• •
收稿日期:2025-08-28
修回日期:2025-11-09
出版日期:2025-11-10
通讯作者:
季石宇
作者简介:曹磊(1987—),男,硕士,高级工程师,cao_l2@hdec.com
Lei CAO( ), Zixiao XU, Chenxuan XU, Zhuo SHAO, Shiyu JI(
), Zixiao XU, Chenxuan XU, Zhuo SHAO, Shiyu JI( )
)
Received:2025-08-28
Revised:2025-11-09
Online:2025-11-10
Contact:
Shiyu JI
摘要:
水系Zn/MnO₂电池凭借高理论容量、高安全性和低成本等优势,已成为最具潜力的水系锌离子电池体系之一。尽管其阴极储能机制复杂,但H+/Zn2+界面传输行为已被证实对容量与循环稳定性具有关键影响。本文系统综述了Zn2+脱嵌、可逆质子反应、H+/Zn2+共嵌入及Mn4+/Mn2+两电子反应等多种反应机制,并总结了晶体结构调控、碳复合、表面修饰和缺陷工程等策略在优化H+/Zn2+传输动力学、提升反应可逆性与电池性能方面的研究进展。最后,对该体系正极材料的未来发展前景进行了展望,以期推动其实际应用。
中图分类号:
曹磊, 许自晓, 徐晨轩, 邵卓, 季石宇. 水系Zn/MnO2体系中H+/Zn2+界面传输调控及反应机理研究进展[J]. 化工学报, DOI: 10.11949/0438-1157.20250959.
Lei CAO, Zixiao XU, Chenxuan XU, Zhuo SHAO, Shiyu JI. Research progress on the H+/Zn2+ interfacial transport regulation and reaction mechanisms in aqueous Zn/MnO2 system[J]. CIESC Journal, DOI: 10.11949/0438-1157.20250959.
| 机理类型 | 典型电压平台 | 优势 | 挑战/局限性 |
|---|---|---|---|
| Zn2+脱嵌 | 1.3~1.4 V | 结构相对明确 | Zn2+扩散动力学慢,结构坍塌 |
| H+转化沉积 | 1.3~1.4 V & 1.2~1.3 V | 容量较高 | Mn溶解,副产物积累 |
| H+/Zn2+共嵌入 | 双平台(H+:较高; Zn2+:较低) | 容量高, 机理互补 | 两种离子相互作用复杂 |
