• •
收稿日期:2025-08-20
修回日期:2025-09-28
出版日期:2025-11-10
通讯作者:
常琛朝
作者简介:王成志(1990—),男,博士,助理研究员,wangchengzhihh@163.com
基金资助:
Chengzhi WANG1,2( ), Zhongliang ZHANG1,2, Chenchao CHANG3(
), Zhongliang ZHANG1,2, Chenchao CHANG3( )
)
Received:2025-08-20
Revised:2025-09-28
Online:2025-11-10
Contact:
Chenchao CHANG
摘要:
氮氧化物(NO x )作为主要大气污染物之一,其排放控制技术一直是环境催化领域的研究热点。选择性催化还原(SCR)技术是目前最有效的脱硝方法之一,而催化剂的性能直接决定了SCR技术的应用效果。微波法作为一种高效、节能的制备技术,凭借均匀加热、促进活性组分高度分散与小尺寸颗粒形成、调控催化剂微观结构、且绿色环保的独特优势,在脱硝催化剂制备中展现出较强的应用前景。本文系统综述了微波法在脱硝催化剂制备及作为反应场热源中的应用进展,重点讨论了微波法对催化剂结构、性能的影响及其作用机制,总结了微波辅助制备的各类脱硝催化剂(包括金属氧化物、分子筛、金属有机骨架材料、碳材料)的性能特点,并对微波法在脱硝催化剂制备中的发展趋势和面临的挑战进行了展望。
中图分类号:
王成志, 张忠良, 常琛朝. 微波技术在脱硝催化剂制备及NH3-SCR中的应用进展[J]. 化工学报, DOI: 10.11949/0438-1157.20250941.
Chengzhi WANG, Zhongliang ZHANG, Chenchao CHANG. Progress in Microwave Technology for Denitration Catalyst Preparation and NH3-SCR Application[J]. CIESC Journal, DOI: 10.11949/0438-1157.20250941.
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| MnO x | 微波水热法 | 100 | 3 | 100 | 制备效率高,活性好、N2选择性高;但抗硫性能仍然较差 | [ |
| CeO2 | 微波焙烧 | 300 | 120 | 70 | 比表面积大、晶粒尺寸小且晶粒分散均匀;温窗较窄 | [ |
| γ-Fe2O3 | 微波热解法 | 700 | 10.8 | 96 | 工艺简单、晶相单一、结晶程度高;煅烧过程中存在一定程度的烧结现象,会导致部分孔结构遭到破坏 | [ |
| V2O5@AC | 微波浸渍法 | 300 | - | 99.7 | 诱导V2O5的高分散性和活性位点的暴露,产生更多的配位不饱和V和缺陷;会使部分催化剂比表面积和孔体积减小 | [ |
| VWTi-多孔纤维陶瓷 | 微波干燥 | 385 | 40.2 | 98 | 分散均匀,改变载体TiO₂的形貌、晶粒和孔结构以增大比表面积;高功率微波会降低TiO2中锐钛矿相比例,对活性有不利影响 | [ |
| Mn2CoO4@rGO | 微波辐照法 | 125 | 30 | 95 | 微波可快速同步实现氧化石墨烯还原及Mn2CoO4纳米片的规则生长,具有大比表面积、Lewis酸位点;负载量需严格控制,过高将抑制活性 | [ |
| Ce-La-Fe/γ-Al2O3 | 微波水热法 | - | - | 95.2 | 助力形成Ce-La-Fe-O固溶体,增加比表面积、表面酸位点及氧空位;制备过程复杂 | [ |
| Fe0.65Ce0.05Ti0.30Oz | 微波水热法 | 255 | 15 | 100 | 使活性温窗向低温偏移;但会降低催化剂的高温活性及比表面积 | [ |
| Fe0.85Ce0.10W0.05Oz | 微波辅助溶胶-凝胶法 | 291 | 10 | 100 | 优化催化剂性能且优于传统水热法,表面吸附氧含量多;高温活性差且NH3氧化副反应较强 | [ |
| Fe0.8Mg0.2Oz | 微波辅助共沉淀法 | - | - | 99.1 | 高温使颗粒团聚、微孔坍塌,温窗较窄不利于应用 | [ |
| NdV/Ti | 微波辅助沉积-沉淀法 | 500 | - | 100 | 促使Nd与VO3⁻快速反应并在TiO2表面形成均匀沉淀,利于钒酸盐分散 | [ |
| Fe2O3@稀土精矿 | 微波焙烧 | - | 20 | 80.6 | 微波处理后可增加Ce3+和Fe2+含量,产生更多氧空位;温度过高,稀土精矿易烧结 | [ |
| Ce x Zr1-x O2 | 微波焙烧 | 350 | 120 | 88.5 | 微波非热效应能够较大的提高脱硝效率,并且使反应的窗口温度前移 | [ |
| (Ce,La)PO4 | 微波焙烧 | 20 | 95 | 降低样品结晶度、增强活性物质分散性、增大比表面积、增加酸性位点 | [ | |
| CeO2-TiO2 | 微波辅助共沉淀法 | 200 | 120 | 97 | 微波可加速催化剂的结晶速率、增大比表面积、增加酸性位点及表面化学吸附氧浓度、提高Ce3⁺/Ce4⁺比例 | [ |
| MnV2O x /TiO2 | 微波辅助沉积-沉淀法 | 阶段1: 700, 阶段2: 800 | 阶段1:2, 阶段2:6 | 86 | 能够快速加热并促进均匀沉积;但其高功率和较长时间可能对催化剂结构产生一定影响 | [ |
| Mn-Fe/Al2O3 | 微波干燥 | 350 | 15 | 100 | 微波导致催化剂孔隙发达,减缓硫酸铵盐沉积的抑制效应 | [ |
表1 微波辅助制备金属氧化物催化剂研究列表
Table 1 List of research on microwave-assisted preparation of metal oxide catalysts
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| MnO x | 微波水热法 | 100 | 3 | 100 | 制备效率高,活性好、N2选择性高;但抗硫性能仍然较差 | [ |
| CeO2 | 微波焙烧 | 300 | 120 | 70 | 比表面积大、晶粒尺寸小且晶粒分散均匀;温窗较窄 | [ |
| γ-Fe2O3 | 微波热解法 | 700 | 10.8 | 96 | 工艺简单、晶相单一、结晶程度高;煅烧过程中存在一定程度的烧结现象,会导致部分孔结构遭到破坏 | [ |
| V2O5@AC | 微波浸渍法 | 300 | - | 99.7 | 诱导V2O5的高分散性和活性位点的暴露,产生更多的配位不饱和V和缺陷;会使部分催化剂比表面积和孔体积减小 | [ |
| VWTi-多孔纤维陶瓷 | 微波干燥 | 385 | 40.2 | 98 | 分散均匀,改变载体TiO₂的形貌、晶粒和孔结构以增大比表面积;高功率微波会降低TiO2中锐钛矿相比例,对活性有不利影响 | [ |
| Mn2CoO4@rGO | 微波辐照法 | 125 | 30 | 95 | 微波可快速同步实现氧化石墨烯还原及Mn2CoO4纳米片的规则生长,具有大比表面积、Lewis酸位点;负载量需严格控制,过高将抑制活性 | [ |
| Ce-La-Fe/γ-Al2O3 | 微波水热法 | - | - | 95.2 | 助力形成Ce-La-Fe-O固溶体,增加比表面积、表面酸位点及氧空位;制备过程复杂 | [ |
| Fe0.65Ce0.05Ti0.30Oz | 微波水热法 | 255 | 15 | 100 | 使活性温窗向低温偏移;但会降低催化剂的高温活性及比表面积 | [ |
| Fe0.85Ce0.10W0.05Oz | 微波辅助溶胶-凝胶法 | 291 | 10 | 100 | 优化催化剂性能且优于传统水热法,表面吸附氧含量多;高温活性差且NH3氧化副反应较强 | [ |
| Fe0.8Mg0.2Oz | 微波辅助共沉淀法 | - | - | 99.1 | 高温使颗粒团聚、微孔坍塌,温窗较窄不利于应用 | [ |
