化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5101-5113.DOI: 10.11949/0438-1157.20250364
沈赟1,2( ), 张岱2, 徐晓峰1, 曹约强1,2(
), 张岱2, 徐晓峰1, 曹约强1,2( ), 周静红1,2(
), 周静红1,2( ), 李伟2, 周兴贵1,2
), 李伟2, 周兴贵1,2
收稿日期:2025-04-09
修回日期:2025-05-14
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
曹约强,周静红
作者简介:沈赟(2000—),男,硕士研究生,shenyun0902@163.com
基金资助:
Yun SHEN1,2( ), Dai ZHANG2, Xiaofeng XU1, Yueqiang CAO1,2(
), Dai ZHANG2, Xiaofeng XU1, Yueqiang CAO1,2( ), Jinghong ZHOU1,2(
), Jinghong ZHOU1,2( ), Wei LI2, Xinggui ZHOU1,2
), Wei LI2, Xinggui ZHOU1,2
Received:2025-04-09
Revised:2025-05-14
Online:2025-10-25
Published:2025-11-25
Contact:
Yueqiang CAO, Jinghong ZHOU
摘要:
制备了系列不同金属负载量的Ni-Ag/SiO2双金属催化剂,揭示了草酸二甲酯(DMO)选择性加氢制乙醇酸甲酯(MG)的活性位点协同机制。采用氮气物理吸附、氢气程序升温还原、X射线衍射、X射线光电子能谱等表征技术对催化剂的形貌和活性位点结构进行了详细分析。结果表明,制得的Ni-Ag/SiO2双金属催化剂中Ni物种主要以层状硅酸镍结构存在,且Ag颗粒负载在其表面。结合催化性能测试与原位红外光谱、程序升温脱附等表征实验探究了催化剂的构-效关系,发现不同Ni/Ag的双金属催化剂表现出显著差异的DMO和H2的吸附活化行为。其中,Ni含量增加促进了DMO吸附活化,而Ag含量增加有利于H2吸附活化,表明Ni-Ag/SiO2双金属催化剂中Ni0/Ni δ+界面位点和Ag位点可能分别与DMO和H2吸附活化相关。当调控Ni/Ag(质量比)至5∶1,可有效平衡DMO与H2吸附活化,其中5Ni-1Ag/SiO2双金属催化剂上DMO转化率和MG选择性分别达到了99.6% 和91.5%,且连续运行350 h无明显衰减。研究结果可为煤基合成气制MG的高效催化剂设计提供借鉴。
中图分类号:
沈赟, 张岱, 徐晓峰, 曹约强, 周静红, 李伟, 周兴贵. Ni-Ag/SiO2催化草酸二甲酯加氢制乙醇酸甲酯反应机理研究[J]. 化工学报, 2025, 76(10): 5101-5113.
Yun SHEN, Dai ZHANG, Xiaofeng XU, Yueqiang CAO, Jinghong ZHOU, Wei LI, Xinggui ZHOU. Mechanistic insights into the hydrogenation of dimethyl oxalate to methyl glycolate over Ni-Ag/SiO2 catalyst[J]. CIESC Journal, 2025, 76(10): 5101-5113.
| 样品 | 负载量①/% | SBET② / (m2/g) | Vpore③/ (cm3/g) | DBJH③/ nm | QCO④/ (mmol/g) | (mmol/g) | (mmol/g) | |
|---|---|---|---|---|---|---|---|---|
| Ni | Ag | |||||||
| SiO2 | — | — | 212 | 0.30 | 4.4 | — | — | — |
| 5Ni-1Ag | 4.6 | 0.98 | 247 | 0.61 | 8.24 | 0.0027 | 0.1241 | 0.1214 |
| 10Ni-1Ag | 9.8 | 0.98 | 267 | 0.58 | 7.00 | 0.0064 | 0.1289 | 0.1225 |
| 15Ni-1Ag | 14.7 | 0.97 | 285 | 0.54 | 5.90 | 0.0102 | 0.1344 | 0.1242 |
| 10Ni-1.5Ag | 9.8 | 1.5 | 261 | 0.58 | 6.88 | 0.0104 | 0.1913 | 0.1809 |
| 10Ni-2Ag | 9.8 | 2 | 257 | 0.57 | 6.81 | 0.0121 | 0.2975 | 0.2854 |
表1 Ni-Ag/SiO2 催化剂的理化特征
Table 1 Physicochemical properties of Ni-Ag/SiO2 catalysts
| 样品 | 负载量①/% | SBET② / (m2/g) | Vpore③/ (cm3/g) | DBJH③/ nm | QCO④/ (mmol/g) | (mmol/g) | (mmol/g) | |
|---|---|---|---|---|---|---|---|---|
| Ni | Ag | |||||||
| SiO2 | — | — | 212 | 0.30 | 4.4 | — | — | — |
| 5Ni-1Ag | 4.6 | 0.98 | 247 | 0.61 | 8.24 | 0.0027 | 0.1241 | 0.1214 |
| 10Ni-1Ag | 9.8 | 0.98 | 267 | 0.58 | 7.00 | 0.0064 | 0.1289 | 0.1225 |
| 15Ni-1Ag | 14.7 | 0.97 | 285 | 0.54 | 5.90 | 0.0102 | 0.1344 | 0.1242 |