Mn4+/Mn2+两电子 反应 | ~1.95 V(高电压) | 超高容量, 高电压 | 需酸性环境,Mn溶解严重 |
表1 水系Zn/MnO2电池主要储能机理对比
Table 1 Comparison of the main energy storage mechanisms of aqueous Zn/MnO2 batteries
| 机理类型 | 典型电压平台 | 优势 | 挑战/局限性 |
|---|---|---|---|
| Zn2+脱嵌 | 1.3~1.4 V | 结构相对明确 | Zn2+扩散动力学慢,结构坍塌 |
| H+转化沉积 | 1.3~1.4 V & 1.2~1.3 V | 容量较高 | Mn溶解,副产物积累 |
| H+/Zn2+共嵌入 | 双平台(H+:较高; Zn2+:较低) | 容量高, 机理互补 | 两种离子相互作用复杂 |
Mn4+/Mn2+两电子 反应 | ~1.95 V(高电压) | 超高容量, 高电压 | 需酸性环境,Mn溶解严重 |
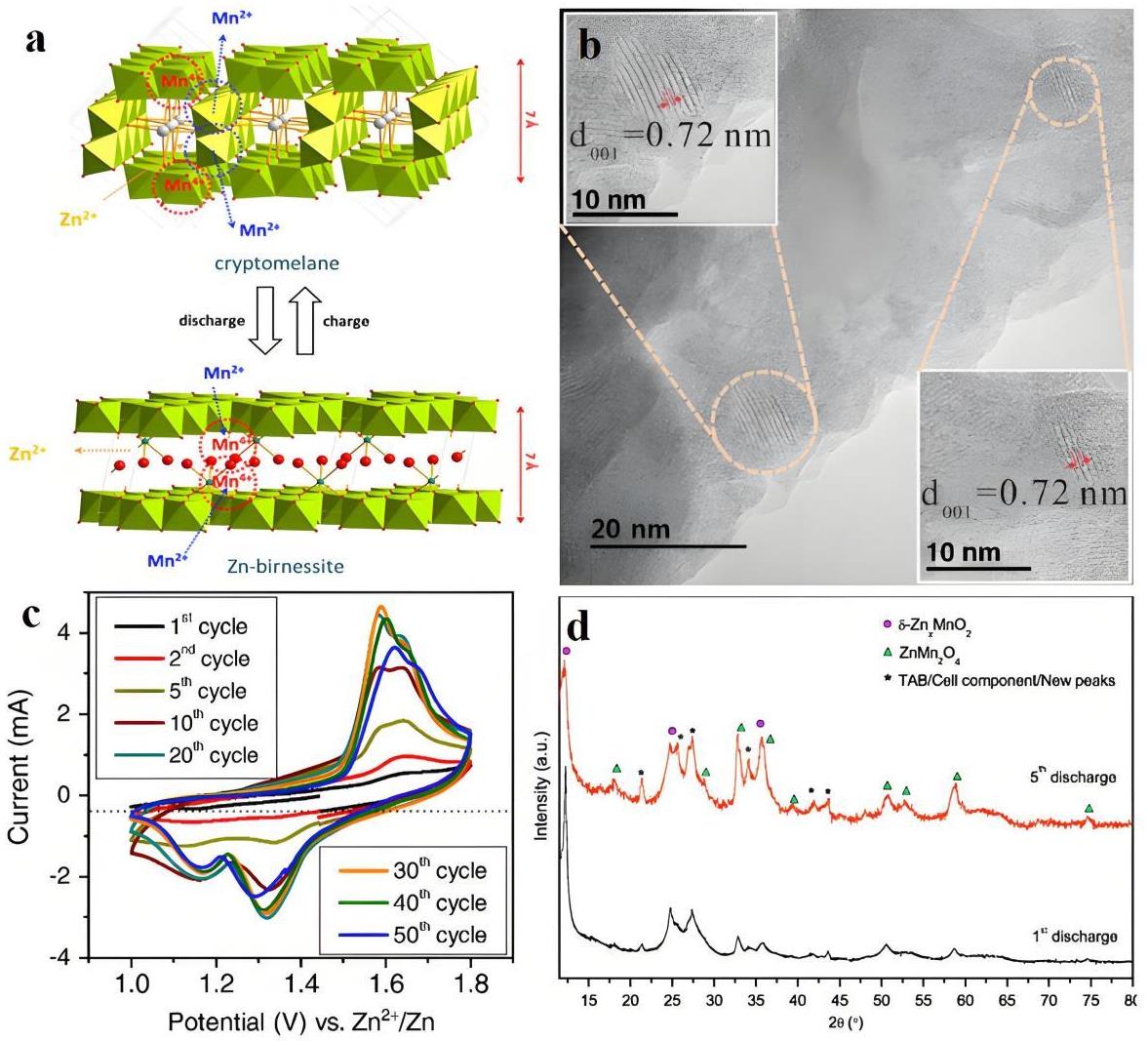
图1 (a) Zn2+嵌入α-MnO2的机理示意图[27];(b) δ-MnO2晶格结构的HR-TEM图;(c) Zn/δ-MnO2电池的CV曲线;(d) Zn/δ-MnO2电池第1和第5次放电循环后正极的XRD谱图[31]
Fig.1 (a) Schematic of the mechanism of Zn2+ intercalation into α-MnO2[27]; (b) HR (High resolution)-TEM image of the lattice structure of δ-MnO2; (c) CV curves of Zn/δ-MnO2 batteries; (d) Ex-situ XRD patterns of the cathodes recovered from the Zn/δ-MnO2 cells after 1st and 5th discharge cycles[31]
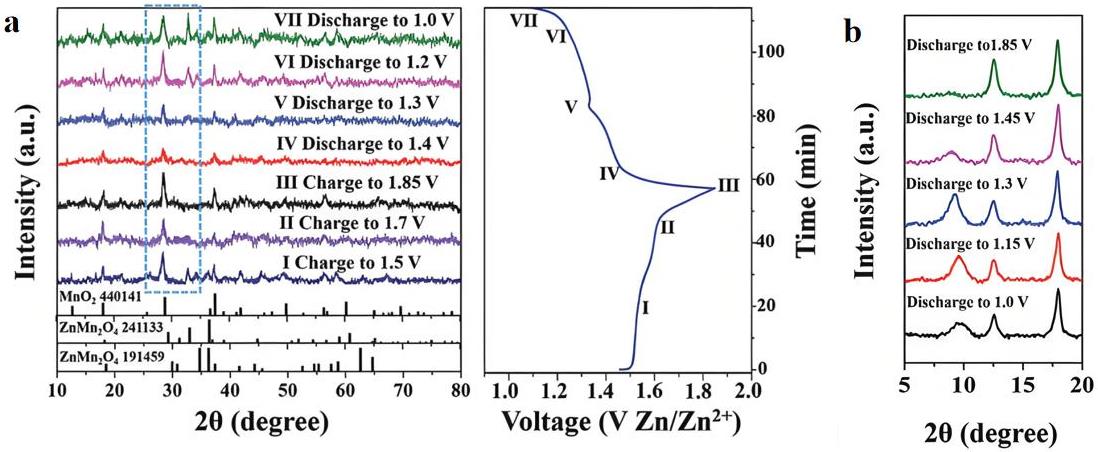
图2 (a) MGS在不同阶段的非原位XRD图谱;(b) MGS在不同阶段从5°到20°的SAXD
