化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3639-3650.DOI: 10.11949/0438-1157.20241329
唐银香1( ), 朱风1, 范莹莹2, 龙雨欣1, 代雍1, 邓春玲1, 黄小凤1(
), 朱风1, 范莹莹2, 龙雨欣1, 代雍1, 邓春玲1, 黄小凤1( )
)
收稿日期:2024-11-20
修回日期:2025-01-07
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
黄小凤
作者简介:唐银香(2000—),女,硕士研究生,2814857817@qq.com
基金资助:
Yinxiang TANG1( ), Feng ZHU1, Yingying FAN2, Yuxin LONG1, Yong DAI1, Chunling DENG1, Xiaofeng HUANG1(
), Feng ZHU1, Yingying FAN2, Yuxin LONG1, Yong DAI1, Chunling DENG1, Xiaofeng HUANG1( )
)
Received:2024-11-20
Revised:2025-01-07
Online:2025-07-25
Published:2025-08-13
Contact:
Xiaofeng HUANG
摘要:
羰基硫(COS)和二硫化碳(CS2)常共存于钢铁行业的含硫污染物中。通过超声辅助浸渍法制备不同改性电石渣用于低温下共脱除COS和CS2,研究不同活性组分、组分含量及煅烧温度对改性电石渣共脱除COS和CS2的影响,借助XRD、N2-BET、XPS、FTIR和TG-DTA等方法进行表征,以探究其脱除机理。结果表明,当焙烧温度为750℃、活性组分为6.25%(质量分数)KOH时制备的改性电石渣催化水解效果最佳,总硫容达到180.68 mg/g;在共脱除COS和CS2的过程中,负载的KOH为催化水解提供—OH基团,水解产物硫化氢(H2S)一部分进一步发生氧化反应,一部分与金属离子结合,并最终生成硫酸盐和金属硫化物达到固硫作用。本文可为电石渣的高附加值资源化利用、COS和CS2高效催化水解剂的开发提供理论支撑。
中图分类号:
唐银香, 朱风, 范莹莹, 龙雨欣, 代雍, 邓春玲, 黄小凤. 制备条件对改性电石渣低温共脱除COS和CS2的影响[J]. 化工学报, 2025, 76(7): 3639-3650.
Yinxiang TANG, Feng ZHU, Yingying FAN, Yuxin LONG, Yong DAI, Chunling DENG, Xiaofeng HUANG. Effect of preparation conditions on low-temperature co-removal of COS and CS2 from modified calcium carbide slag[J]. CIESC Journal, 2025, 76(7): 3639-3650.
| 化学组成 | 质量分数/% |
|---|---|
| CaO | 84.825 |
| SiO2 | 3.079 |
| Al2O3 | 1.904 |
| Cl- | 0.655 |
| SO3 | 0.567 |
| Na2O | 0.142 |
| Fe2O3 | 0.130 |
表1 电石渣化学组成
Table 1 Chemical composition of calcium carbide slag
| 化学组成 | 质量分数/% |
|---|---|
| CaO | 84.825 |
| SiO2 | 3.079 |
| Al2O3 | 1.904 |
| Cl- | 0.655 |
| SO3 | 0.567 |
| Na2O | 0.142 |
| Fe2O3 | 0.130 |
| 样品 | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) | 总硫容/(mg/g) |
|---|---|---|---|---|---|
| 改性未焙烧电石渣 | 636 | 31 | 41.96 | 0.19 | 42.15 |
| 改性焙烧电石渣 | 1744 | 56 | 180.18 | 0.50 | 180.68 |
表2 改性电石渣对COS和CS2的同时脱除的穿透时间与穿透硫容
Table 2 Penetration time and penetration sulfur capacity of modified calcium carbide slag for simultaneous removal of COS and CS2
| 样品 | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) | 总硫容/(mg/g) |
|---|---|---|---|---|---|
| 改性未焙烧电石渣 | 636 | 31 | 41.96 | 0.19 | 42.15 |
| 改性焙烧电石渣 | 1744 | 56 | 180.18 | 0.50 | 180.68 |
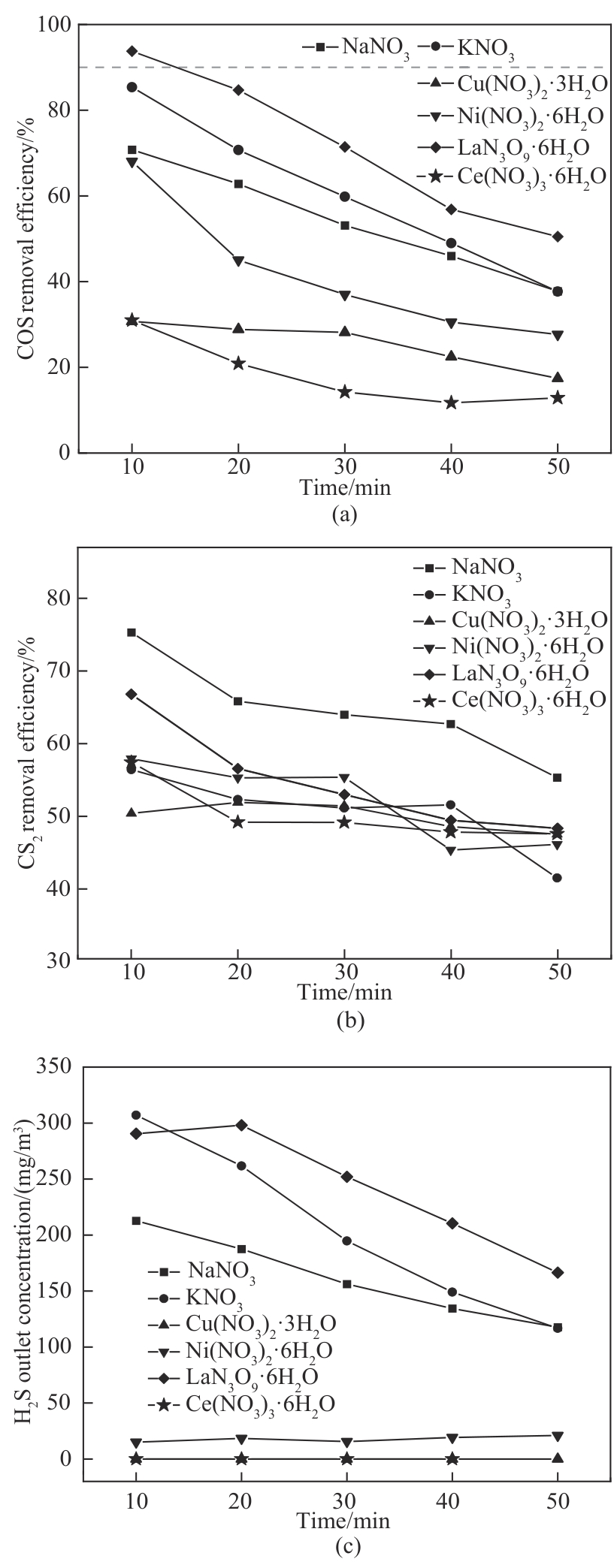
图8 不同金属种类改性电石渣对COS和CS2的同时脱除及H2S出口情况
Fig.8 Simultaneous removal of COS and CS2 and H2S export from modified calcium carbide slag with different metal species