| 10Ni-1.5Ag | 9.8 | 1.5 | 261 | 0.58 | 6.88 | 0.0104 | 0.1913 | 0.1809 |
| 10Ni-2Ag | 9.8 | 2 | 257 | 0.57 | 6.81 | 0.0121 | 0.2975 | 0.2854 |
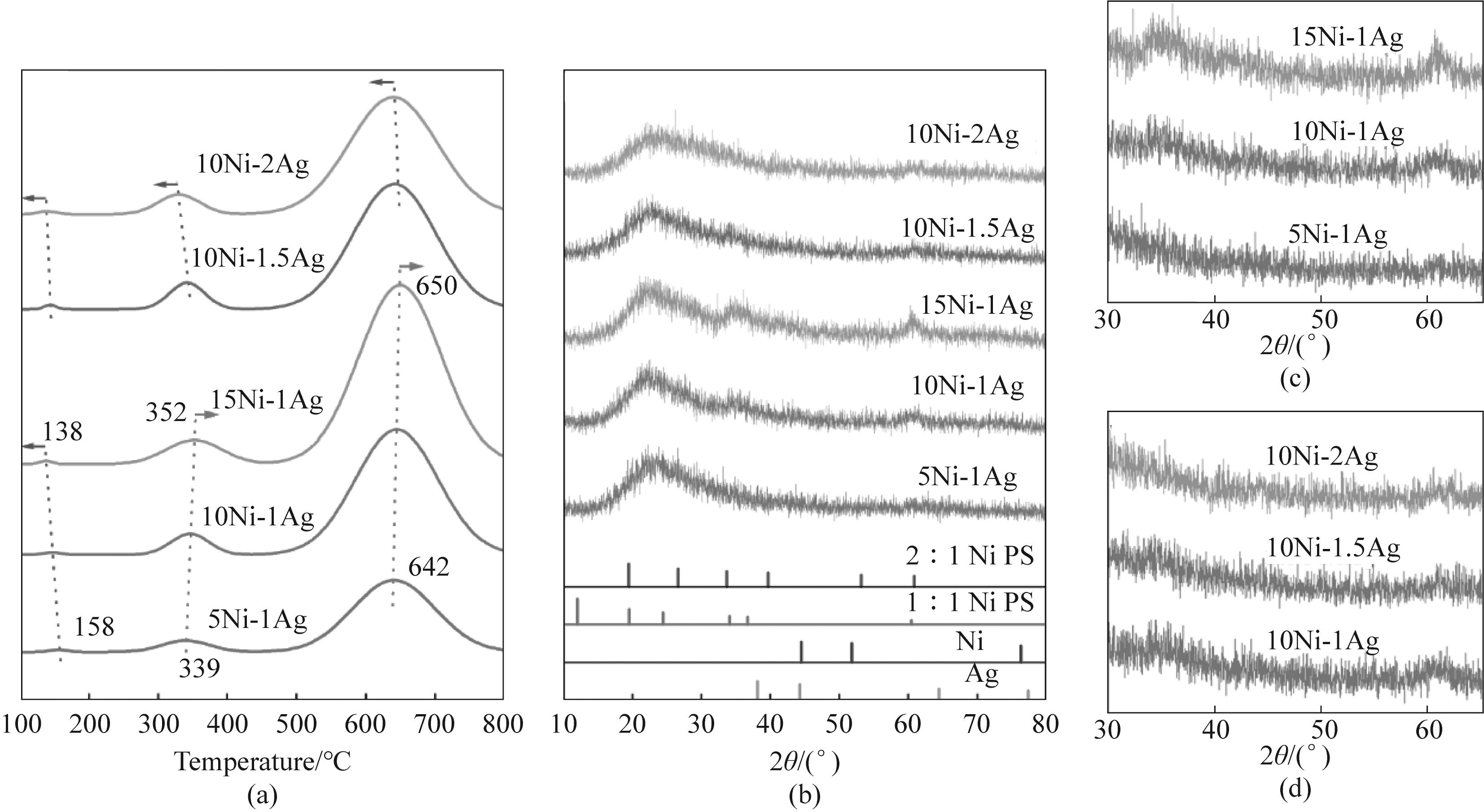
图2 Ni-Ag/SiO2催化剂的(a) H2-TPR 谱图,(b) XRD谱图和[(c)、(d)] XRD局部放大谱图
Fig.2 (a) H2-TPR patterns, (b) XRD patterns and [(c), (d)] XRD local amplified patterns of Ni-Ag/SiO2 catalysts
| 样品 | 低温区还原峰耗氢量/(10-3 mmol/g) | 中温区还原峰耗氢量/(10-2 mmol/g) | 高温区还原峰耗氢量/(mmol/g) |
|---|---|---|---|
| 5Ni-1Ag | 4.380 | 3.375 | 0.465 |
| 10Ni-1Ag | 4.311 | 4.915 | 0.831 |
| 15Ni-1Ag | 4.368 | 8.149 | 1.166 |
| 10Ni-1.5Ag | 5.948 | 5.893 | 0.818 |
| 10Ni-2Ag | 6.534 | 5.825 | 0.815 |
表2 H2-TPR结果中Ni-Ag/SiO2催化剂各峰耗氢量
Table 2 Amount of hydrogen consumption corresponding to the H2-TPR peaks of Ni-Ag/SiO2 catalysts
| 样品 | 低温区还原峰耗氢量/(10-3 mmol/g) | 中温区还原峰耗氢量/(10-2 mmol/g) | 高温区还原峰耗氢量/(mmol/g) |
|---|---|---|---|
| 5Ni-1Ag | 4.380 | 3.375 | 0.465 |
| 10Ni-1Ag | 4.311 | 4.915 | 0.831 |
| 15Ni-1Ag | 4.368 | 8.149 | 1.166 |
| 10Ni-1.5Ag | 5.948 | 5.893 | 0.818 |
| 10Ni-2Ag | 6.534 | 5.825 | 0.815 |
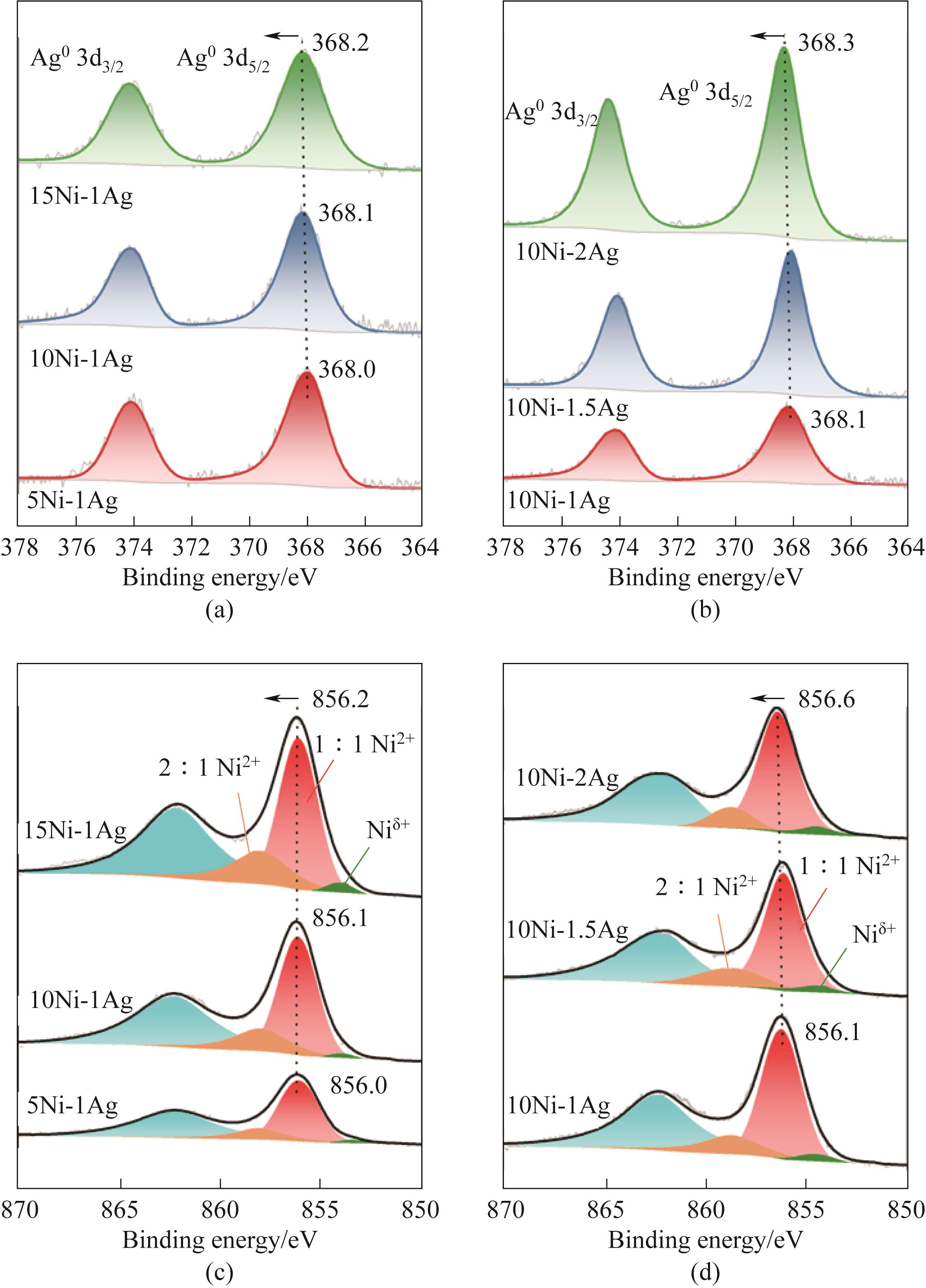
图4 Ni-Ag/SiO2 催化剂中的[(a)、(b)] Ag 3d XPS谱图和[(c)、(d)] Ni 2p3/2 XPS谱图
Fig.4 [(a),(b)] Ag XPS spectra and [(c),(d)] Ni 2p3/2 XPS spectra of Ni-Ag/SiO2 catalysts
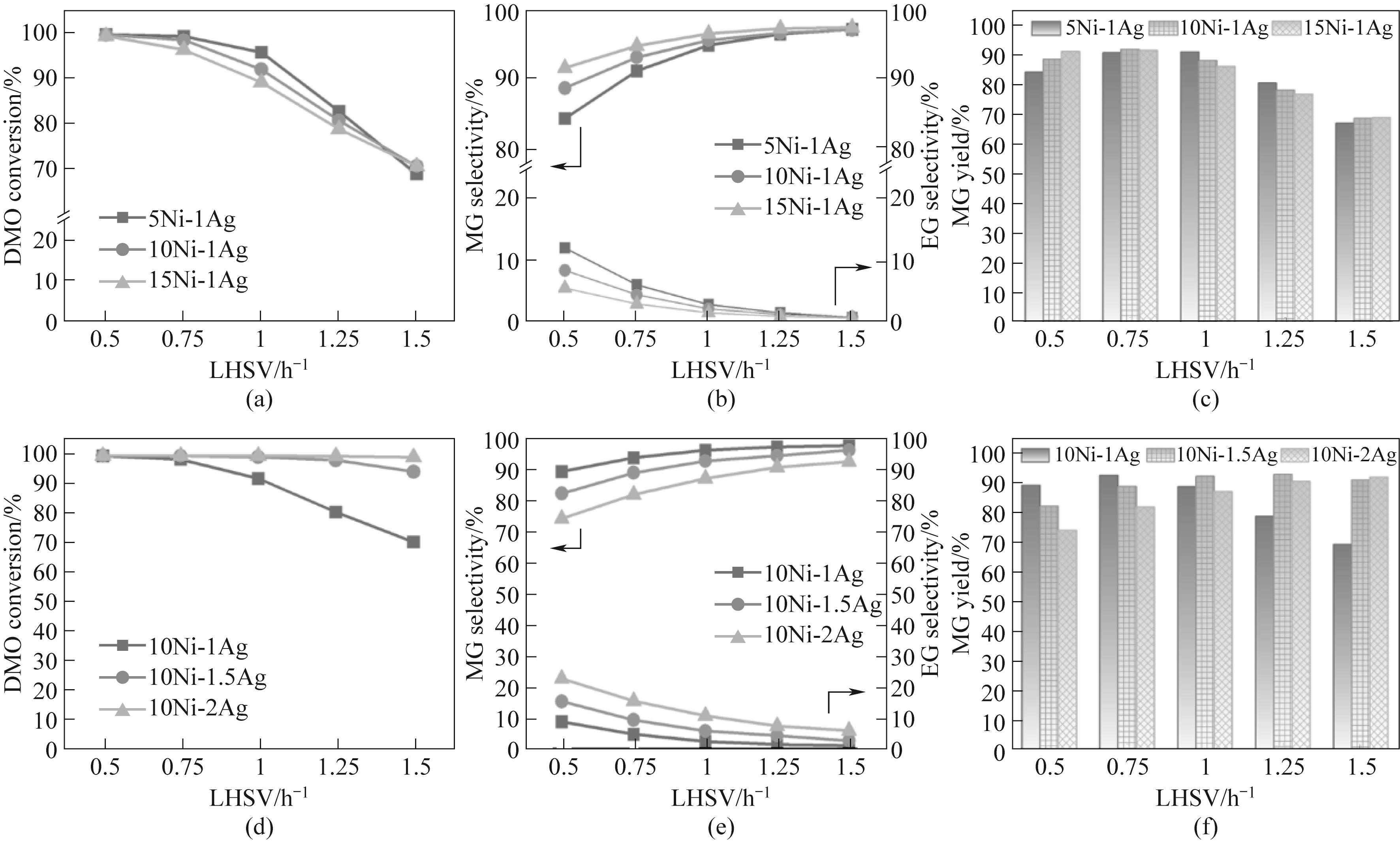
图5 (a)~(c)不同 Ni 负载量和(d)~(f)不同 Ag负载量的Ni-Ag/SiO2催化剂的性能与液时空速的关系(反应条件:220℃,2.0 MPa,H2/DMO = 50)
Fig.5 Performance of Ni-Ag/SiO2 catalysts with (a)—(c) different Ni loadings and (d)—(f) different Ag loadings as a function of LHSV (reaction conditions: 220℃, 2.0 MPa, H2/DMO = 50)
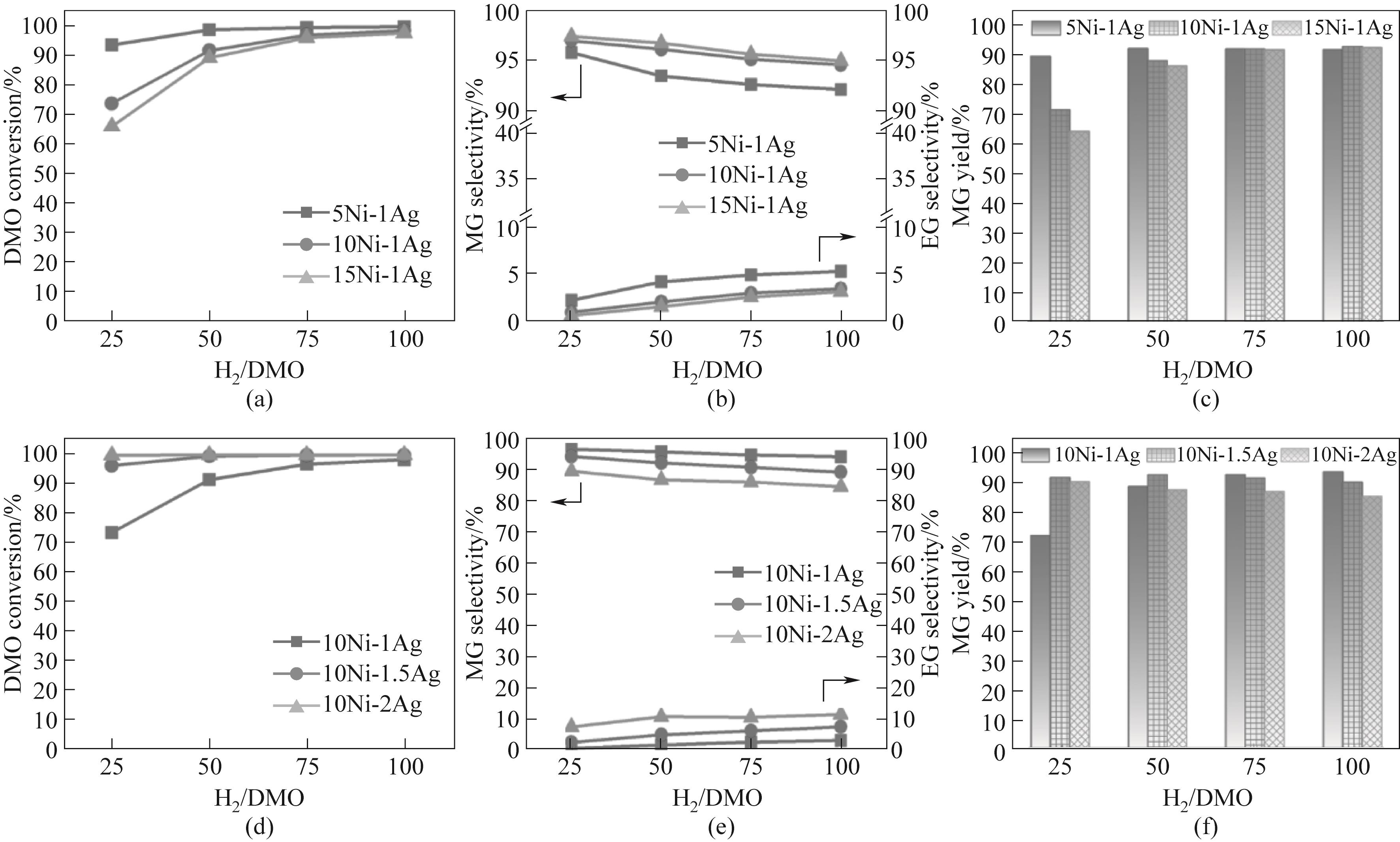
图6 (a)~(c)不同 Ni 负载量和(d)~(f)不同Ag负载量的Ni-Ag/SiO2催化剂的性能与氢酯比的关系(反应条件:220℃,2.0 MPa,LHSV = 1 h-1)
Fig.6 Performance of Ni-Ag/SiO2 catalysts with (a)—(c) different Ni loadings and (d)—(f) different Ag loadings as a function of H2/DMO (reaction conditions: 220℃, 2.0 MPa, LHSV = 1 h-1)
| 催化剂 | LHSV/h-1 | H/D | DMO 转化率/% | 选择性/% | MG 收率% | ||
|---|---|---|---|---|---|---|---|
| MG | EG | 其他 | |||||
| 10Ni/SiO2 | 0.5 | 50 | 12.6 | 83.1 | 0.5 | 16.4 | 10.5 |
| 1 | 50 | 10.4 | 93.3 | 0.1 | 6.6 | 9.7 | |
| 10Ni-0.5Ag/SiO2 | 0.5 | 50 | 87.7 | 96.7 | 1.6 | 1.7 | 84.8 |
| 0.75 | 50 | 70.6 | 97.5 | 0.8 | 1.7 | 68.9 | |
| 1 | 50 | 58.0 | 97.5 | 0.6 | 1.9 | 56.5 | |
| 1.25 | 50 | 47.9 | 97.4 | 0.5 | 2.1 | 46.6 | |
| 1.5 | 50 | 39.9 | 97.3 | 0.4 | 2.3 | 38.8 | |
| 1 | 100 | 73.5 | 97.9 | 0.9 | 1.3 | 71.9 | |
| 1 | 75 | 64.5 | 97.9 | 0.7 | 1.4 | 63.2 | |
| 1 | 50 | 55.0 | 97.5 | 0.7 | 1.9 | 53.6 | |
| 1 | 25 | 40.2 | 96.6 | 0.5 | 3.0 | 38.8 | |
表3 不同Ni、Ag负载量的Ni-Ag/SiO2催化剂的性能考评结果