Fig.2 (a) Ex situ XRD patterns of MGS at different stages; (b) SAXD patterns of MGS from 5° to 20° at various stages
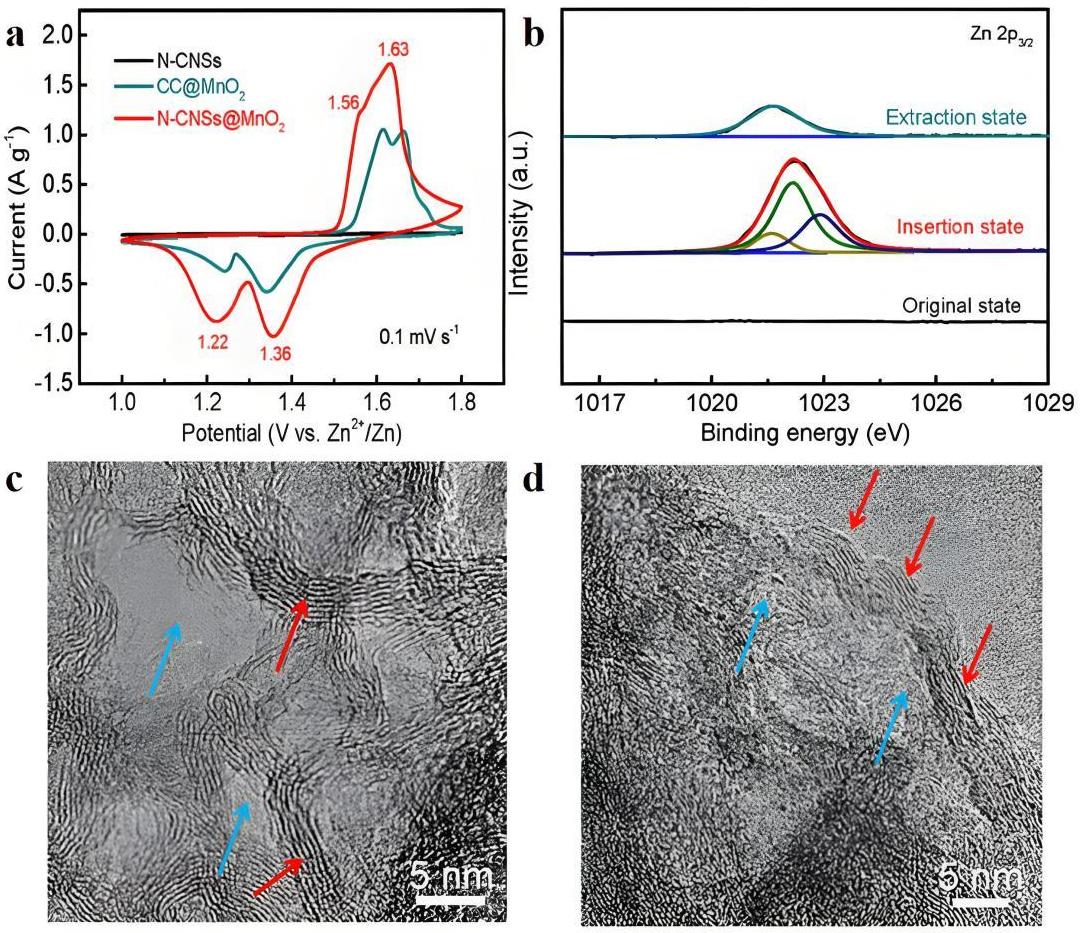
图3 (a) N-CNSs和N-CNSs@MnO2电极在0.1 mV·s-1扫描速率下的CV曲线;(b) N-CNSs@MnO2电极在初始、嵌入、脱出状态下的Zn 2p3/2能级光谱;N-CNSs@MnO2阴极(c)嵌入和(d)脱出状态的HR-TEM图像。红色箭头表示δ-MnO2的层状结构。蓝色箭头表示N-CNSs@MnO2中的纳米孔[37]
Fig.3 (a) CV curves of N-CNSs and N-CNSs@MnO2 electrodes at a scan rate of 0.1 mV·s-1; (b) Zn 2p3/2 spectra of N-CNSs@MnO2 electrode in initial, intercalated and de-intercalated states; HR-TEM images of N-CNSs@MnO2 cathode at (c) insertion and (d) extraction states. The red and blue arrows represent the layer structure of δ-MnO2 and nanopores in N-CNSs@MnO2[37], respectively.

图4 MnO2电极在第一个循环过程中放电至1.0 V (a) 和充电到1.8 V (b) 的TEM/HR-TEM图像;(c) α-MnO2电极在第一次放电至1.0 V时的XRD图谱;(d) α-MnO2电极在第一个循环过程中放电到1.0 V,然后再充电到1.8 V的XRD图谱[29]
Fig.4 TEM/HR-TEM images of MnO2 electrode discharged to 1 V (a) and charged to 1.8 V (b) during the first cycle; (c) XRD pattern of α-MnO2 electrode discharged to 1 V in the first cycle; (d) XRD patterns collected from α-MnO2 electrodes discharged to 1 V and charged back to 1.8 V in the 1st[29]