| 样品 | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) | 总硫容/(mg/g) |
|---|---|---|---|---|---|
| NaOH改性电石渣 | 60 | 12 | 6.44 | 0.12 | 6.56 |
| KOH改性电石渣 | 1744 | 56 | 180.18 | 0.50 | 180.68 |
表3 NaOH、KOH改性电石渣同时脱除COS和CS2的穿透时间与穿透硫容
Table 3 Penetration time and penetration sulfur capacity of NaOH and KOH modified calcium carbide slag for simultaneous removal of COS and CS2
| 样品 | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) | 总硫容/(mg/g) |
|---|---|---|---|---|---|
| NaOH改性电石渣 | 60 | 12 | 6.44 | 0.12 | 6.56 |
| KOH改性电石渣 | 1744 | 56 | 180.18 | 0.50 | 180.68 |
| KOH含量/%(质量分数) | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) |
|---|---|---|---|---|
| 0 | 2 | 5 | 0.10 | 0.02 |
| 2.50 | 540 | 25 | 70.51 | 0.33 |
| 3.75 | 600 | 26 | 80.53 | 0.34 |
| 6.25 | 1744 | 56 | 180.18 | 0.50 |
| 8.75 | 640 | 151 | 89.21 | 2.07 |
表4 不同KOH含量改性电石渣同时脱除COS和CS2的穿透时间与穿透硫容
Table 4 Penetration time and penetration sulfur capacity for simultaneous removal of COS and CS2 from modified calcium carbide slag with different KOH contents
| KOH含量/%(质量分数) | COS穿透时间/min | CS2穿透时间/min | COS穿透硫容/(mg/g) | CS2穿透硫容/(mg/g) |
|---|---|---|---|---|
| 0 | 2 | 5 | 0.10 | 0.02 |
| 2.50 | 540 | 25 | 70.51 | 0.33 |
| 3.75 | 600 | 26 | 80.53 | 0.34 |
| 6.25 | 1744 | 56 | 180.18 | 0.50 |
| 8.75 | 640 | 151 | 89.21 | 2.07 |
| KOH含量/%(质量分数) | 比表面积/(m2/g) |
|---|---|
| 0 | 1.68 |
| 2.50 | 4.87 |
| 3.75 | 4.53 |
| 6.25 | 4.27 |
| 8.75 | 3.19 |
表5 不同KOH含量改性电石渣的比表面积
Table 5 Specific surface area of modified calcium carbide slag with different KOH content
| KOH含量/%(质量分数) | 比表面积/(m2/g) |
|---|---|
| 0 | 1.68 |
| 2.50 | 4.87 |
| 3.75 | 4.53 |
| 6.25 | 4.27 |
| 8.75 | 3.19 |
| [1] | Xiong Y X, Wang H X, Ren J, et al. Carbide slag recycling to fabricate shape-stable phase change composites for thermal energy storage[J]. Journal of Energy Storage, 2023, 60: 106694. |
| [2] | 张亚斌, 苏杨, 张慧荣, 等. 钢渣、电石渣增强硫化砷渣稳定化/固化机制[J]. 化工学报, 2024, 75(7): 2656-2669. |
| Zhang Y B, Su Y, Zhang H R, et al. Mechanism of enhanced arsenic sulfide stabilization/solidification by using steel slag and carbide slag[J]. CIESC Journal, 2024, 75(7): 2656-2669. | |
| [3] | 高文英. 含碱工业废弃物脱硫性能实验研究[D]. 济南: 山东大学, 2007. |
| Gao W Y. Experimental study on the desulphurization characteristics of the alkaliferous industry wastes[D]. Jinan: Shandong University, 2007. | |