Table 3 Performance evaluation results of Ni-Ag/SiO2 catalysts with different Ni and Ag loadings
| 催化剂 | LHSV/h-1 | H/D | DMO 转化率/% | 选择性/% | MG 收率% | ||
|---|---|---|---|---|---|---|---|
| MG | EG | 其他 | |||||
| 10Ni/SiO2 | 0.5 | 50 | 12.6 | 83.1 | 0.5 | 16.4 | 10.5 |
| 1 | 50 | 10.4 | 93.3 | 0.1 | 6.6 | 9.7 | |
| 10Ni-0.5Ag/SiO2 | 0.5 | 50 | 87.7 | 96.7 | 1.6 | 1.7 | 84.8 |
| 0.75 | 50 | 70.6 | 97.5 | 0.8 | 1.7 | 68.9 | |
| 1 | 50 | 58.0 | 97.5 | 0.6 | 1.9 | 56.5 | |
| 1.25 | 50 | 47.9 | 97.4 | 0.5 | 2.1 | 46.6 | |
| 1.5 | 50 | 39.9 | 97.3 | 0.4 | 2.3 | 38.8 | |
| 1 | 100 | 73.5 | 97.9 | 0.9 | 1.3 | 71.9 | |
| 1 | 75 | 64.5 | 97.9 | 0.7 | 1.4 | 63.2 | |
| 1 | 50 | 55.0 | 97.5 | 0.7 | 1.9 | 53.6 | |
| 1 | 25 | 40.2 | 96.6 | 0.5 | 3.0 | 38.8 | |
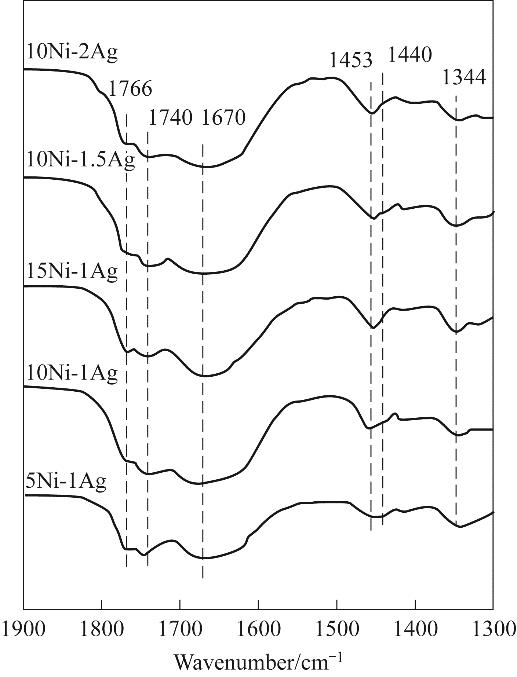
图7 DMO在不同Ni、Ag负载量Ni-Ag/SiO2催化剂上吸附的 in-situ FTIR谱图
Fig.7 In-situ FTIR patterns of DMO adsorbed on Ni-Ag/SiO2 catalysts with different Ni and Ag loadings
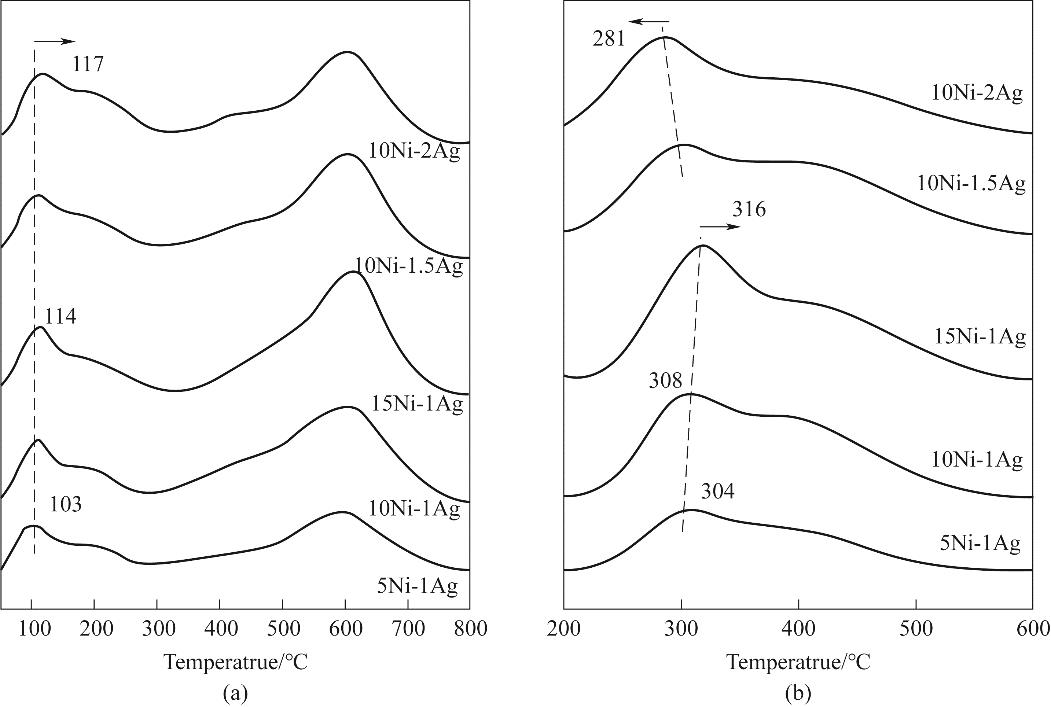
图8 不同Ni、Ag负载量Ni-Ag/SiO2催化剂的(a)H2-TPD谱图和(b)DMO-TPD谱图
Fig.8 (a) H2-TPD patterns and (b) DMO-TPD patterns of Ni-Ag/SiO2 catalysts with different Ni and Ag loadings
| 催化剂 | H2-TPD | DMO-TPD | A(H2)/A(DMO) |
|---|---|---|---|
| 5Ni-1Ag/SiO2 | 0.70 | 0.35 | 1.98 |
| 10Ni-1Ag/SiO2 | 0.88 | 0.67 | 1.31 |
| 15Ni-1Ag/SiO2 | 0.95 | 0.78 | 1.23 |
| 10Ni-1.5Ag/SiO2 | 1.07 | 0.68 | 1.59 |
| 10Ni-2Ag/SiO2 | 1.27 | 0.69 | 1.85 |
表4 不同Ni、Ag负载量的Ni-Ag/SiO2催化剂的TPD面积
Table 4 TPD area of Ni-Ag/SiO2 catalysts with different Ni and Ag loadings
| 催化剂 | H2-TPD | DMO-TPD | A(H2)/A(DMO) |
|---|---|---|---|
| 5Ni-1Ag/SiO2 | 0.70 | 0.35 | 1.98 |
| 10Ni-1Ag/SiO2 | 0.88 | 0.67 | 1.31 |
| 15Ni-1Ag/SiO2 | 0.95 | 0.78 | 1.23 |
| 10Ni-1.5Ag/SiO2 | 1.07 | 0.68 | 1.59 |
| 10Ni-2Ag/SiO2 | 1.27 | 0.69 | 1.85 |
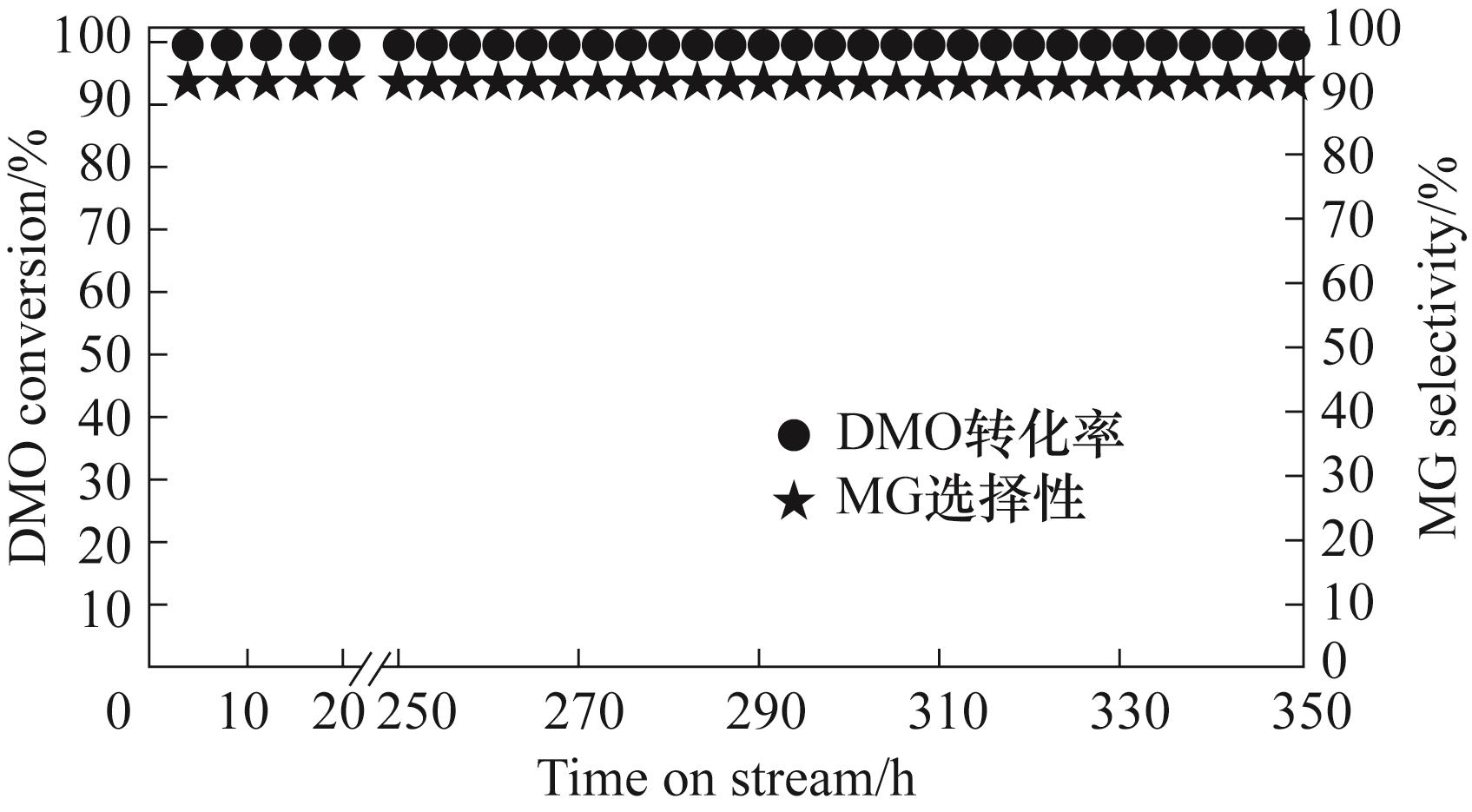
图9 5Ni-1Ag/SiO2催化剂稳定性考评结果(反应条件:220℃,2.0 MPa,LHSV= 0.75 h-1,H2/DMO=50)
Fig.9 5Ni-1Ag/SiO2 catalyst stability assessment results (reaction conditions: T=220℃, P=2.0 MPa, LHSV= 0.75 h-1, H2/DMO=50)
| [1] | Yang Q C, Fan Y J, Liu C L, et al. A promising alternative potential solution for sustainable and economical development of coal to ethylene glycol industry: dimethyl oxalate to methyl glycolate process[J]. Energy, 2023, 277: 127668. |
| [2] | Zhou R J, Yan W Q, Cao Y Q, et al. Probing the structure sensitivity of dimethyl oxalate partial hydrogenation over Ag nanoparticles: a combined experimental and microkinetic study[J]. Chemical Engineering Science, 2022, 259: 117830. |