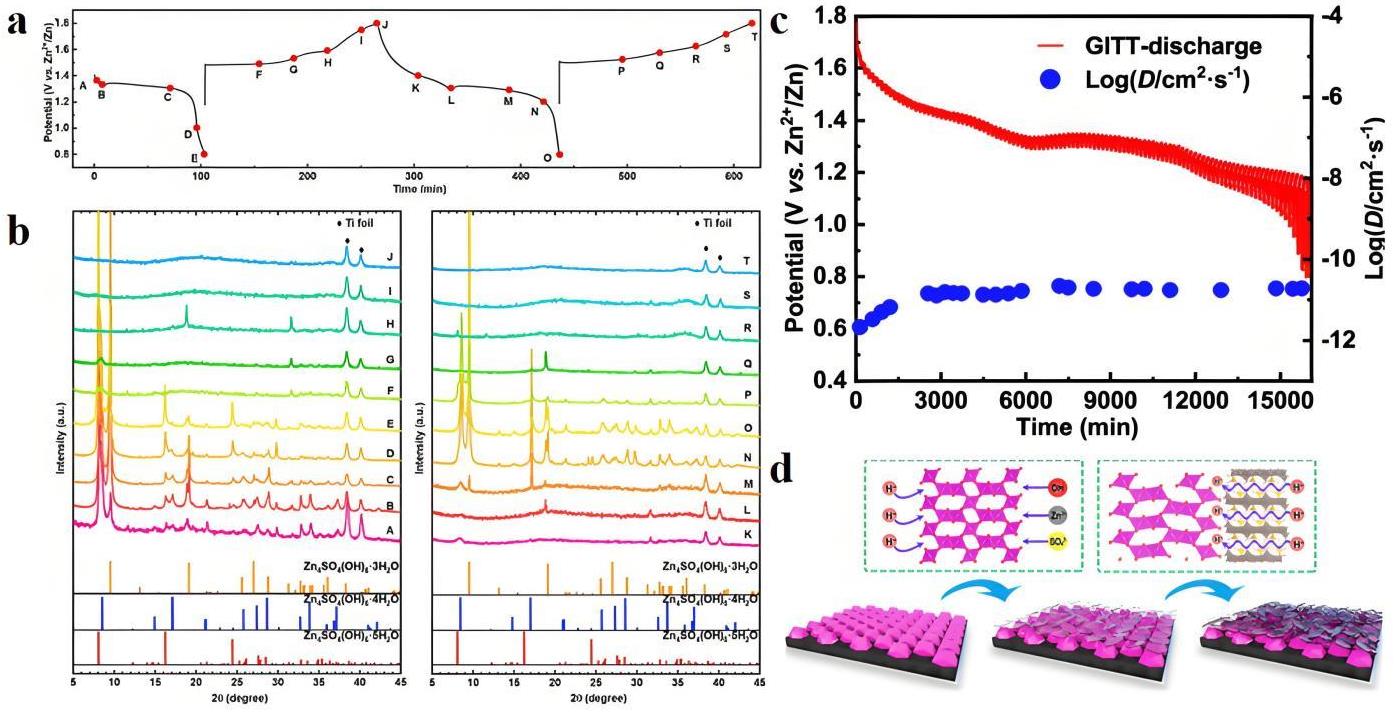
图5 (a) PFM电极的GCD曲线;(b) PFM电极在不同放电、充电状态下的非原位XRD图谱;(c) PFM电极的GITT曲线和扩散系数;(d) Zn/PEM电池中H+嵌入存储机制示意图[38]
Fig.5 (a) GCD curves of PFM electrode; (b) Ex situ XRD patterns of PFM electrode at different discharge–charge states; (c) GITT curves and the corresponding diffusion coefficients of PFM electrode; (d) Schematic of the proposed H+ ion insertion energy storage mechanism for the cell with the PEM electrode[38]
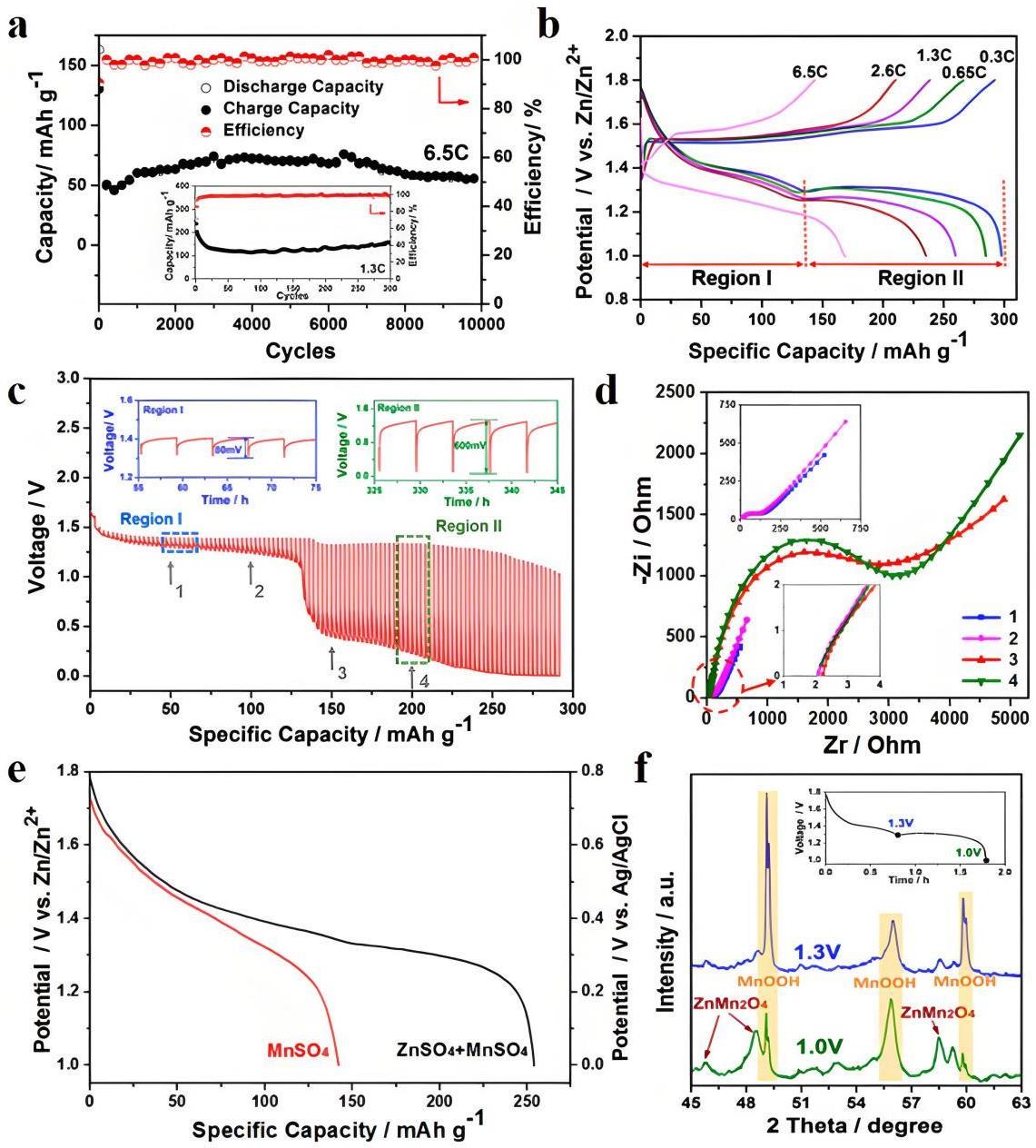
图6 (a) Zn/MnO2@CFP电池在2 M ZnSO4 + 0.2 M MnSO4电解液中的循环性能及其在6.5 C和1.3 C速率下相应的库伦效率;(b) Zn/MnO2@CFP电池在不同倍率下第一个循环的充放电曲线;(c) MnO2@CFP正极的GITT曲线;(d) 不同放电深度下的EIS;(e) MnO2@CFP正极在添加或不添加ZnSO4的0.2 M MnSO4电解液中的放电曲线;(f) MnO2@CFP正极在1.3 V和1.0 V放电深度时的非原位XRD图[30]
Fig.6 (a) Cycling performance of Zn/MnO2@CFP cells in 2 M ZnSO4+0.2 M MnSO4 electrolyte and corresponding coulombic efficiencies at 6.5 C and 1.3 C rates; (b) First cycle charge-discharge curves of Zn/MnO2@CFP batteries at different rates; (c) GITT curves of MnO2@CFP cathode; (d) Corresponding