| [4] | Wang F, Li H, Gao J Y, et al. Treating waste with waste: facile KHCO 3 - modified calcium carbide slag for simultaneous removal of NO and SO2 [J]. Fuel, 2023, 351: 128967. |
| [5] | 朱风, 陈凯琳, 黄小凤, 等. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| Zhu F, Chen K L, Huang X F, et al. Performance study of KOH modified carbide slag for removal of carbonyl sulfide[J]. CIESC Journal, 2023, 74(6): 2668-2679. | |
| [6] | González Fá A J, Orazi V, Jasen P, et al. Adsorption of carbonyl sulfide on Pt-doped vacancy-defected SWCNT: a DFT study[J]. Applied Surface Science, 2020, 525: 146331. |
| [7] | 刘娜, 宁平, 李凯, 等. HCN、COS和CS2催化水解及其水解产物协同净化的研究进展[J]. 化工进展, 2018, 37(1): 301-310. |
| Liu N, Ning P, Li K, et al. Research progress in catalytic hydrolysis of HCN, COS and CS2 and synergetic purification of hydrolysates[J]. Chemical Industry and Engineering Progress, 2018, 37(1): 301-310. | |
| [8] | 梁键星, 李咸伟, 刘道清, 等. 协同催化水解羰基硫和二硫化碳的低温催化剂的研究进展[J]. 材料导报, 2021, 35(21): 21028-21036. |
| Liang J X, Li X W, Liu D Q, et al. A review of catalysts with activities for simultaneous hydrolyses of carbonyl sulfide and carbon disulfide at low temperatures[J]. Materials Reports, 2021, 35(21): 21028-21036. | |
| [9] | Gu J N, Liang J X, Hu S J, et al. Enhanced removal of COS from blast furnace gas via catalytic hydrolysis over Al2O3-based catalysts: insight into the role of alkali metal hydroxide[J]. Separation and Purification Technology, 2022, 295: 121356. |
| [10] | Wang F, Chen H Y, Sun X, et al. Single atom Fe in favor of carbon disulfide (CS2) adsorption and thus the removal efficiency[J]. Separation and Purification Technology, 2021, 258(P2): 118086. |
| [11] | Zhang J L, Li B C, Zhang W L, et al. Preparation of copper-based catalysts by ultrasonic co-impregnation to catalyze the hydrogenation of sec-butyl acetate[J]. Journal of Dispersion Science and Technology, 2020, 41(3): 338-347. |
| [12] | 唐兆吉, 姜艳, 杨占林, 等. 浸渍方式对Mo-Ni/γ-Al2O3加氢处理催化剂性能的影响[J]. 现代化工, 2017, 37(7): 105-108, 110. |
| Tang Z G, Jiang Y, Yang Z L, et al. Influence of impregnation method on the performance of Mo-Ni/γ-Al2O3 hydrotreating catalyst[J]. Modern Chemical Industry, 2017, 37(7): 105-108, 110. | |
| [13] | Bandosz T J. Effect of pore structure and surface chemistry of virgin activated carbons on removal of hydrogen sulfide[J]. Carbon, 1999, 37(3): 483-491. |