| NdV/Ti | 微波辅助沉积-沉淀法 | 500 | - | 100 | 促使Nd与VO3⁻快速反应并在TiO2表面形成均匀沉淀,利于钒酸盐分散 | [ |
| Fe2O3@稀土精矿 | 微波焙烧 | - | 20 | 80.6 | 微波处理后可增加Ce3+和Fe2+含量,产生更多氧空位;温度过高,稀土精矿易烧结 | [ |
| Ce x Zr1-x O2 | 微波焙烧 | 350 | 120 | 88.5 | 微波非热效应能够较大的提高脱硝效率,并且使反应的窗口温度前移 | [ |
| (Ce,La)PO4 | 微波焙烧 | 20 | 95 | 降低样品结晶度、增强活性物质分散性、增大比表面积、增加酸性位点 | [ | |
| CeO2-TiO2 | 微波辅助共沉淀法 | 200 | 120 | 97 | 微波可加速催化剂的结晶速率、增大比表面积、增加酸性位点及表面化学吸附氧浓度、提高Ce3⁺/Ce4⁺比例 | [ |
| MnV2O x /TiO2 | 微波辅助沉积-沉淀法 | 阶段1: 700, 阶段2: 800 | 阶段1:2, 阶段2:6 | 86 | 能够快速加热并促进均匀沉积;但其高功率和较长时间可能对催化剂结构产生一定影响 | [ |
| Mn-Fe/Al2O3 | 微波干燥 | 350 | 15 | 100 | 微波导致催化剂孔隙发达,减缓硫酸铵盐沉积的抑制效应 | [ |
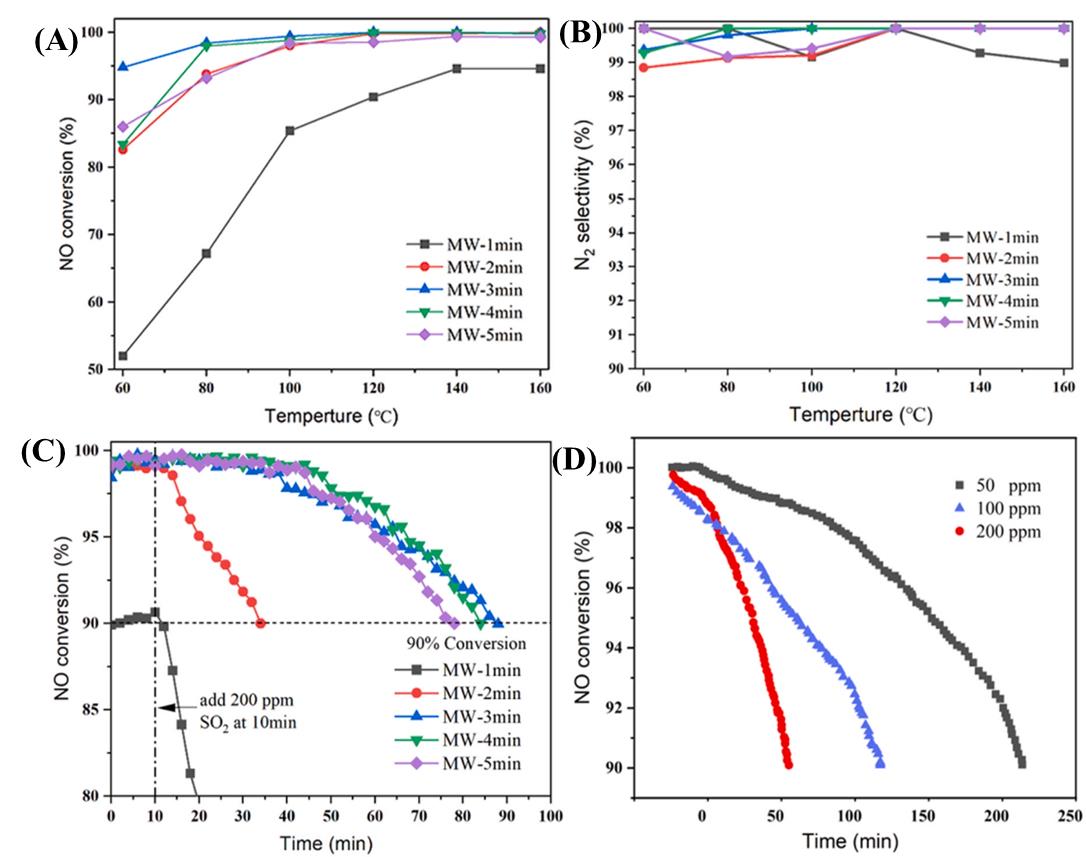
图2 不同微波反应时间合成的NH3-SCR催化剂的性能:(A)NO转化率,(B)N2选择性,(C)催化剂抗硫中毒性测试,(D)MW-3 min催化剂在不同SO2浓度下的NH3-SCR性能[29]
Fig. 2 Performance of NH3-SCR catalysts synthesized with different microwave reaction times:(A) NO conversion, (B) N2 selectivity, (C) catalyst resistance to sulfur neutrality test, and (D) NH3-SCR performance of MW-3 min catalysts at different SO2 concentrations[29]
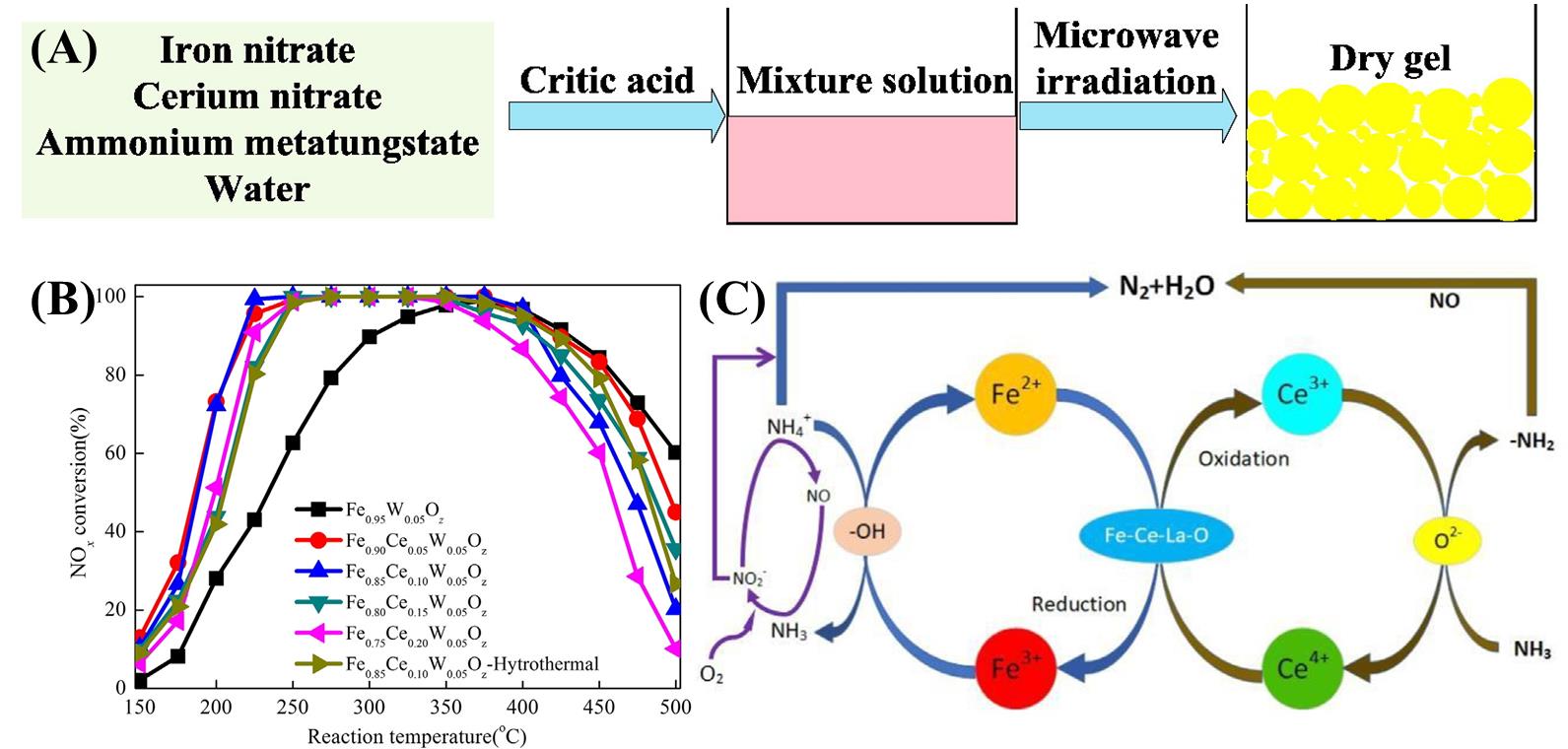
图4 (A)Fe0.85Ce0.10W0.05Oz催化剂的合成示意图,(B)水热法与微波合成Fe0.85Ce0.10W0.05Oz催化剂活性[23],(C)Ce-La-Fe/γ-Al2O3催化剂的反应机理[39]
Fig. 4 (A) Schematic diagram of the synthesis of Fe0.85Ce0.10W0.05Oz catalysts, (B) catalyst activity synthesized by hydrothermal method versus microwave [23], (C) reaction mechanism of Ce-La-Fe/γ-Al2O3 catalyst [39]
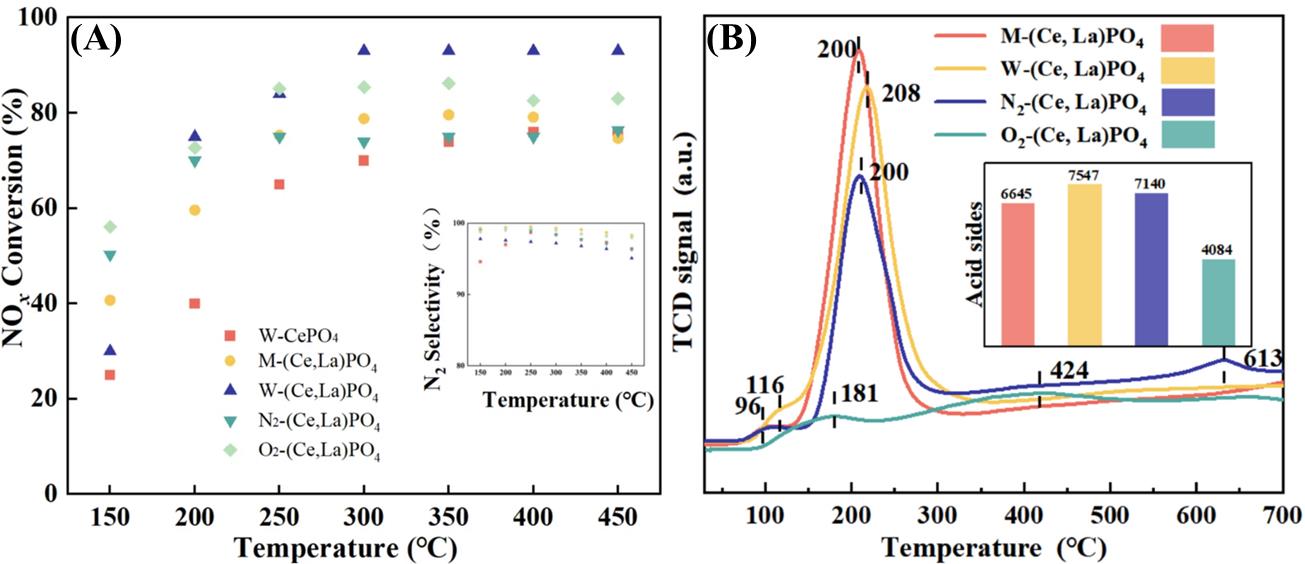
图6 (A)不同煅烧条件的NH3-SCR活性,(B)不同煅烧条件催化剂的NH3-TPD结果[45]
Fig. 6 (A) NH3-SCR activity for different calcination conditions, (B) NH3-TPD results for catalysts with different calcination conditions [45]
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| CuII-SSZ-13 | 微波水热法 | 400 | 540 | 100 | 微波辅助水热合成可缩短CuII-SSZ-13的结晶时间,影响其成核与生长,形成稳定骨架结构并增强Al-O-Si键 | [ |
| Cu/SSZ-13 | 微波活化 | 250 | 30 | 90 | 拓宽活性温窗、增加活性位点;但功率过大或时间过长会消耗大量能量并可能对催化剂表面造成局部损伤 | [ |
| CuCe@ZIF-7 | 微波水热法 | 900 | 240 | 95 | 快速、高效制备前驱体 | [ |
| Cu-SSZ-13 | 微波水热法 | 400 | 540 | 100 | 缩短SSZ-13的结晶时间,促进其成核与生长,使颗粒分散性优异、形貌规则,增强离子交换能力及NH3/NO吸附能力 | [ |
| Cu-ZSM-5 | 微波干燥 | 800 | 10 | 87 | 影响催化剂的孔道结构及活性物质的晶粒大小与分布;但会破坏微孔结构导致有效活性位点减少 | [ |
| Cu-SSZ-13 | 微波水热法 | - | 360 | 100 | 缩短SSZ-13分子筛晶化时间,促进晶核形成与生长,提高结晶度 | [ |
| Fe-Al-SBA-15 | 微波水热法 | - | - | 95 | 促进Fe-Al-SBA-15形成更多低聚Fe x Oᵧ簇和四面体骨架铝 | [ |
| MCM-41 | 微波晶化法 | 阶段1: 300, 阶段2: 60 | 阶段1: 15,阶段2: 25 | - | 快速合成、晶粒直径小、分散均匀、比表面积大、具有良好高温和水热稳定性,且操作便利、节能、环境污染少,无转晶现象 | [ |
表2 微波辅助制备分子筛类催化剂研究列表
Table 2 Research on microwave-assisted preparation of zeolite catalysts
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| CuII-SSZ-13 | 微波水热法 | 400 | 540 | 100 | 微波辅助水热合成可缩短CuII-SSZ-13的结晶时间,影响其成核与生长,形成稳定骨架结构并增强Al-O-Si键 | [ |
| Cu/SSZ-13 | 微波活化 | 250 | 30 | 90 | 拓宽活性温窗、增加活性位点;但功率过大或时间过长会消耗大量能量并可能对催化剂表面造成局部损伤 | [ |
| CuCe@ZIF-7 | 微波水热法 | 900 | 240 | 95 | 快速、高效制备前驱体 | [ |
| Cu-SSZ-13 | 微波水热法 | 400 | 540 | 100 | 缩短SSZ-13的结晶时间,促进其成核与生长,使颗粒分散性优异、形貌规则,增强离子交换能力及NH3/NO吸附能力 | [ |
| Cu-ZSM-5 | 微波干燥 | 800 | 10 | 87 | 影响催化剂的孔道结构及活性物质的晶粒大小与分布;但会破坏微孔结构导致有效活性位点减少 | [ |
| Cu-SSZ-13 | 微波水热法 | - | 360 | 100 | 缩短SSZ-13分子筛晶化时间,促进晶核形成与生长,提高结晶度 | [ |
| Fe-Al-SBA-15 | 微波水热法 | - | - | 95 | 促进Fe-Al-SBA-15形成更多低聚Fe x Oᵧ簇和四面体骨架铝 | [ |
| MCM-41 | 微波晶化法 | 阶段1: 300, 阶段2: 60 | 阶段1: 15,阶段2: 25 | - | 快速合成、晶粒直径小、分散均匀、比表面积大、具有良好高温和水热稳定性,且操作便利、节能、环境污染少,无转晶现象 | [ |
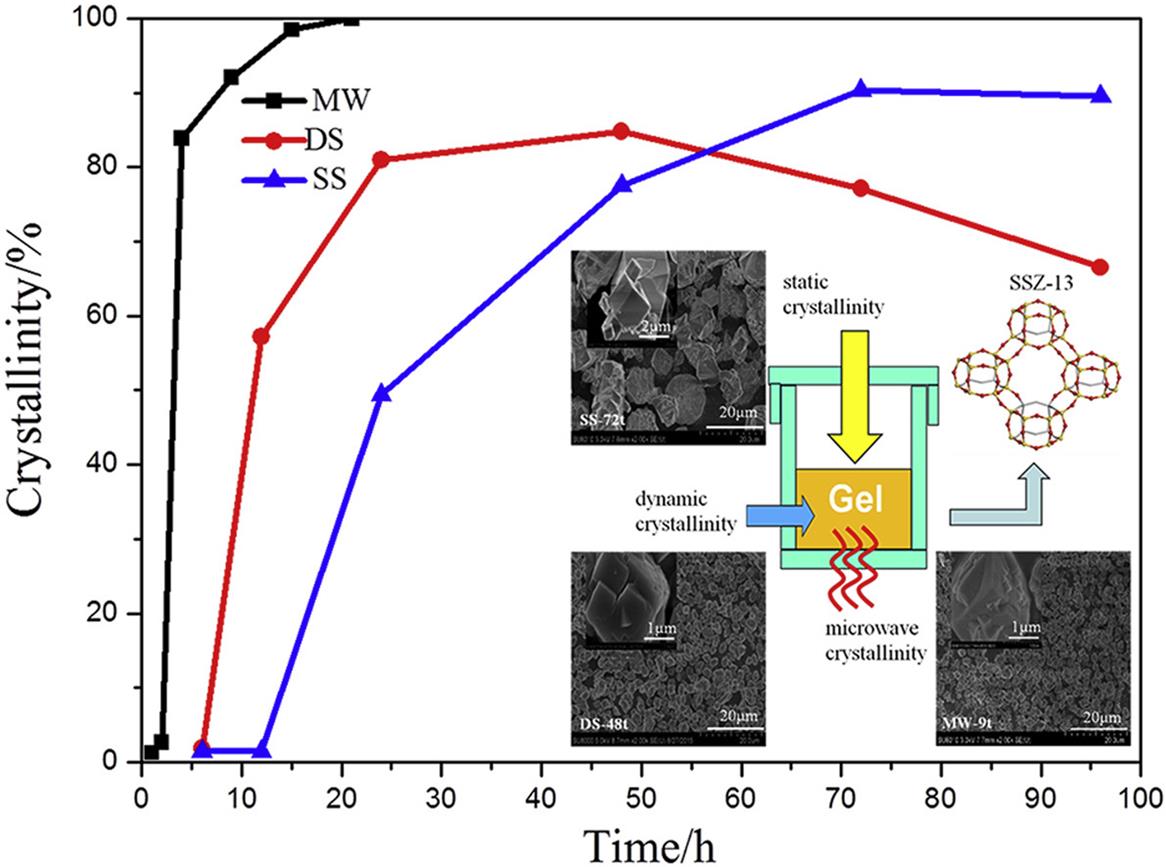
图7 微波、动态和静态水热法合成Cu-SSZ-13催化剂的形貌及脱硝效率[52]
Fig. 7 Morphology and denitrification efficiency of Cu-SSZ-13 catalyst synthesized by microwave, dynamic and static hydrothermal methods[52]