| [3] | Xie T H, Ai S, Huang Y C, et al. Synthesis and purification of glycolic acid from the mixture of methyl levulinate and methyl glycolate via acid-mediated hydrolysis reactions and extraction[J]. Separation and Purification Technology, 2021, 268: 118718. |
| [4] | An J W, Wang X H, Zhao J X, et al. Density-functional theory study on hydrogenation of dimethyl oxalate to methyl glycolate over copper catalyst: effect of copper valence state[J]. Molecular Catalysis, 2020, 482: 110667. |
| [5] | Huang H J, Wang B, Wang Y, et al. Partial hydrogenation of dimethyl oxalate on Cu/SiO2 catalyst modified by sodium silicate[J]. Catalysis Today, 2020, 358: 68-73. |
| [6] | Sun Y, Wang H, Shen J H, et al. Highly effective synthesis of methyl glycolate with heteropolyacids as catalysts[J]. Catalysis Communications, 2009, 10(5): 678-681. |
| [7] | Luo Z W, Xu X F, Dong G L, et al. Regulating mesopore structures of support toward enhanced selective hydrogenation of dimethyl oxalate to methyl glycolate on Ag catalysts[J]. Chemical Engineering Journal, 2022, 450: 138397. |
| [8] | Dong G L, Luo Z W, Cao Y Q, et al. Understanding size-dependent hydrogenation of dimethyl oxalate to methyl glycolate over Ag catalysts[J]. Journal of Catalysis, 2021, 401: 252-261. |
| [9] | Qu R Y, Junge K, Beller M. Hydrogenation of carboxylic acids, esters, and related compounds over heterogeneous catalysts: a step toward sustainable and carbon-neutral processes[J]. Chemical Reviews, 2023, 123(3): 1103-1165. |
| [10] | Chen H M, Tan J J, Zhu Y L, et al. An effective and stable Ni2P/TiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Catalysis Communications, 2016, 73: 46-49. |
| [11] | Zhu J, Cao L Q, Li C Y, et al. Nanoporous Ni3P evolutionarily structured onto a Ni foam for highly selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. ACS Applied Materials & Interfaces, 2019, 11(41): 37635-37643. |
| [12] | Ouyang M Y, Wang J, Peng B, et al. Effect of Ti on Ag catalyst supported on spherical fibrous silica for partial hydrogenation of dimethyl oxalate[J]. Applied Surface Science, 2019, 466: 592-600. |
| [13] | Dong G L, Cao Y Q, Zheng S N, et al. Catalyst consisting of Ag nanoparticles anchored on amine-derivatized mesoporous silica nanospheres for the selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. Journal of Catalysis, 2020, 391: 155-162. |
| [14] | Luo Z W, Ge X H, Fang D, et al. In situ exsolution to fabricate interfacial Ni0/Ni δ + sites for regulating reaction pathways in hydrogenation[J]. Journal of Catalysis, 2024, 434: 115528. |
| [15] | 方笛, 罗祖伟, 曹约强, 等. 催化剂制备方法对Ni-Ag/SiO2 催化草酸二甲酯加氢制乙醇酸甲酯性能的影响[J]. 燃料化学学报, 2024, 52(10): 1495-1507. |