| [14] | Ma W P, Zhu G Y, Li H Q, et al. Carbon emission free preparation of calcium hydroxide with calcium carbide slag (CCS) through micro-bubble impurities removal[J]. Journal of Cleaner Production, 2023, 423: 138669. |
| [15] | 董永刚, 曹建新, 刘飞, 等. 电石渣理化性质的分析与表征[J]. 环境科学与技术, 2008, 31(9): 95-98. |
| Dong Y G, Cao J X, Liu F, et al. Analysis and characterization of physiochemical property of carbide slag[J]. Environmental Science & Technology, 2008, 31(9): 95-98. | |
| [16] | Ishizuka T, Tsuchiai H, Murayama T, et al. Preparation of active absorbent for dry-type flue gas desulfurization from calcium oxide, coal fly ash, and gypsum[J]. Industrial & Engineering Chemistry Research, 2000, 39(5): 1390-1396. |
| [17] | Guo F S, Li S R, Hou Y D, et al. Metalated carbon nitrides as base catalysts for efficient catalytic hydrolysis of carbonyl sulfide[J]. Chemical Communications, 2019, 55(75): 11259-11262. |
| [18] | Zhao S Z, Kang D J, Liu Y P, et al. Spontaneous formation of asymmetric oxygen vacancies in transition-metal-doped CeO2 nanorods with improved activity for carbonyl sulfide hydrolysis[J]. ACS Catalysis, 2020, 10(20): 11739-11750. |
| [19] | Rodríguez J, Remesal E, Ramírez P, et al. Water-gas shift reaction on K/Cu(111) and Cu/K/TiO2(110) surfaces: alkali promotion of water dissociation and production of H2 [J]. ACS Catalysis, 2019, 9(12): 10751-10760. |
| [20] | Wang B N, Wang X Z, Yang S, et al. Research progress on catalysts for organic sulfur hydrolysis: review of activity and stability[J]. Chinese Journal of Chemical Engineering, 2024, 71: 203-216. |
| [21] | 赖君玲, 赖晓晨, 柳叶, 等. 干水和碱性干碱的制备及催化水解COS的研究[J]. 石油化工高等学校学报, 2017, 30(5): 12-16. |
| Lai J L, Lai X C, Liu Y, et al. Preparation of dry water and dry bases for catalytic hydrolysis of carbonyl sulfide[J]. Journal of Petrochemical Universities, 2017, 30(5): 12-16. | |
| [22] | 何贞泉, 田森林, 张秋林, 等. 前驱体对Cu/Al2O3的HCN催化水解性能的影响[J]. 环境工程学报, 2016, 10(9): 5044-5050. |
| He Z Q, Tian S L, Zhang Q L, et al. Influence of precursors on catalytic hydrolysis performance of Cu/Al2O3 catalyst toward HCN[J]. Chinese Journal of Environmental Engineering, 2016, 10(9): 5044-5050. | |
| [23] | Yi H H, He D, Tang X L, et al. Effects of preparation conditions for active carbon-based catalyst on catalytic hydrolysis of carbon disulfide[J]. Fuel, 2012, 97: 337-343. |