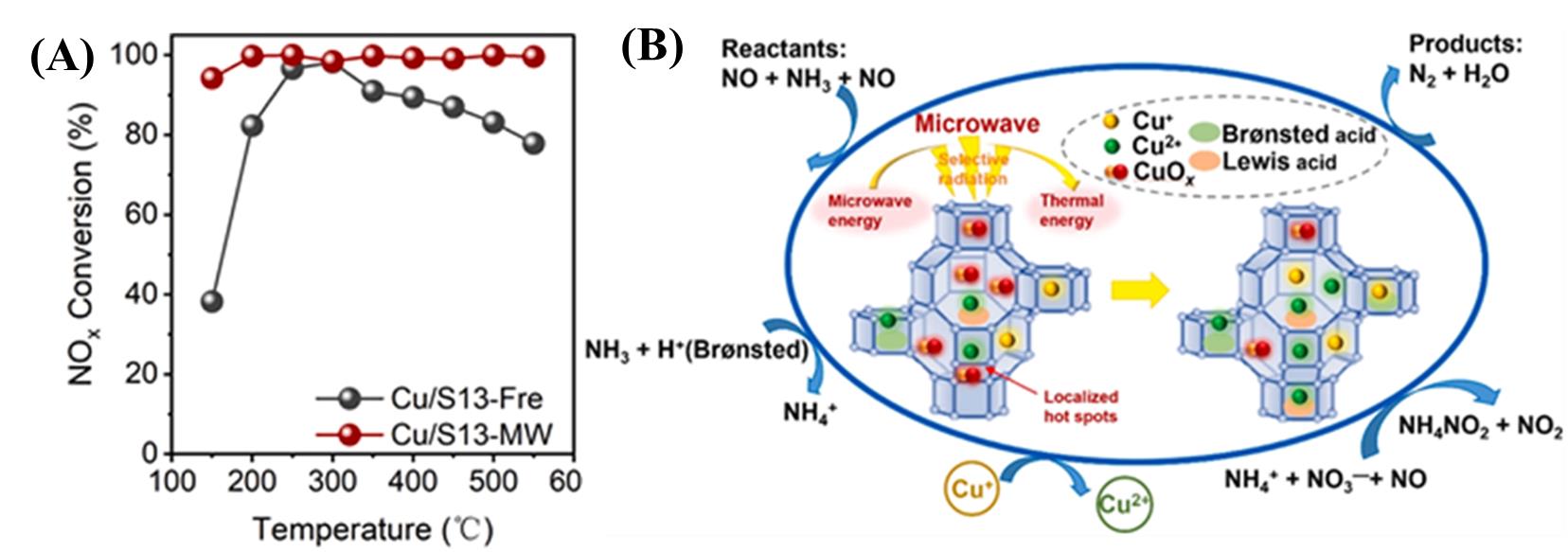
图8 (A)微波活化前后Cu/SSZ-13沸石上的催化活性及(B)反应机理图[50]
Fig. 8 (A)Catalytic activity and (B) reaction mechanism diagram on Cu/SSZ-13 zeolite before and after microwave activation[50]
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| Cu-BTC | 微波合成法 | 600 | 30 | 97.8 | 与非热等离子体协同作用可激活Cu-BTC产生配位不饱和位点,增加羰基含量,提升表面催化活性和化学吸附性能 | [ |
| CuFe2O4 | 微波合成法 | 900 | 240 | 92 | 分散性和稳定性良好 | [ |
| Ce-Cu-BTC | 微波辅助加热-分步浸渍法 | - | 120 | 91 | 促进形成高比表面积、规则多孔结构及丰富活性位点,增强Ce与Cu的协同作用;但热稳定性差,并且在高于300 ℃时骨架易坍塌 | [ |
| Ti0.2-Ni0.8-MOF | 微波辐照 | 100 | 120 | 100 | 更高的合成效率、更好的结晶度、晶粒细小且更加均匀 | [ |
| 活性焦 | 微波辐照 | 500 | 30 | 90 | 可提高活性焦的比表面积和孔容,减小孔径,激活表面官能团 | [ |
| 活性焦负载Fe/Mn/Cu | 微波辐照 | 200 | - | 85.6 | 增强活性焦极化、提高等离子体强度、增加含氧官能团 | [ |
| CuO/AC | 微波活化 | 700 | 7 | 52 | 可增加介孔比例、优化孔结构和表面碱性基团;但脱硝性能不理想 | [ |
| 活性炭负载Cu-Fe | 微波活化 | - | - | 86 | 微波处理的椰壳活性炭作为载体,经Cu、Fe改性后可提供丰富表面活性位点,利于NO和Hg0的吸附与转化;但活性炭吸附作用贡献无法考量 | [ |
| Mn2CoO4@rGO | 微波水热法 | 125 | 30 | 100 | 可同时实现氧化石墨烯的还原和Mn2CoO4纳米片在还原石墨烯层上的直立规则生长 | [ |
表3 微波辅助制备MOFs、碳基催化剂研究列表
Table 3 List of studies on microwave-assisted preparation of MOFs and carbon-based catalysts
| 催化剂 | 制备方法 | 微波功率/W | 微波时间/分钟 | NO x 转化率/% | 利弊 | 参考文献 |
|---|---|---|---|---|---|---|
| Cu-BTC | 微波合成法 | 600 | 30 | 97.8 | 与非热等离子体协同作用可激活Cu-BTC产生配位不饱和位点,增加羰基含量,提升表面催化活性和化学吸附性能 | [ |
| CuFe2O4 | 微波合成法 | 900 | 240 | 92 | 分散性和稳定性良好 | [ |
| Ce-Cu-BTC | 微波辅助加热-分步浸渍法 | - | 120 | 91 | 促进形成高比表面积、规则多孔结构及丰富活性位点,增强Ce与Cu的协同作用;但热稳定性差,并且在高于300 ℃时骨架易坍塌 | [ |
| Ti0.2-Ni0.8-MOF | 微波辐照 | 100 | 120 | 100 | 更高的合成效率、更好的结晶度、晶粒细小且更加均匀 | [ |
| 活性焦 | 微波辐照 | 500 | 30 | 90 | 可提高活性焦的比表面积和孔容,减小孔径,激活表面官能团 | [ |
| 活性焦负载Fe/Mn/Cu | 微波辐照 | 200 | - | 85.6 | 增强活性焦极化、提高等离子体强度、增加含氧官能团 | [ |
| CuO/AC | 微波活化 | 700 | 7 | 52 | 可增加介孔比例、优化孔结构和表面碱性基团;但脱硝性能不理想 | [ |
| 活性炭负载Cu-Fe | 微波活化 | - | - | 86 | 微波处理的椰壳活性炭作为载体,经Cu、Fe改性后可提供丰富表面活性位点,利于NO和Hg0的吸附与转化;但活性炭吸附作用贡献无法考量 | [ |
| Mn2CoO4@rGO | 微波水热法 | 125 | 30 | 100 | 可同时实现氧化石墨烯的还原和Mn2CoO4纳米片在还原石墨烯层上的直立规则生长 | [ |
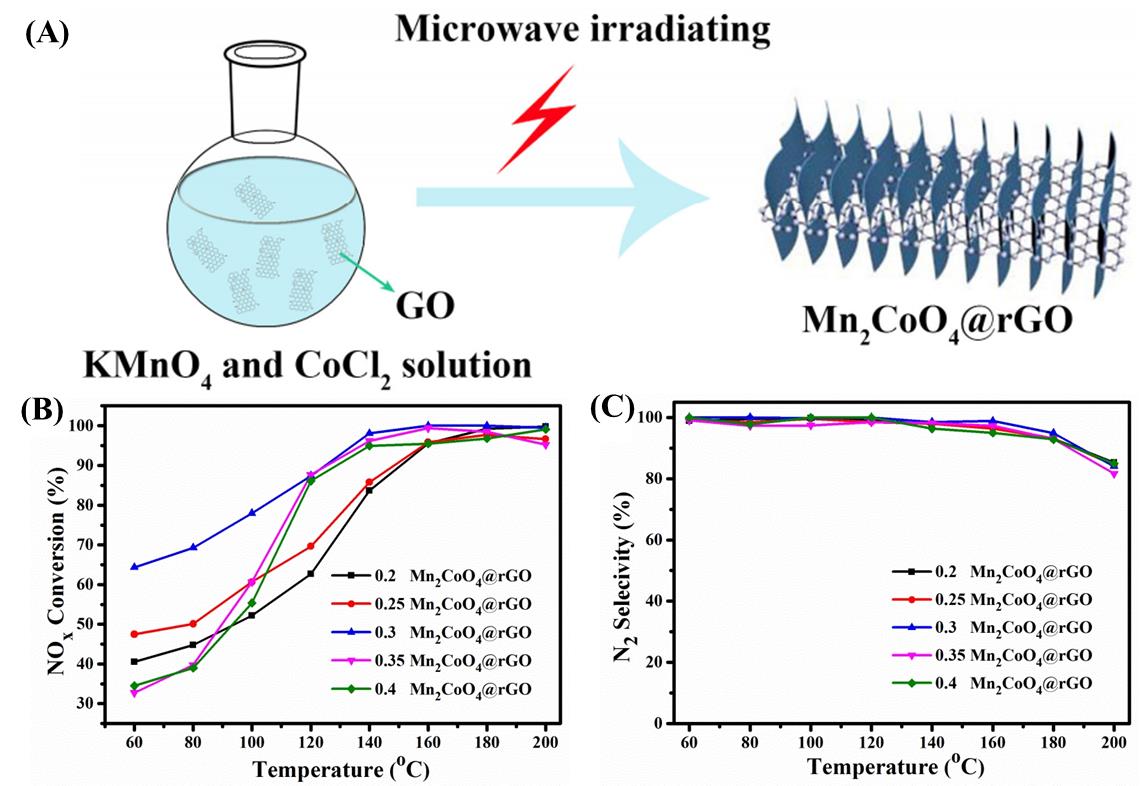
图10 (A)Mn2CoO4@rGO催化剂的合成示意图,(B)NH3-SCR性能,(C)催化剂的N2选择性[40]
Fig. 10 (A) Schematic of the synthesis of Mn2CoO4@rGO catalyst, (B) NH3-SCR performance, (C) N2 selectivity of the catalyst[40]
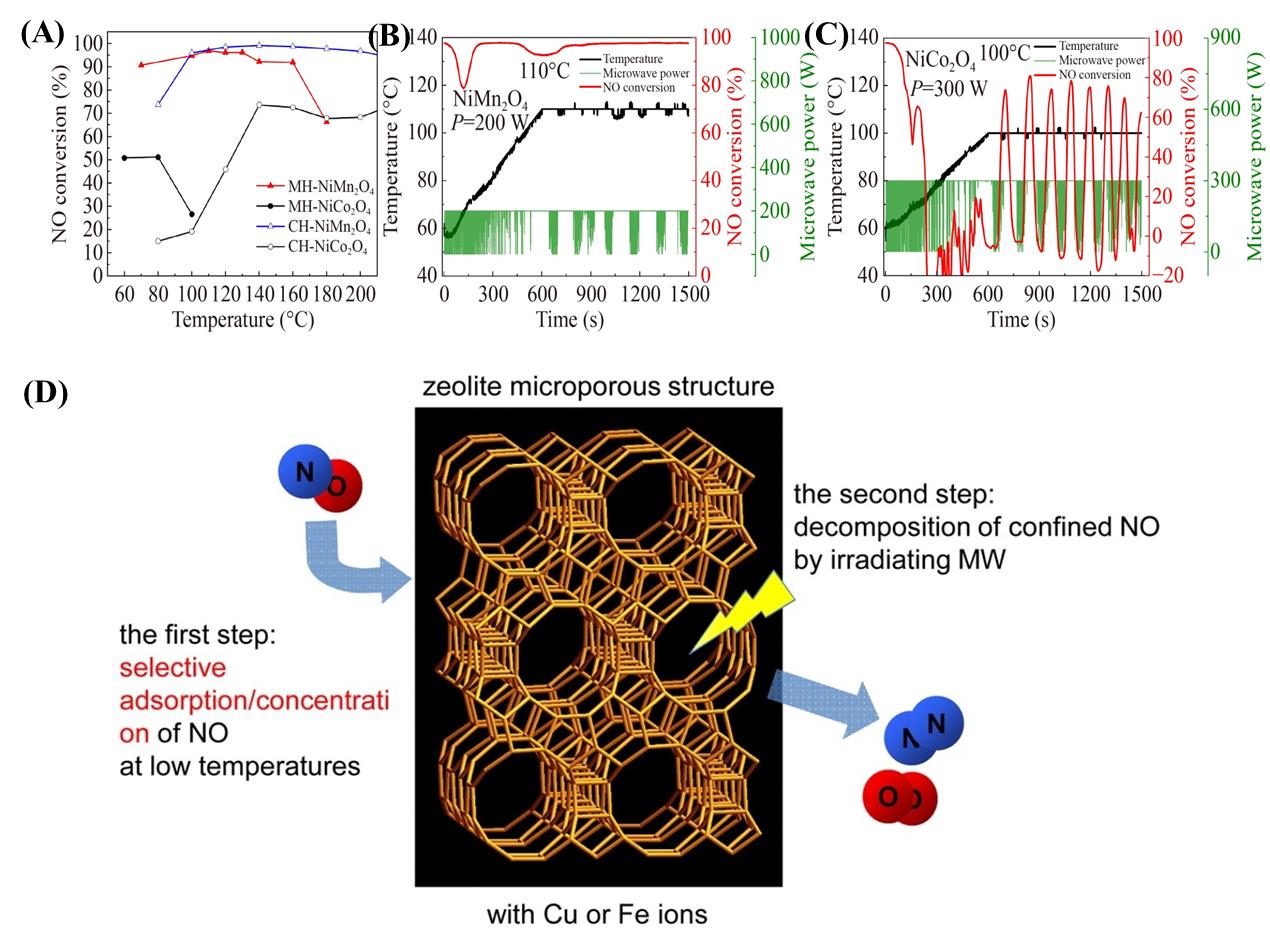
图11 (A)催化剂在微波加热(MH)和常规加热(CH)下的活性;微波加热下(B)NiMn2O4和(C)NiCo2O4催化剂的NH3-SCR活性[71];(D)使用掺杂MeO x (Me=Mn、Ni、Cu)的沸石去除NO的催化还原过程[70]
Fig. 11 (A) Activity of catalysts under microwave heating (MH) and conventional heating (CH); NH3-SCR activity of (B) NiMn2O4; (C) NiCo2O4 catalysts under microwave heating[71]; (D) Catalytic reduction process for NO removal using zeolite doped with MeO x (Me = Mn, Ni, Cu) [70]
| [1] | Li X L, Niu Y F, Li J, et al. Trace Co doping improves NH3-SCR performance and poisoning resistance of Ce-Mn-based catalysts[J]. Chemical Engineering Journal, 2023, 454: 140180. |
| [2] | Zhu K M, Yan W Q, Liu S J, et al. One-step hydrothermal synthesis of MnO x -CeO2/reduced graphene oxide composite aerogels for low temperature selective catalytic reduction of NO x [J]. Applied Surface Science, 2020, 508: 145024. |
| [3] | Wang S, Liu J, Jin Z S, et al. Gas-Phase Regeneration of Metal-Poisoned V2O5-WO3/TiO2 NH3-SCR Catalysts via a Masking and Reconstruction Strategy [J]. Environmental Science & Technology, 2024, 58(30), 13574-13584. |
| [4] | Lyu F Y, Qiao J X, Xu X S, et al. Simple preparation of V2O5-WO3/TiO2/SiC catalytic membrane with highly efficient dust removal and NO reduction[J]. Separation and Purification Technology, 2024, 343: 127155. |
