catalysis for the water-gas shift reaction[J]. International Journal of Hydrogen Energy, 2019, 44(39): 21837-21846. |
| [9] | Kowalik P, Antoniak-Jurak K, Próchniak W, et al. The evaluation of synthesis route impact on structure, morphology and LT-WGS activity of Cu/ZnO/Al2O3 catalysts[J]. Catalysis Letters, 2017, 147(6): 1422-1433. |
| [10] | Huber F, Venvik H, Rønning M, et al. Preparation and characterization of nanocrystalline, high-surface area Cu-Ce-Zr mixed oxide catalysts from homogeneous co-precipitation[J]. Chemical Engineering Journal, 2008, 137(3): 686-702. |
| [11] | Talkhoncheh S K, Haghighi M, Minaei S, et al. Synthesis of CuO/ZnO/Al2O3/ZrO2/CeO2 nanocatalysts via homogeneous precipitation and combustion methods used in methanol steam reforming for fuel cell grade hydrogen production[J]. RSC Advances, 2016, 6(62): 57199-57209. |
| [12] | de A A Soler-Illia G J, Candal R J, Regazzoni A E, et al. Synthesis of mixed copper-zinc basic carbonates and Zn-doped tenorite by homogeneous alkalinization[J]. Chemistry of Materials, 1997, 9(1): 184-191. |
| [13] | Matijevic E. Preparation and properties of uniform size colloids[J]. Chemistry of Materials, 1993, 5(4): 412-426. |
| [14] | Lee J, Ryou Y, Chan X J, et al. How Pt interacts with CeO2 under the reducing and oxidizing environments at elevated temperature: the origin of improved thermal stability of Pt/CeO2 compared to CeO2 [J]. The Journal of Physical Chemistry C, 2016, 120(45): 25870-25879. |
| [15] | An K, Alayoglu S, Musselwhite N, et al. Enhanced CO oxidation rates at the interface of mesoporous oxides and Pt nanoparticles[J]. Journal of the American Chemical Society, 2013, 135(44): 16689-16696. |
| [16] | Nagai Y, Hirabayashi T, Dohmae K, et al. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide-support interaction[J]. Journal of Catalysis, 2006, 242(1): 103-109. |
| [17] | Jeong M H, So J, Oh J, et al. Cerium-modified Pt/Al2O3 for NH3 synthesis by NO reduction with H2 [J]. Applied Surface Science, 2023, 638: 158067. |
| [18] | Fu X H, Liu Y X, Deng J G, et al. Intermetallic compound PtMn y -derived Pt-MnO x supported on mesoporous CeO2: highly efficient catalysts for the combustion of toluene[J]. Applied Catalysis A: General, 2020, 595: 117509. |
| [19] | Pérez-Pastenes H, Viveros-García T. A new insight over oxygen storage capacity, SMSI, and dispersion effects on VOC oxidation using Pt/Al2O3-CeO2 catalysts[J]. Topics in Catalysis, 2022, 65(13): 1530-1540. |
| [20] | Shi H H, Yang P X, Huang L, et al. Single-atom Pt-CeO2/Co3O4 catalyst with ultra-low Pt loading and high performance for toluene removal[J]. Journal of Colloid and Interface Science, 2023, 641: 972-980. |
| [21] | Li S S, Li X, Dan Y, et al. Designed synthesis of nanostructured Al2O3 stabilized homogeneous CeO2-ZrO2 solid solution as highly active support for Pd-only three-way catalyst[J]. Molecular Catalysis, 2019, 477: 110513. |
| [22] | Choi Y S, Kim J R, Hwang J H, et al. Effect of reduction temperature on the activity of Pt-Sn/Al2O3 catalysts for propane dehydrogenation[J]. Catalysis Today, 2023, 411: 113957. |