| [24] | Li K L, Wang C, Ning P, et al. Surface characterization of metal oxides-supported activated carbon fiber catalysts for simultaneous catalytic hydrolysis of carbonyl sulfide and carbon disulfide[J]. Journal of Environmental Sciences, 2020, 96: 44-54. |
| [25] | Li X, Wang X Q, Wang L L, et al. Efficient removal of carbonyl sulfur and hydrogen sulfide from blast furnace gas by one-step catalytic process with modified activated carbon[J]. Applied Surface Science, 2022, 579: 152189. |
| [26] | 翁诗甫. 傅里叶变换红外光谱分析[M]. 2版. 北京: 化学工业出版社, 2010. |
| Weng S F. Fourier Transform Infrared Spectrum Analysis[M]. 2nd ed. Beijing: Chemical Industry Press, 2010. | |
| [27] | Tanpure S, Ghanwat V, Shinde B, et al. The eggshell waste transformed green and efficient synthesis of K-Ca(OH)2 catalyst for room temperature synthesis of chalcones[J]. Polycyclic Aromatic Compounds, 2022, 42(4): 1322-1340. |
| [28] | 马铭宇, 王超, 李运甲, 等. 高炉煤气中羰基硫水解吸附催化剂的制备及性能研究[J]. 化工学报, 2022, 73(1): 275-283. |
| Ma M Y, Wang C, Li Y J, et al. Preparation and performance study of catalyst for COS hydrolysis and adsorption in blast furnace gas[J]. CIESC Journal, 2022, 73(1): 275-283. | |
| [29] | 刘霜, 齐天勤机, 张永春. KOH改性活性炭及其对微量乙烷的吸附性能[J]. 现代化工, 2019, 39(3): 176-180. |
| Liu S, Qi T Q J, Zhang Y C. KOH modified activated carbon and its adsorption performance to trace ethane[J]. Modern Chemical Industry, 2019, 39(3): 176-180. | |
| [30] | Song X, Li K, Wang C, et al. Regeneration performance and mechanism of modified walnut shell biochar catalyst for low temperature catalytic hydrolysis of organic sulfur[J]. Chemical Engineering Journal, 2017, 330: 727-735. |
| [31] | Wang X, Li Y J, Zhang W, et al. Simultaneous SO2 and NO removal by pellets made of carbide slag and coal char in a bubbling fluidized-bed reactor[J]. Process Safety and Environmental Protection, 2020, 134: 83-94. |
| [32] | 元宁, 李嘉, 张晋玲, 等. 煤气化灰渣制备活性炭及其CO2吸附分离研究[J/OL]. 洁净煤技术, . |
| Yuan N, Li J, Zhang J L, et al. Preparation of activated carbon from coal gasification slag and its CO2 adsorption and separation performance[J/OL]. Clean Coal Technology, . | |
| [33] | Yamada T, Okigawa Y, Hasegawa M. Potassium-doped n-type bilayer graphene[J]. Applied Physics Letters, 2018, 112(4): 043105. |
| [34] | Liang J X, Xue Y X, Gu J N, et al. Sustainably recycling spent lithium-ion batteries to prepare magnetically separable cobalt ferrite for catalytic degradation of bisphenol A via peroxymonosulfate activation[J]. Journal of Hazardous Materials, 2022, 427: 127910. |