EIS at different depth of discharge as indexed by arrows in panel C; (e) Discharge curves of MnO2@CFP cathode in 0.2 M MnSO4 solution with or without ZnSO4 as electrolytes; (f) Ex situ XRD patterns of the MnO2@CFP cathode at depth of discharge at 1.3 and 1.0 V[30]

图7 (a) 在C/3的电流密度下,初始沉积、部分和完全放电MnO2正极的XRD图;(b) 在3C的电流密度下,初始沉积、部分和完全放电MnO2正极的XRD图;(c) Zn/MnO2电池氧化还原反应和晶体结构示意图[41];(d) MON正极在0.1 C电流下在不同电解液中的放电曲线;(e) 根据ICP结果定量Zn2+和H+对容量的贡献;(f) 第一次和第二次放电平台的DFT计算中Zn/MON电池的电压[42]
Fig.7 (a) XRD patterns of initially deposited, partially and fully discharged MnO2 cathodes at a current density of C/3; (b) XRD patterns of initially deposited, partially and fully discharged MnO2 cathodes at a current density of 3 C; (c) Schematic diagram of redox reaction and crystal structure of Zn/MnO2 battery[41]; (d) Discharge curves of MON cathode in different electrolytes at a current density of 0.1 C; (e) Quantification of Zn2+ and H+ contributions to capacity based on ICP results; (f) Graphical illustration of the voltage of Zn/MON cells in DFT calculations for the first and second discharge plateaus[42]