| [23] | Xu W F, Niu P Y, Guo H Q, et al. Hydrogenolysis of glycerol to 1, 3-propanediol over a Al2O3-supported platinum tungsten catalyst with two-dimensional open structure[J]. Reaction Kinetics, Mechanisms and Catalysis, 2021, 133(1): 173-189. |
| [24] | Wang Y, Liu H H, Wang S Y, et al. Remarkable enhancement of dichloromethane oxidation over potassium-promoted Pt/Al2O3 catalysts[J]. Journal of Catalysis, 2014, 311: 314-324. |
| [25] | Yan S, Zhao M, Wang J L, et al. The preparation of Pd/CeO2-ZrO2-Al2O3 catalyst with superior structural stability: effect of zirconia incorporation method[J]. Journal of Materials Science, 2020, 55(23): 9993-10008. |
| [26] | Mao M Y, Lv H Q, Li Y Z, et al. Metal support interaction in Pt nanoparticles partially confined in the mesopores of microsized mesoporous CeO2 for highly efficient purification of volatile organic compounds[J]. ACS Catalysis, 2016, 6(1): 418-427. |
| [27] | Lyu Y, Xu J Y, Chen S, et al. Simultaneous catalytic oxidation of toluene and CO over Cu-V/Al-Ce catalysts: physicochemical properties-activity relationship and simultaneous oxidation mechanism[J]. Journal of Hazardous Materials, 2024, 466: 133507. |
| [28] | Wang C, Zhang C H, Hua W C, et al. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts[J]. Chemical Engineering Journal, 2017, 315: 392-402. |
| [29] | Zhang L, Chu P Q, Wang Y H, et al. Promotion effect of strong CeO2/Co3O4 interfacial interaction for light alkane catalytic removal[J]. Applied Catalysis B: Environment and Energy, 2025, 369: 125150. |
| [30] | Su H, Gao W, Li L Q, et al. Oxygen vacancy-rich CeO2 quantum dots boost the activity and durability of Pt/C for methanol oxidation and oxygen reduction reactions[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(33): 12317-12325. |
| [31] | Wang C, Sasmaz E, Wen C, et al. Pd supported on SnO2-MnO x -CeO2 catalysts for low temperature CO oxidation[J]. Catalysis Today, 2015, 258: 481-486. |
| [32] | 顾欧昀, 廖永涛, 陈锐杰, 等. 铜锰复合氧化物催化剂上甲苯的催化燃烧[J]. 化工学报, 2016, 67(7): 2832-2840. |
| Gu O Y, Liao Y T, Chen R J, et al. Catalytic combustion of toluene over Cu-Mn mixed oxide catalyst[J]. CIESC Journal, 2016, 67(7): 2832-2840. | |
| [33] | Yang P, Yang S S, Shi Z N, et al. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts[J]. Applied Catalysis B: Environmental, 2015, 162: 227-235. |
| [34] | 王健礼, 王康才, 曹红岩. Pt/γ-Al2O3/Ce x Zr1- x O2催化剂低温催化燃烧去除饮食油烟[J]. 物理化学学报, 2009, 25(4): 689-693. |
| Wang J L, Wang K C, Cao H Y, et al. Low temperature catalytic combustion of cooking fume over Pt/γ-Al2O3/Ce x Zr1- x O2 catalyst[J]. Acta Physico-Chimica Sinica, 2009, 25(4): 689-693. | |
| [35] | 杨黄根, 晏全, 韦庆敏, 等. 制备方法对铈锆铝复合氧化物还原热处理性能的影响[J]. 功能材料, 2019, 50(11): 11182-11189. |
| Yang H G, Yan Q, Wei Q M, et al. Effect of preparation method on the performance of CeO2-ZrO2-Al2O3 composite oxide after reductive treatment[J]. Journal of Functional Materials, 2019, 50(11): 11182-11189. | |
| [36] | Lan L, Huang X, Zhou W Q, et al. Development of a thermally stable Pt catalyst by redispersion between CeO2 and Al2O3 [J]. RSC Advances, 2021, 11(12): 7015-7024. |