| [35] | Pan Y K, Chen M Q, Su Z, et al. Two-dimensional CaO/carbon heterostructures with unprecedented catalytic performance in room-temperature H2S oxidization[J]. Applied Catalysis B: Environmental, 2021, 280: 119444. |
| [36] | 黄俊, 刘羿良, 吴鹏, 等. TiAl基羰基硫水解催化剂的中毒机制与抗氧性能研究[J]. 化工学报, 2022, 73(10): 4461-4471. |
| Huang J, Liu Y L, Wu P, et al. Poisoning mechanism and antioxidant performance of TiAl-based carbonyl sulfur hydrolysis catalyst[J]. CIESC Journal, 2022, 73(10): 4461-4471. | |
| [37] | 王广建, 田爱秀, 陈晓婷, 等. COS水解催化剂及其脱硫机理研究进展[J]. 炼油技术与工程, 2017, 47(9): 37-40. |
| Wang G J, Tian A X, Chen X T, et al. Study on COS hydrolysis catalyst and its desulfurization mechanisms[J]. Petroleum Refinery Engineering, 2017, 47(9): 37-40. | |
| [38] | Wang Y, Zhang G J, Shi X, et al. New insights in the hydrolysis mechanism of carbon disulfide (CS2): a density functional study[J]. Structural Chemistry, 2023, 34(1): 71-82. |
| [1] | 毛建拥, 葛纪军, 徐盼, 毕荣山. HDI制备过程中副产物水解氯生成机理实验研究[J]. 化工学报, 2025, 76(S1): 384-392. |
| [2] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [3] | 李秋英, 花亦怀, 程昊, 张涵玮, 刘文睿, 白昊川, 王凯, 邱利民. 集成ORC系统的高效氢液化流程设计研究[J]. 化工学报, 2025, 76(7): 3651-3658. |
| [4] | 赵世颖, 左志帅, 贺梦颖, 安华良, 赵新强, 王延吉. Co-Pt/HAP的制备及其催化1,2-丙二醇氨化反应[J]. 化工学报, 2025, 76(7): 3305-3315. |
| [5] | 李愽龙, 蒋雨希, 任傲天, 秦雯琪, 傅杰, 吕秀阳. TS-1/In-TS-1催化果糖一步法醇解制备乳酸甲酯连续化试验[J]. 化工学报, 2025, 76(6): 2678-2686. |
| [6] | 卢丽丽, 李晨, 陈柳云, 谢新玲, 罗轩, 苏通明, 秦祖赠, 纪红兵. BiOBr的形貌调控及其光催化CO2还原性能的研究[J]. 化工学报, 2025, 76(6): 2687-2700. |
| [7] | 龚丽芳, 任美慧, 蒋吉春, 郭光召, 胡红云, 黄永达, 姚洪. 垃圾焚烧烟气中芳香烃化合物在线监测和选择性催化还原脱除研究[J]. 化工学报, 2025, 76(6): 3018-3028. |
| [8] | 王一非, 任婧杰, 毕明树, 叶昊天. 基于本质安全与经济性的环己烷氧化工艺参数多目标优化研究[J]. 化工学报, 2025, 76(6): 2722-2732. |
| [9] | 彭健, 沈鲁恺, 王立坤, 忻利宏, 刘涌, 赵高凌, 马赛男, 韩高荣. 钨酸盐纳米材料的制备及其在电致变色领域的研究进展[J]. 化工学报, 2025, 76(6): 2451-2468. |
| [10] | 杨盛华, 孙阳杰, 薛晓君, 米杰, 王建成, 冯宇. 缺陷型金属氧化物脱除气体污染物研究进展[J]. 化工学报, 2025, 76(6): 2469-2482. |
| [11] | 张涵川, 尚超, 吕文祥, 黄德先, 张亚宁. 基于无监督时序聚类的催化裂化装置工况划分识别与产率预测方法[J]. 化工学报, 2025, 76(6): 2781-2790. |
| [12] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [13] | 宋粉红, 王文光, 郭亮, 范晶. C元素修饰g-C3N4对TiO2的调控及复合材料光催化产氢性能研究[J]. 化工学报, 2025, 76(6): 2983-2994. |
| [14] | 姬海燕, 刘家印, 吴海军, 何璟琳, 靳紫恒, 魏钿航, 江霞. 低温等离子体在生物质气化制氢中的应用研究进展[J]. 化工学报, 2025, 76(6): 2419-2433. |
| [15] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号