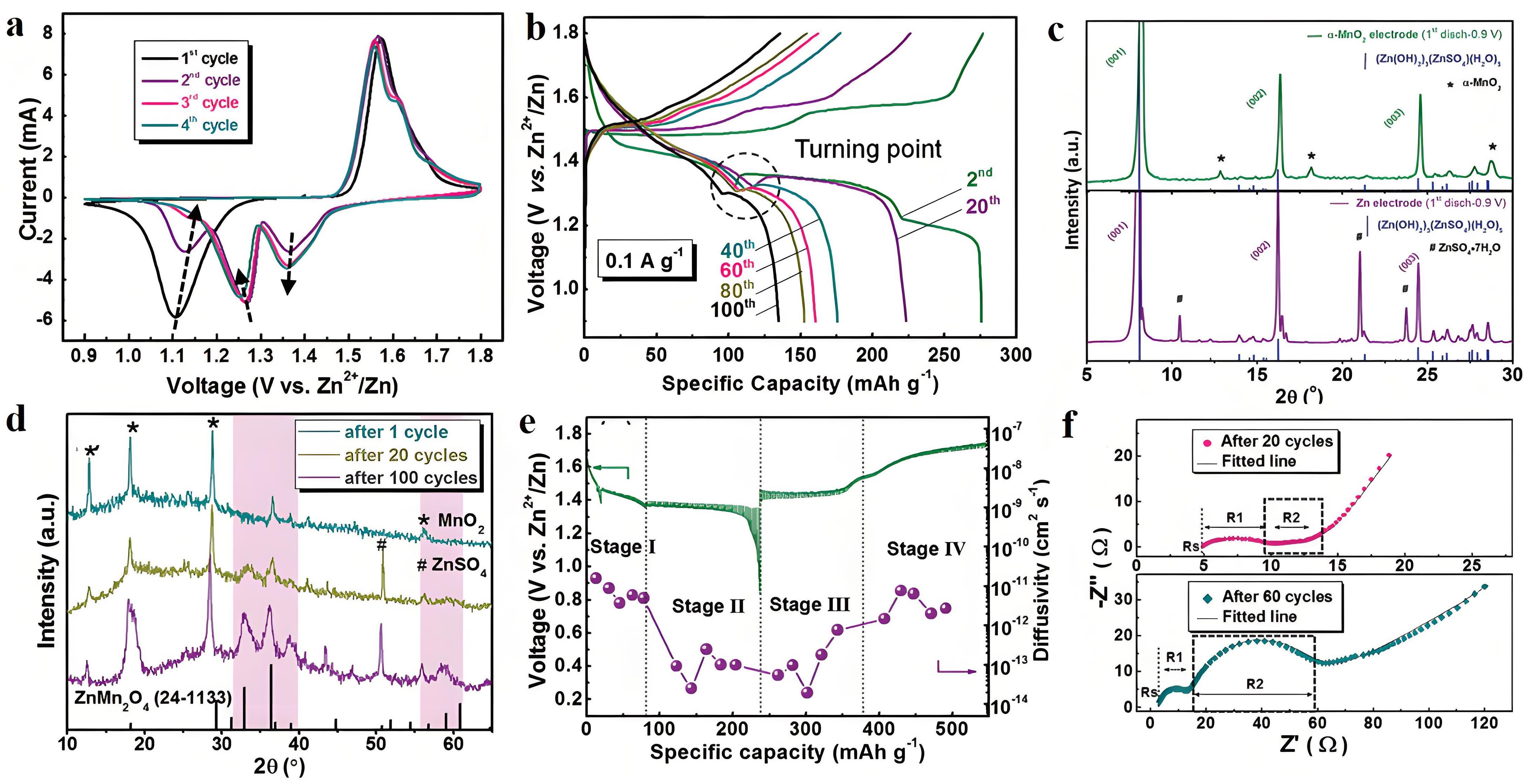
图8 (a) CV曲线;(b) 不同循环后的GCD曲线;(c) α-MnO2和Zn电极在完全放电状态下的XRD图谱;(d) 不同循环α-MnO2正极的非原位XRD图谱;(e) GITT曲线和扩散率与充电/放电状态的关系;(f) 20次和60次循环后的Nyquist图[44]
Fig.8 (a) CV curves; (b) GCD curves after different cycles; (c) XRD patterns of α-MnO2 and Zn electrodes in fully discharged state; (d) Ex-situ XRD patterns of α-MnO2 cathodes after different cycles; (e) GITT curve and diffusivity vs charge/discharge state; (f) Nyquist plots after 20 and 60 cycles[44]
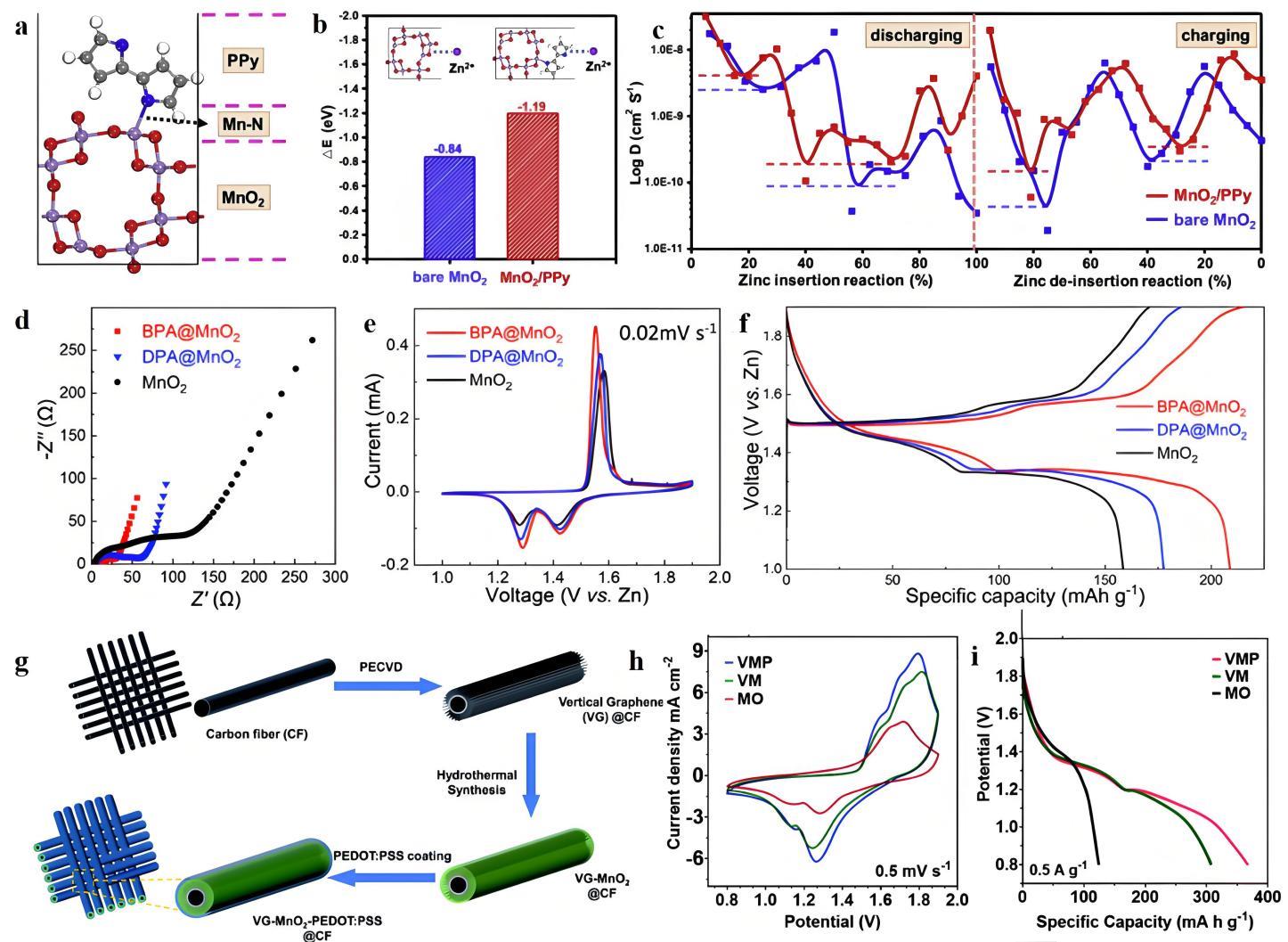
图9 (a) PPy覆盖无序MnO2表面的最佳构型;(b) 初始MnO2和MnO2/PPy的Zn2+吸附能;(c) 初始MnO2和MnO2/PPy在不同放电/充电状态下的Zn2+扩散系数[47];(d) BPA和DPA改性的MnO2电极与原始MnO2电在完全充电和放电状态下的Nyquist图;(e) 0.02 mV∙s-1时的CV曲线;(f) 0.1 C 放电速率下第10次循环的GCD曲线[48];(g) VMP电极的合成示意图;(h) VMP、VM和MO正极的CV曲线;(I) VMP、VM和MO正极的GCD曲线[50]
Fig.9 (a) Optimal configuration of the disordered MnO2 surface covered by PPy; (b) Zn2+ adsorption energy of bare MnO2 and MnO2/PPy; (c) Zn2+ diffusion coefficients at different discharge/charge state of bare MnO2 and MnO2/PPy [47]; (d) Nyquist