| [5] | Jung M G, Shin J H, Kwon D W, et al. Promotional effects of Me (Sb, La, Ce, Mo) additives on the NH3-SCR activity and SO2 durability of V2O5-WO3/TiO2 catalysts[J]. Process Safety and Environmental Protection, 2024, 183: 911-924. |
| [6] | Wang T P, Hu Z, Zhou J L, et al. Efficient enhancement of the anti-KCl-poisoning performance for V2O5-WO3/TiO2 catalysts by Ce(SO4)2 modification[J]. Journal of Solid State Chemistry, 2023, 319: 123807. |
| [7] | Guo M Y, Gao J, Niu K, et al. SCR performance and mechanism study with multiple poisoning gases on CeO x promoted V2O5-WO3/TiO2 monolithic honeycomb catalyst of NO x removal[J]. Separation and Purification Technology, 2025, 363: 132087. |
| [8] | Yuan B, Qian Z, Zhangc Z, et al. A critical review on the technique and mechanism of microwave-based denitrification in flue gas[J]. Journal of Environmental Sciences, 2022, 120: 144-157. |
| [9] | Niu Z R, Gao F Y, Wu W J, et al. Preparation and optimization of Mn-based catalysts for low-temperature NH3-SCR: Component selection, synthesis strategy and influencing factors[J]. Separation and Purification Technology, 2025, 357: 130103. |
| [10] | 李晓雪. MOFs/TiO2催化剂多温区脱硝性能研究[D]. 北京: 华北电力大学, 2023. |
| Li X X. Study on Multi temperature Zone Denitration Performance of MOFs/TiO2 Catalyst [D]. Beijing: North China Electric Power University, 2023. | |
| [11] | 黄磊. Ni基双金属MOF的制备及低温CO-SCR催化应用研究[D]. 大连: 大连理工大学, 2021. |
| Huang L. Study on Ni-based bimetallic MOF synthesis and low temperature selective catalytic NO reduction by CO[D]. Dalian: Dalian University of Technology, 2021. | |
| [12] | Wei W, Shao Z S, Qiao R J, et al. Recent development of microwave applications for concrete treatment[J]. Construction and Building Materials, 2021, 269: 121224. |
| [13] | Liu Y X, Shan Y, Wang Y. Novel simultaneous removal technology of NO and SO2 using a semi-dry microwave activation persulfate system[J]. Environmental Science & Technology, 2020, 54(3): 2031-2042. |
| [14] | Dong Y N, Hu T D, Pudukudy M, et al. Influence of microwave-assisted synthesis on the structural and textural properties of mesoporous MIL-101(Fe) and NH2-MIL-101(Fe) for enhanced tetracycline adsorption[J]. Materials Chemistry and Physics, 2020, 251: 123060. |
| [15] | Gao Y, Wang F, Tang J, et al. Ferrocenedicarboxylate modified Bi-MOF for water decontamination via different advanced oxidation processes: Multifunction, mechanisms and biotoxicity test[J]. Chemical Engineering Journal, 2024, 495: 153651. |
| [16] | Chang M, Wang F, Liu Z Y, et al. Mining iron from stainless steel pickling wastewater to produce quasi-MIL-100(Fe) for boosted photocatalytic peroxymonosulfate activation[J]. Nano Research, 2025, 18(10): 94907382. |
| [17] | Li S Y, Song L Y, Li J, et al. Promotional mechanisms of activity and SO2 tolerance of NdVOx/TiO2 catalysts for selective catalytic reduction of NO x with NH3 [J]. ACS Catalysis, 2023, 13(5): 2867-2884. |
| [18] | Luo M Y, Bo L L, Huang S N, et al. Effect of polyethylene glycol addition on Cu-Mn-Ce-O x /TiO2/CH catalyst in microwave catalytic combustion of toluene[J]. Applied Catalysis A: General, 2025, 696: 120185. |
| [19] | 李佳璇. 气氛条件对微波辐照Mn-Fe/AC催化剂脱硝性能的影响[D]. 太原: 太原理工大学, 2021. |
| Li J X. Influence of atmosphere conditions on denitration performance of Mn-Fe/AC catalyst irradiated by microwave[D]. Taiyuan: Taiyuan University of Technology, 2021. | |
| [20] | 易钰涵, 康建刚, 余春沐, 等. 烧结烟气条件下微波催化直接分解NO脱硝试验[J]. 烧结球团, 2024, 49(2): 99-106. |