| Fang D, Luo Z W, Cao Y Q, et al. Influence of Ni-Ag/SiO2 catalyst preparation method on its performance in hydrogenation of dimethyl oxalate to methyl glycolate[J]. Journal of Fuel Chemistry and Technology, 2024, 52(10): 1495-1507. | |
| [16] | Luo Z W, Shen Y, Fang D, et al. Insights into support effects of Ag/SiO2 catalysts for dimethyl glycolate semi-hydrogenation to methyl glycolate[J]. Molecular Catalysis, 2024, 559: 114109. |
| [17] | Yan W Q, Zhang J B, Xiao L, et al. Toward rational catalyst design for partial hydrogenation of dimethyl oxalate to methyl glycolate: a descriptor-based microkinetic analysis[J]. Catalysis Science & Technology, 2019, 9(20): 5763-5773. |
| [18] | Cao Y Q, Chen B, Guerrero-Sánchez J, et al. Controlling selectivity in unsaturated aldehyde hydrogenation using single-site alloy catalysts[J]. ACS Catalysis, 2019, 9(10): 9150-9157. |
| [19] | Giannakakis G, Flytzani-Stephanopoulos M, Charles H Sykes E. Single-atom alloys as a reductionist approach to the rational design of heterogeneous catalysts[J]. Accounts of Chemical Research, 2019, 52(1): 237-247. |
| [20] | Wang S, Zhao Z J, Chang X, et al. Activation and spillover of hydrogen on sub-1 nm palladium nanoclusters confined within sodalite zeolite for the semi-hydrogenation of alkynes[J]. Angewandte Chemie International Edition, 2019, 58(23): 7668-7672. |
| [21] | Jiang L Z, Liu K L, Hung S F, et al. Facet engineering accelerates spillover hydrogenation on highly diluted metal nanocatalysts[J]. Nature Nanotechnology, 2020, 15(10): 848-853. |
| [22] | Qi H M, Wang X L, Lei M, et al. Highly efficient catalytic hydrogenation of nitrobenzene on cobalt- immobilized nitrogen-doped carbon: a dual-sites synergistic effect between cobalt single atoms and cobalt nanoparticles[J]. Chemical Engineering Journal, 2024, 500: 157057. |
| [23] | Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209-1212. |
| [24] | Yang D, Tao S, Zhu H Y, et al. Construction of Rh-N4 single atoms and Rh clusters dual-active sites for synergistic heterogeneous hydroformylation of olefins with ultra-high turnover frequency[J]. Chemical Engineering Journal, 2024, 479: 147505. |
| [25] | Müslehiddinoğlu J, Vannice M A. CO adsorption on supported and promoted Ag epoxidation catalysts[J]. Journal of Catalysis, 2003, 213(2): 305-320. |
| [26] | Li J, Xiong H C, Liu X Z, et al. Weak CO binding sites induced by Cu-Ag interfaces promote CO electroreduction to multi-carbon liquid products[J]. Nature Communications, 2023, 14(1): 698. |