plots of BPA and DPA modified MnO2 electrodes vs pristine MnO2 electrodes in fully charged and discharged states; (e) CV curves at 0.02 mV s-1; (f) GCD curves of the 10th cycle at 0.1 C discharge rate[48]; (g) Schematic diagram of the synthesis of VMP electrodes; (h) CV curves of VMP, VM (VG-MnO2) and MO (MnO2@CF) cathodes; (i) GCD curves of VMP, VM and MO cathodes[50]
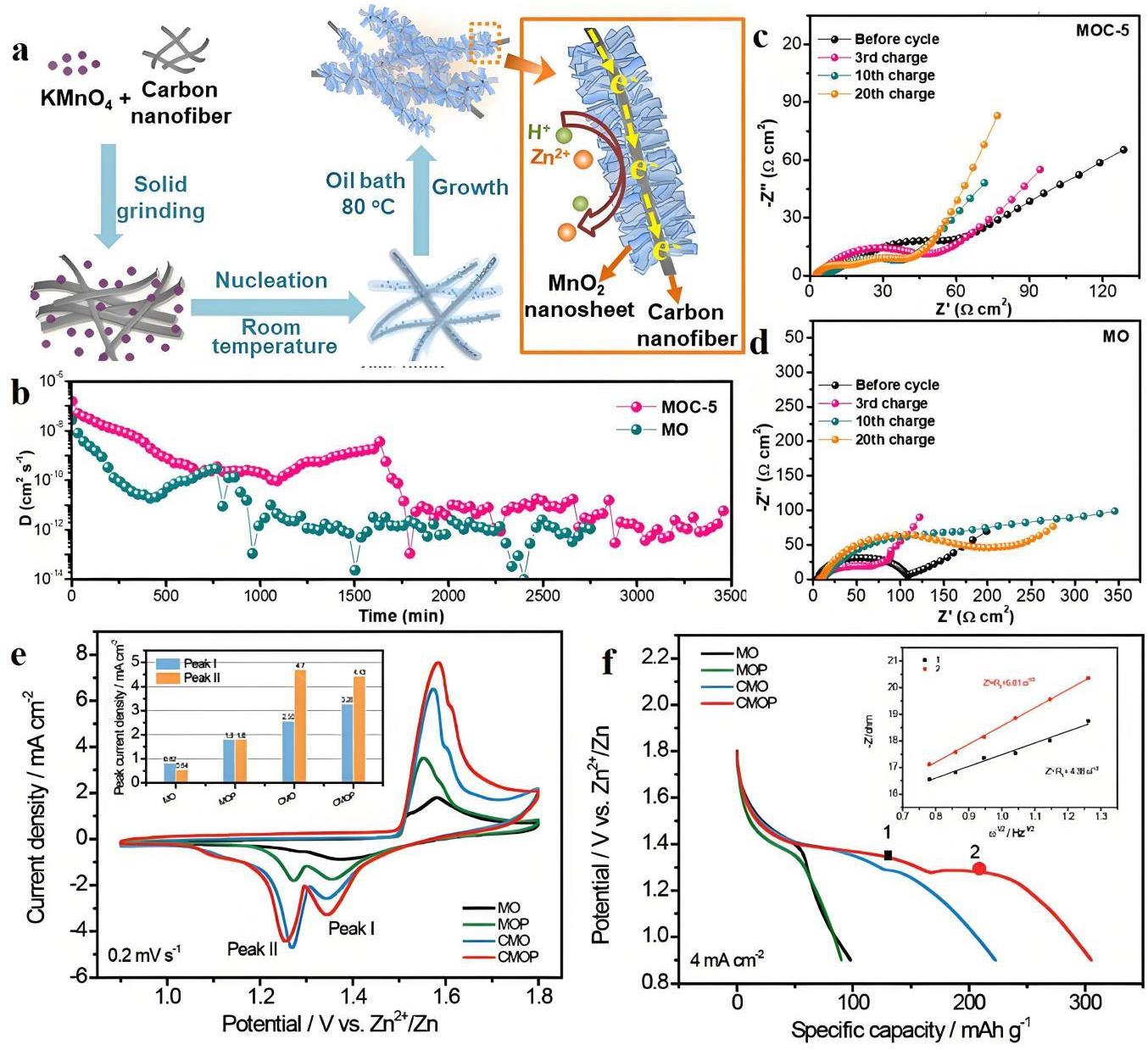
图10 (a) MOC (CNF@MnO2) 电极合成示意图;(b) 据GITT结果计算出的MOC-5 (the feeding weight ratio of KMnO4/CNF of 5:1) 和MO (δ-MnO2) 电极的离子扩散系数;(c) MOC-5电极在给定循环完全充电状态下的Nyquist图;(d) MO电极在给定循环完全充电状态下的Nyquist图[53];(e) CV曲线和MO、MOP (MnO2/PEDOT)、CMO (CNT/MnO2) 和CMOP (CNT/MnO2/PEDOT) 电极在0.2 mV·s-1时的相对峰值电流密度(插图)的比较;(f) 4 mA·cm-2时的GCD曲线。插图是在(e)中标记的CMOP电极不同放电状态下相应的线性拟合Z'-ω-1/2曲线[54]
Fig.10 (a) Schematic diagram of the synthetic procedure of MOC; (b) Calculated ion diffusion coefficients of MOC-5 and MO based on the GITT results; (c) Nyquist plots of MOC-5 electrode in fully charged state for a given cycle; (d) Nyquist plots of MO electrode in fully charged state for a given