| Yi Y H, Kang J G, Yu C M, et al. Research on direct decomposition of NO by microwave catalysis under condition of sintering flue gas[J]. Sintering and Pelletizing, 2024, 49(2): 99-106. | |
| [21] | Yan H Y, Qu H X, Bai H P, et al. Property, active species and reaction mechanism of NO and NH3 over mesoporous Fe-Al-SBA-15 via microwave assisted synthesis for NH3-SCR[J]. Journal of Molecular Catalysis A: Chemical, 2015, 403: 1-9. |
| [22] | 孙中豪, 张慧茹, 米雪, 等. CuFe2O4催化剂的制备及其NH3-SCR催化性能研究[J]. 功能材料, 2022, 53(2): 2080-2086. |
| Sun Z H, Zhang H R, Mi X, et al. Synthesis of CuFe2O4 catalysts and catalytic performance for NH3-SCR[J]. Journal of Functional Materials, 2022, 53(2): 2080-2086. | |
| [23] | Xiong Z B, Peng B, Zhou F, et al. Magnetic iron-cerium-tungsten mixed oxide pellets prepared through critic acid Sol-gel process assisted by microwave irradiation for selective catalytic reduction of NO x with NH3 [J]. Powder Technology, 2017, 319: 19-25. |
| [24] | 孟昭磊, 李保卫, 付金艳, 等. 稀土精矿负载Fe2O3矿物催化材料的NH3-SCR脱硝性能研究[J]. 过程工程学报, 2021, 21(3): 363-372. |
| Meng Z L, Li B W, Fu J Y, et al. Study on NH3-SCR denitration performance of rare earth concentrate supported Fe2O3 mineral catalytic material[J]. The Chinese Journal of Process Engineering, 2021, 21(3): 363-372. | |
| [25] | 李江涛, 李红霞, 赵然. 微波焙烧对纳米CexZr1-xO2固溶体微观结构及其NH3-SCR催化性能的影响[J]. 内蒙古科技大学学报, 2023, 42(1): 5-9. |
| Li J T, Li H X, Zhao R. Effect of microwave roasting on the microstructure of Ce x Zr1- x O2 nanosolid solution and its NH3-SCR catalytic performance[J]. Journal of Inner Mongolia University of Science and Technology, 2023, 42(1): 5-9. | |
| [26] | 许嘉淇. 新型锰基低温脱硝催化剂的制备及性质研究[D]. 邯郸: 河北工程大学, 2023. |
| Xu J Q. Preparation and characterization of novel manganese-based low-temperature denitrification catalyst[D]. Handan: Hebei University of Engineering, 2023. | |
| [27] | Gu T, Wang C Z, Chen D H, et al. Effect of morphology on the performance of MnO x catalysts for selective catalytic reduction of NO with NH3 [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 694: 134197. |
| [28] | Mo D H, Qin Q J, Huang C H, et al. Regulating the distribution of iron active sites on γ-Fe2O3 via Mn-modified α-Fe2O3 for NH3-SCR[J]. Applied Catalysis B: Environment and Energy, 2024, 349: 123869. |
| [29] | Pei Z Z, Wang H P, Zhao H Y, et al. Investigation on denitrification performance of microwave synthesized high-efficiency MnO x catalysts for low-temperature NH3-SCR[J]. Journal of Alloys and Compounds, 2024, 1008: 176533. |
| [30] | Jiang Y, Xu Y C, Sun X, et al. Dual-edged sword effects of sulfation/hydration on Ce-doped TiO2(001) single-atom catalyst for NH3-SCR reaction: a first-principles study[J]. Fuel, 2024, 363: 131039. |
| [31] | Han L P, Cai S X, Gao M, et al. Selective catalytic reduction of NO x with NH3 by using novel catalysts: state of the art and future prospects[J]. Chemical Reviews, 2019, 119(19): 10916-10976. |
| [32] | 李江涛. 微波电磁焙烧纳米CeO2及其复合氧化物的形貌调控及其NH3-SCR催化性能的研究[D]. 包头: 内蒙古科技大学, 2023. |
| Li J T. Morphological modulation of microwave electromagnetic roasted nano-CeO2 and its composite oxides and their NH3-SCR catalytic performance[D]. Baotou: Inner Mongolia University of Science & Technology, 2023. | |
| [33] | Liu F D, He H, Zhang C B, et al. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and characterization[J]. Applied Catalysis B: Environmental, 2010, 96(3/4): 408-420. |
| [34] | Yang H P, Li J L, Sang H R, et al. Promoting NO removal performance of Fenton-like enhanced SCR reactions via modulating V/Fe in Fe-V oxides[J]. Applied Surface Science, 2024, 653: 159379. |