| [37] | Lan L, Xiang J H, Huang L, et al. Synthesis of a highly stable Pt/CeO2/Al2O3 catalyst for gasoline engine emission control by adjusting Pt distribution[J]. The Canadian Journal of Chemical Engineering, 2022, 100(4): 827-837. |
| [38] | Gao Y X, Wang W D, Chang S J, et al. Morphology effect of CeO2 support in the preparation, metal-support interaction, and catalytic performance of Pt/CeO2 catalysts[J]. ChemCatChem, 2013, 5(12): 3610-3620. |
| [39] | Xi K, Wang Y, Jiang K, et al. Support interaction of Pt/CeO2 and Pt/SiC catalysts prepared by nano platinum colloid deposition for CO oxidation[J]. Journal of Rare Earths, 2020, 38(4): 376-383. |
| [40] | Liu H H, Wang Y, Jia A P, et al. Oxygen vacancy promoted CO oxidation over Pt/CeO2 catalysts: a reaction at Pt-CeO2 interface[J]. Applied Surface Science, 2014, 314: 725-734. |
| [41] | André R F, Rousse G, Sassoye C, et al. From Ce(OH)3 to nanoscaled CeO2: identification and crystal structure of a cerium oxyhydroxide intermediate phase[J]. Chemistry of Materials, 2023, 35(13): 5040-5048. |
| [42] | Ikuma Y, Oosawa H, Shimada E, et al. Effect of microwave radiation on the formation of Ce2O(CO3)2·H2O in aqueous solution[J]. Solid State Ionics, 2002, 151(1/2/3/4): 347-352. |
| [43] | 罗尧尧, 王天, 刘秉国, 等. 微反应器中Ce2O(CO3)2·H2O前体的合成及CeO2的制备研究[J]. 中国稀土学报, 2020, 38(4): 474-482. |
| Luo Y Y, Wang T, Liu B G, et al. Synthesis of cerium oxide carbonate hydrate precursor in microreactor and preparation of ceria[J]. Journal of the Chinese Society of Rare Earths, 2020, 38(4): 474-482. | |
| [44] | Shishido T, Yamamoto M, Li D L, et al. Water-gas shift reaction over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation[J]. Applied Catalysis A: General, 2006, 303(1): 62-71. |
| [45] | Nie M X, Xu Z Y, Luo L, et al. One-pot synthesis of ultrafine trimetallic PtPdCu alloy nanoparticles decorated on carbon nanotubes for bifunctional catalysis of ethanol oxidation and oxygen reduction[J]. Journal of Colloid and Interface Science, 2023, 643: 26-37. |
| [1] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [2] | 张茹, 朱传强, 张栋, 黄政, 肖雨果, 李明, 李长明. 采用高分子非催化还原脱硝的垃圾焚烧工艺伴生固废含氮污染物特征研究[J]. 化工学报, 2025, 76(9): 4944-4959. |
| [3] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [4] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [5] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [6] | 杨敏, 段新伟, 吴俊宏, 米杰, 王建成, 武蒙蒙. Sm2O3/γ-Al2O3催化剂的COS催化水解性能及失活机制[J]. 化工学报, 2025, 76(8): 4061-4070. |
| [7] | 周媚, 曾浩桀, 蒋火炎, 蒲婷, 曾星星, 刘宝玉. 二次晶化法改性合成MTW分子筛及其在苯和环己烯烷基化反应中的催化性能[J]. 化工学报, 2025, 76(8): 4071-4080. |
| [8] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [9] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [10] | 赵世颖, 左志帅, 贺梦颖, 安华良, 赵新强, 王延吉. Co-Pt/HAP的制备及其催化1,2-丙二醇氨化反应[J]. 化工学报, 2025, 76(7): 3305-3315. |
| [11] | 卢煦旸, 徐强, 康浩鹏, 史健, 曹泽水, 郭烈锦. 化学链制氢系统中磁铁矿氧载体的CO还原特性研究[J]. 化工学报, 2025, 76(7): 3286-3294. |
| [12] | 李秋英, 花亦怀, 程昊, 张涵玮, 刘文睿, 白昊川, 王凯, 邱利民. 集成ORC系统的高效氢液化流程设计研究[J]. 化工学报, 2025, 76(7): 3651-3658. |
| [13] | 唐银香, 朱风, 范莹莹, 龙雨欣, 代雍, 邓春玲, 黄小凤. 制备条件对改性电石渣低温共脱除COS和CS2的影响[J]. 化工学报, 2025, 76(7): 3639-3650. |
| [14] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [15] | 杨盛华, 孙阳杰, 薛晓君, 米杰, 王建成, 冯宇. 缺陷型金属氧化物脱除气体污染物研究进展[J]. 化工学报, 2025, 76(6): 2469-2482. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号