| [27] | 董桂霖, 罗祖伟, 曹约强, 等. 液相还原温度对草酸酯加氢制乙醇酸甲酯银硅催化剂性能的影响[J]. 化工学报, 2022, 73(1): 232-240. |
| Dong G L, Luo Z W, Cao Y Q, et al. Effect of liquid-phase reduction temperature on performance of silver-silica catalysts for hydrogenation of dimethyl oxalate to methyl glycolate[J]. CIESC Journal, 2022, 73(1): 232-240. | |
| [28] | Kuhaudomlap S, Mekasuwandumrong O, Praserthdam P, et al. Influence of highly stable Ni2+ species in Ni phyllosilicate catalysts on selective hydrogenation of furfural to furfuryl alcohol[J]. ACS Omega, 2022, 8(1): 249-261. |
| [29] | Yang F F, Wang H, Han J Y, et al. Enhanced selective deoxygenation of m-cresol to toluene on Ni/SiO2 catalysts derived from nickel phyllosilicate[J]. Catalysis Today, 2019, 330: 149-156. |
| [30] | Kong X, Zhu Y F, Zheng H Y, et al. Ni nanoparticles inlaid nickel phyllosilicate as a metal-acid bifunctional catalyst for low-temperature hydrogenolysis reactions[J]. ACS Catalysis, 2015, 5(10): 5914-5920. |
| [31] | Zheng J W, Lin H Q, Zheng X L, et al. Highly efficient mesostructured Ag/SBA-15 catalysts for the chemoselective synthesis of methyl glycolate by dimethyl oxalate hydrogenation[J]. Catalysis Communications, 2013, 40: 129-133. |
| [32] | Qu Z P, Huang W X, Cheng M J, et al. Restructuring and redispersion of silver on SiO2 under oxidizing/reducing atmospheres and its activity toward CO oxidation[J]. The Journal of Physical Chemistry. B, 2005, 109(33): 15842-15848. |
| [33] | Zhou J F, Duan X P, Ye L M, et al. Enhanced chemoselective hydrogenation of dimethyl oxalate to methyl glycolate over bimetallic Ag-Ni/SBA-15 catalysts[J]. Applied Catalysis A: General, 2015, 505: 344-353. |
| [34] | Hengne A M, Malawadkar A V, Biradar N S, et al. Surface synergism of an Ag-Ni/ZrO2 nanocomposite for the catalytic transfer hydrogenation of bio-derived platform molecules[J]. RSC Advances, 2014, 4(19): 9730-9736. |
| [35] | Lazar M, Mihet M, Dan M, et al. Preparation and characterization of nickel based multicomponent catalysts[J]. Journal of Physics: Conference Series, 2009, 182: 012049. |
| [36] | Burattin P, Che M, Louis C. Ni/SiO2 materials prepared by deposition-precipitation: influence of the reduction conditions and mechanism of formation of metal particles[J]. The Journal of Physical Chemistry B, 2000, 104(45): 10482-10489. |
| [37] | Wang J Y, Fu Y, Kong W B, et al. Design of a carbon-resistant Ni@S-2 reforming catalyst: controllable Ni nanoparticles sandwiched in a peasecod-like structure[J]. Applied Catalysis B: Environmental, 2021, 282: 119546. |