| [35] | Wang J K, Li Q, Qin Q J, et al. Fabrication of magnetic core–shell CZ/Fe3O4 catalysts for catalytic CO-SCR[J]. Industrial & Engineering Chemistry Research, 2024, 63(16): 7031-7043. |
| [36] | 王栋, 张信莉, 路春美, 等. 微波热解制备γ-Fe2O3催化剂及其SCR脱硝性能[J]. 化工学报, 2014, 65(12): 4805-4813. |
| Wang D, Zhang X L, Lu C M, et al. Microwave-assisted preparation of γ-Fe2O3 as SCR catalysts[J]. CIESC Journal, 2014, 65(12): 4805-4813. | |
| [37] | Cheng J, Xu R N, Song L Y, et al. Unveiling the role of microwave induction on V2O5@AC catalysts with enhanced activity for low temperature NH3-SCR reaction: an experimental and DFT study[J]. Environmental Science: Nano, 2023, 10(5): 1313-1328. |
| [38] | Miao L F, Wu X L, Ji Z L, et al. Microwave-assisted preparation of porous fibrous ceramic-based catalytic filter elements for the simultaneous removal of NO x and dust from high-temperature gases[J]. Separation and Purification Technology, 2021, 278: 119549. |
| [39] | Ren Z Y, Fan H, Wang R. A novel ring-like Fe2O3-based catalyst: Tungstophosphoric acid modification, NH3-SCR activity and tolerance to H2O and SO2 [J]. Catalysis Communications, 2017, 100: 71-75. |
| [40] | Tang X L, Li C L, Yi H H, et al. Facile and fast synthesis of novel Mn2CoO4@rGO catalysts for the NH3-SCR of NO x at low temperature[J]. Chemical Engineering Journal, 2018, 333: 467-476. |
| [41] | Wang Y, Zhao R, Sun J W, et al. Mechanistic study of Ce–La–Fe/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 [J]. International Journal of Hydrogen Energy, 2022, 47(13): 8261-8274. |
| [42] | Xiong Z B, Wu C, Hu Q, et al. Promotional effect of microwave hydrothermal treatment on the low-temperature NH3-SCR activity over iron-based catalyst[J]. Chemical Engineering Journal, 2016, 286: 459-466. |
| [43] | Xu L T, Yang Q L, Hu L H, et al. Insights over titanium modified FeMgO x catalysts for selective catalytic reduction of NO x with NH3: influence of precursors and crystalline structures[J]. Catalysts, 2019, 9(6): 560. |
| [44] | Xu L T, Niu S L, Lu C M, et al. Influence of calcination temperature on Fe0.8Mg0.2O z catalyst for selective catalytic reduction of NO x with NH3 [J]. Fuel, 2018, 219: 248-258. |
| [45] | Li N, Li J Q, Wang T, et al. Study ondenitrification performance and mechanism of (Ce, La)PO4 under different calcination conditions[J]. Reaction Kinetics, Mechanisms and Catalysis, 2025, 138(3): 1393-1407. |
| [46] | Cheng J, Song L Y, Wu R, et al. Promoting effect of microwave irradiation on CeO2-TiO2 catalyst for selective catalytic reduction of NO by NH3 [J]. Journal of Rare Earths, 2020, 38(1): 59-69. |
| [47] | Li S Y, Song L Y, Zhan Z C, et al. Redox and acid properties of MnV2O x /TiO2 catalysts synthesized by assistance of microwave for NO selective catalytic reduction by ammonia[J]. Chemical Engineering Journal Advances, 2021, 8: 100156. |
| [48] | 蔡森, 归柯庭. 微波辐射对铁基催化剂低温SCR脱硝活性的影响[J]. 动力工程学报, 2015, 35(12): 993-997, 1011. |
| Cai S, Gui K T. Effects of microwave drying on Fe-based catalysts for low-temperature SCR of NO x with NH3 [J]. Journal of Chinese Society of Power Engineering, 2015, 35(12): 993-997, 1011. | |
| [49] | Wang B, Yu H F, Wang M X, et al. Microwave synthesis conditions dependent catalytic performance of hydrothermally aged CuII-SSZ-13 for NH3-SCR of NO[J]. Catalysis Today, 2021, 376: 19-27. |
| [50] | Cheng H D, Tang X L, Yi H H, et al. Microwave activation of Cu/SSZ-13 zeolite for widening active temperature window of NO x from diesel exhaust[J]. Journal of Environmental Chemical Engineering, 2025, 13(1): 115152. |
| [51] | 孙中豪, 郝润龙, 赵毅. 改性沸石咪唑催化剂的NH3-SCR催化性能研究[J]. 功能材料, 2022, 53(7): 7006-7012. |
| Sun Z H, Hao R L, Zhao Y. Study on modified zeolite imidazole catalysts and catalytic performance for NH3-SCR[J]. Journal of Functional Materials, 2022, 53(7): 7006-7012. | |
| [52] | Han L N, Zhao X G, Yu H F, et al. Preparation of SSZ-13 zeolites and their NH3-selective catalytic reduction activity[J]. Microporous and Mesoporous Materials, 2018, 261: 126-136. |
| [53] | 唐剑骁, 马丽萍, 王冬东, 等. 微波对Cu-ZSM-5催化剂结构及低温NH3-SCR脱硝活性的影响[J]. 人工晶体学报, 2017, 46(8): 1569-1574. |
| Tang J X, Ma L P, Wang D D, et al. Influence of microwave on the structure and NH3-SCR denitration activity of Cu-ZSM-5 catalysts[J]. Journal of Synthetic Crystals, 2017, 46(8): 1569-1574. | |
| [54] | 卓佐西, 秦刚华, 刘春红, 等. 微波辅助双模板剂体系快速合成纳米SSZ-13分子筛及NH3-SCR脱硝性能研究[J]. 现代化工, 2023, 43(5): 153-158. |
| Zhuo Z X, Qin G H, Liu C H, et al. Rapid synthesis of nano SSZ-13 molecular sieve by microwave-assisted dual-template system and study on its performance in NH3-SCR denitration[J]. Modern Chemical Industry, 2023, 43(5): 153-158. | |
| [55] | Wei Z S, Zeng G H, Xie Z R, et al. Microwave catalytic NO x and SO2 removal using FeCu/zeolite as catalyst[J]. Fuel, 2011, 90(4): 1599-1603. |
| [56] | 夏启斌, 陈汇勇, 奚红霞, 等. MCM-41中孔分子筛的微波、常温和水热合成比较[J]. 华南理工大学学报(自然科学版), 2007, 35(6): 86-90, 115. |
| Xia Q B, Chen H Y, Xi H X, et al. Comparison of synthesis of meso-pore zeolite MCM-41 by microwave crystallization, room-temperature crystallization and hydrothermal crystallization[J]. Journal of South China University of Technology (Natural Science Edition), 2007, 35(6): 86-90, 115. | |
| [57] | 王达锐, 孙洪敏, 薛明伟, 等. 微波法高效合成全结晶ZSM-5分子筛催化剂及其催化性能[J]. 化工进展, 2023, 42(7): 3582-3588. |
| Wang D R, Sun H M, Xue M W, et al. Efficient synthesis of fully crystalline ZSM-5 zeolite catalyst by microwave method and its catalytic performance[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3582-3588. | |
| [58] | Ko S, Gao F Y, Yao X L, et al. Synthesis of metal–organic frameworks (MOFs) and their application in the selective catalytic reduction of NO x with NH3 [J]. New Journal of Chemistry, 2022, 46(33): 15758-15775. |
| [59] | Song K L, Guo K Y, Mao S M, et al. Insight into the origin of excellent SO2 tolerance and de-NO x performance of quasi-Mn-BTC in the low-temperature catalytic reduction of nitrogen oxide[J]. ACS Catalysis, 2023, 13(7): 5020-5032. |
| [60] | Gong X L, Zhao R, Qin J Q, et al. Ultra-efficient removal of NO in a MOFs-NTP synergistic process at ambient temperature[J]. Chemical Engineering Journal, 2019, 358: 291-298. |
| [61] | 黄樱蕾. Ce-Cu-BTC衍生催化剂的制备及低温CO-SCR脱硝性能研究[D]. 大连: 大连理工大学, 2024. |
| Huang Y L. Preparation and low-temperature CO-SCR denitrification performance of Ce-Cu-BTC derived catalysts[D]. Dalian: Dalian University of Technology, 2024. | |
| [62] | 石勇, 李橙, 黄磊, 等. Tix-Ni1-x-MOFs的制备及其CO选择性催化还原NOx研究[J]. 中国环境科学, 2022, 42(11): 5080-5087. |
| Shi Y, Li C, Huang L, et al. Preparation of Ti x -Ni1- x -MOFs and their selective catalytic reduction of NO x by CO[J]. China Environmental Science, 2022, 42(11): 5080-5087. | |
| [63] | Zhang J H, He Z J, Guo Q, et al. Effects of microwave modification on the desulfurization and denitrification of activated coke[J]. BioResources, 2020, 16(1): 729-746. |