| [38] | Yang H, Li J, Chang Q, et al. Ni nanoparticles inlaid in amorphous silicon nitride-derived nickel phyllosilicate: a highly stable and active catalyst for ammonia decomposition[J]. Fuel, 2025,394:135119. |
| [39] | Pei G X, Liu X Y, Wang A Q, et al. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts[J]. Applied Catalysis A: General, 2017, 545: 90-96. |
| [40] | Cheng S, Meng T, Mao D S, et al. Ni-modified Ag/SiO2 catalysts for selective hydrogenation of dimethyl oxalate to methyl glycolate[J]. Nanomaterials, 2022, 12(3): 407. |
| [41] | Yin A Y, Guo X Y, Prof K F, et al. Ion-exchange temperature effect on Cu/HMS catalysts for the hydrogenation of dimethyl oxalate to ethylene glycol[J]. ChemCatChem, 2010, 2(2): 206-213. |
| [42] | Ghiat I, Boudjemaa A, Saadi A, et al. Efficient hydrogen generation over a novel Ni phyllosilicate photocatalyst[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2019, 382: 111952. |
| [43] | Waterhouse G I N, Bowmaker G A, Metson J B. Oxidation of a polycrystalline silver foil by reaction with ozone[J]. Applied Surface Science, 2001, 183(3/4): 191-204. |
| [44] | Chen H M, Tan J J, Cui J L, et al. Promoting effect of boron oxide on Ag/SiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate[J]. Molecular Catalysis, 2017, 433: 346-353. |
| [45] | Cui G Q, Zhang X, Wang H, et al. ZrO2- x modified Cu nanocatalysts with synergistic catalysis towards carbon-oxygen bond hydrogenation[J]. Applied Catalysis B: Environmental, 2021, 280: 119406. |
| [46] | Cui G Q, Meng X Y, Zhang X, et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid-base sites[J]. Applied Catalysis B: Environmental, 2019, 248: 394-404. |
| [47] | Wang C Z, Chen P J, Li Y K, et al. In situ DRIFTS study of CO coupling to dimethyl oxalate over structured Al-fiber@ns-AlOOH@Pd catalyst[J]. Journal of Catalysis, 2016, 344: 173-183. |
| [48] | Castonguay M, Roy J R, Rochefort A, et al. Orientation and conformation of methyl pyruvate on Ni(111)[J]. Journal of the American Chemical Society, 2000, 122(3): 518-524. |
| [49] | Castonguay M, Roy J R, Lavoie S, et al. Selective C—C bond activation of methyl pyruvate on Ni(111) to yield surface methoxycarbonyl[J]. Journal of the American Chemical Society, 2001, 123(26): 6429-6430. |
| [50] | Rachmady W, Vannice M A. Acetic acid reduction by H2 over supported Pt catalysts: a DRIFTS and TPD/TPR study[J]. Journal of Catalysis, 2002, 207(2): 317-330. |
| [51] | Gao X P, Zhou Y N, Jing F L, et al. Layered double hydroxides derived ZnO-Al2O3 supported Pd-Ag catalysts for selective hydrogenation of acetylene[J]. Chinese Journal of Chemistry, 2017, 35(6): 1009-1015. |