| [64] | Liu H Y, Yang J B, Qiao X L, et al. Microwave plasma-assisted catalytic reduction of NO by active coke over transition-metal oxides[J]. Energy & Fuels, 2020, 34(4): 4384-4392. |
| [65] | Liu Y, Ning P, Li K, et al. Simultaneous removal of NO x and SO2 by low-temperature selective catalytic reduction over modified activated carbon catalysts[J]. Russian Journal of Physical Chemistry A, 2017, 91(3): 490-499. |
| [66] | Sun X, Sun L N, Liu Y, et al. Research on reaction conditions and mechanism for simultaneous removal of NO and Hg0 over CuFe modified activated carbon at low temperature[J]. Journal of the Energy Institute, 2020, 93(1): 87-98. |
| [67] | Cheng J, Song L Y, Wu R, et al. Promoting effect of microwave irradiation on CeO2-TiO2 catalyst for selective catalytic reduction of NO by NH3 [J]. Journal of Rare Earths, 2020, 38(1): 59-69. |
| [68] | Wang J, Zhu C, Li B W, et al. Prepare a catalyst consist of rare earth minerals to denitrate via NH3-SCR[J]. Green Processing and Synthesis, 2020, 9(1): 191-202. |
| [69] | Díaz-Ortiz Á, Prieto P, De la Hoz A. A critical overview on the effect of microwave irradiation in organic synthesis[J]. The Chemical Record, 2019, 19(1): 85-97. |
| [70] | Liu J L, Liang H S, Zhang Y, et al. Facile synthesis of ellipsoid-like MgCo2O4/Co3O4 composites for strong wideband microwave absorption application[J]. Composites Part B: Engineering, 2019, 176: 107240. |
| [71] | Song L Y, Deng S L, Bian C Y, et al. NiB2O4 (B = Mn or Co) catalysts for NH3-SCR of NO x at low-temperature in microwave field[J]. Frontiers of Environmental Science & Engineering, 2023, 17(8): 96. |
| [72] | Peng K, Zhou J C, Xu W T, et al. Microwave irradiation-selective catalytic reduction of NO to N2 by activated carbon at low temperature[J]. Energy & Fuels, 2017, 31(7): 7344-7351. |
| [73] | Xu W T, Zhou J C, Li H, et al. Microwave-assisted catalytic reduction of NO into N2 by activated carbon supported Mn2O3 at low temperature under O2 excess[J]. Fuel Processing Technology, 2014, 127: 1-6. |
| [74] | Ohnishi T, Kawakami K, Nishioka M, et al. Direct decomposition of NO on metal-loaded zeolites with coexistence of oxygen and water vapor under unsteady-state conditions by NO concentration and microwave rapid heating[J]. Catalysis Today, 2017, 281: 566-574. |
| [75] | Wei Z S, Zeng G H, Xie Z R. Microwave catalytic desulfurization and denitrification simultaneously on Fe/Ca-5A zeolite catalyst[J]. Energy & Fuels, 2009, 23(6): 2947-2951. |
| [1] | 袁琳慧, 王瑜. 单服务器浸没射流式液冷系统散热性能[J]. 化工学报, 2025, 76(S1): 160-169. |
| [2] | 赵子祥, 段钟弟, 孙浩然, 薛鸿祥. 大温差两相流动诱导水锤冲击的数值模型[J]. 化工学报, 2025, 76(S1): 170-180. |
| [3] | 黄博, 黄灏, 王文, 贺隆坤. 薄膜型LNG船液货舱温度场计算分析[J]. 化工学报, 2025, 76(S1): 195-204. |
| [4] | 汪思远, 刘国强, 熊通, 晏刚. 窗式空调器轴流风机的风速非均匀分布特性及其对冷凝器流路优化设计的影响规律[J]. 化工学报, 2025, 76(S1): 205-216. |
| [5] | 孔俊龙, 毕扬, 赵耀, 代彦军. 储能电池直冷热管理系统的模拟实验[J]. 化工学报, 2025, 76(S1): 289-296. |
| [6] | 任现超, 谷雅秀, 段少斌, 贾文竹, 李汉林. 翅片式椭圆套管蒸发式冷凝器传热传质性能实验研究[J]. 化工学报, 2025, 76(S1): 75-83. |
| [7] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [8] | 罗海梅, 王泓, 孙照明, 尹艳华. 同向双螺杆传热系数计算模型的分析与验证[J]. 化工学报, 2025, 76(9): 4809-4823. |
| [9] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [10] | 胡金琦, 闵春华, 李小龙, 范元鸿, 王坤. 振动叶片耦合柔性板强化流体混沌混合与传热研究[J]. 化工学报, 2025, 76(9): 4824-4837. |
| [11] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [12] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [13] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [14] | 吴林凯, 林志敏, 王良璧. 基于热质传递效应的准稳态结霜模型改进及数值验证[J]. 化工学报, 2025, 76(8): 4004-4016. |
| [15] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号