| [52] | Zou J L, Duan X P, Liu X, et al. Identifying the activity origin of silver catalysts induced by interfacial electron localization for regioselective CO bond hydrogenation[J]. Chemical Engineering Journal, 2023, 454: 140110. |
| [53] | Velu S, Gangwal S K. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption[J]. Solid State Ionics, 2006, 177(7/8): 803-811. |
| [54] | Chen S, Pan X Y, Miao C X, et al. Study of catalytic hydrodeoxygenation performance for the Ni/KIT-6 catalysts[J]. Journal of Saudi Chemical Society, 2018, 22(5): 614-627. |
| [55] | Liu K R, Yan P F, Jiang H, et al. Silver initiated hydrogen spillover on anatase TiO2 creates active sites for selective hydrodeoxygenation of guaiacol[J]. Journal of Catalysis, 2019, 369: 396-404. |
| [56] | Dong C, Mu R T, Li R T, et al. Disentangling local interfacial confinement and remote spillover effects in oxide-oxide interactions[J]. Journal of the American Chemical Society, 2023, 145(31): 17056-17065. |
| [57] | Shin E J, Keane M A. Gas-phase hydrogenation/hydrogenolysis of phenol over supported nickel catalysts[J]. Industrial & Engineering Chemistry Research, 2000, 39(4): 883-892. |
| [1] | 刘奕扬, 邢志祥, 刘烨铖, 彭明, 李玉洋, 李云浩, 沈宁舟. 加氢站液氢泄漏扩散特性与安全监测数值模拟研究[J]. 化工学报, 2025, 76(9): 4694-4708. |
| [2] | 王小令, 王绍清, 赵云刚, 常方哲, 穆瑞峰. 基于ReaxFF MD模拟的煤加氢热解有机Ca转化机制研究[J]. 化工学报, 2025, 76(8): 4297-4309. |
| [3] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [4] | 刘晗, 崔家馨, 殷梦凡, 郑涛, 张睿, 孟祥海, 刘植昌, 刘海燕, 徐春明. CuAlCl4-二甲苯络合物晶体结构及二元固液相平衡测定[J]. 化工学报, 2025, 76(5): 2241-2250. |
| [5] | 何传超, 周静红, 曹约强, 施尧, 周兴贵. Ag/SiO2催化草酸酯加氢制乙醇酸甲酯的床层-颗粒双尺度耦合模拟研究[J]. 化工学报, 2025, 76(2): 654-666. |
| [6] | 许顺年, 冯晓, 史得军, 孙志国, 张宸玮, 王刚, 高金森, 徐春明. 原油直接加氢改质及其沥青质超分子解缔反应的研究[J]. 化工学报, 2025, 76(2): 812-824. |
| [7] | 李奕菲, 苏沿霏, 尹甜, 姜浩强, 许志明, 张霖宙, 史权, 徐春明. 基于GC×GC-TOF MS的煤液化产物油分子组成结构表征[J]. 化工学报, 2025, 76(2): 543-553. |
| [8] | 翟庆伟, 林锦辉, 李彦锋, 韩东旭, 吴小华, 王鹏, 陈宇杰, 宇波. 新型泵-热协同增压液氢加氢站系统㶲分析[J]. 化工学报, 2025, 76(10): 5390-5401. |
| [9] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [10] | 胡德政, 王榕, 王世栋, 杨文菲, 张宏伟, 袁珮. 兼具加氢和脱硫活性的富含Ni δ+非晶态NiP@γ-Al2O3催化剂的构筑及其用于石油树脂加氢的性能研究[J]. 化工学报, 2024, 75(9): 3152-3162. |
| [11] | 杨露, 刘聪聪, 孟彤彤, 张博远, 杨腾飞, 邓文安, 王晓斌. 分散型催化剂在煤/重油共炼体系中的加氢抑焦作用[J]. 化工学报, 2024, 75(7): 2556-2564. |
| [12] | 黄志鸿, 周利, 柴士阳, 吉旭. 耦合加氢装置优化的多周期氢网络集成[J]. 化工学报, 2024, 75(5): 1951-1965. |
| [13] | 丁禹, 杨昌泽, 李军, 孙会东, 商辉. 原子尺度钼系加氢脱硫催化剂的研究进展与展望[J]. 化工学报, 2024, 75(5): 1735-1749. |
| [14] | 韩宇, 周乐, 张鑫, 罗勇, 孙宝昌, 邹海魁, 陈建峰. 高黏附性Pd/SiO2/NF整体式催化剂的制备及加氢性能研究[J]. 化工学报, 2024, 75(4): 1533-1542. |
| [15] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号