cycle[53]; (e) CV curves and a comparison of the relative peak current densities (inset) of MO, MOP, CMO, and CMOP electrodes at 0.2 mV s-1; (f) GCD curves at 4 mA·cm-2. The inset is the corresponding linear fitting Z′-ω-1/2 curves at different discharged states of CMOP electrode marked in (e)[54]
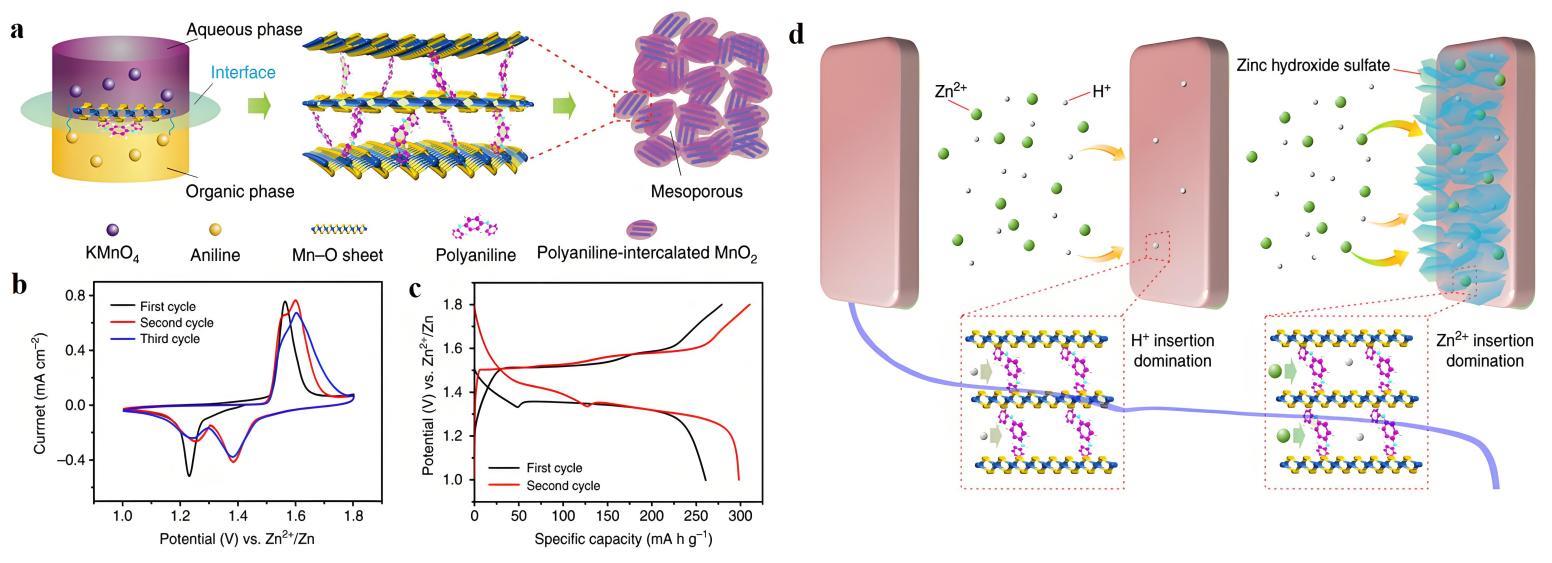
图11 (a) PANI插入MnO2纳米片形成夹层膨胀结构的示意图;(b) Zn/PANI-MnO2纽扣电池在0.1 mV·s-1下的CV曲线;(c) 50 mA·g-1下的GCD曲线;(d) H+和Zn2+相继插入MnO2的机理示意图[56]
Fig.11 (a) Schematic of expanded intercalated structure of PANI-intercalated MnO2 nanolayers; (b) CV curves of Zn/PANI -intercalated MnO2 coin-type cell at 0.1 mV·s-1; (c) GCD curves at 50 mA·g-1; (d) Schematic diagram of the sequential insertion of H+ and Zn2+ into MnO2[56]
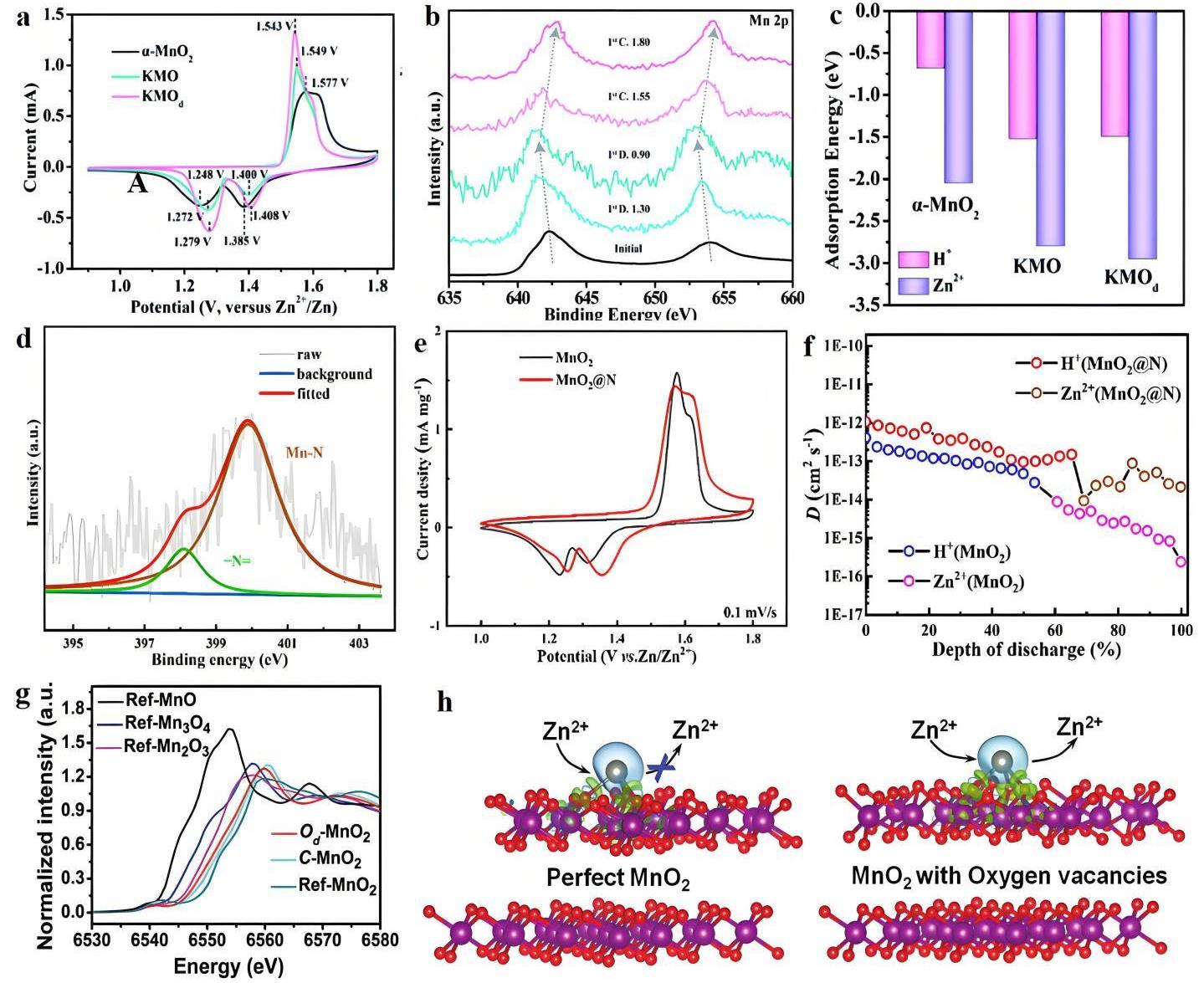
图12 (a) 0.1 mV·s-1时的CV曲线;(b) KMOd电极在不同充/放电状态下的非原位Mn 2p XPS光谱;(c) Zn2+和H+在KMOd、KMO和α-MnO2中的吸附能[63];(d) MnO2@N电极的N 1s的XPS光谱;(e) MnO2和MnO2@N电极在0.1 mV·s-1扫描速率下的CV曲线;(f) MnO2和MnO2@N放电状态下离子扩散系数对应的GITT曲线[64];(g) 参考MnO、Mn3O4、Mn2O3和MnO2,Od-MnO2和C-MnO2的XAS曲线;(h) 初始MnO2和具有氧空位的MnO2的Zn2+嵌入/脱出示意图[65]
Fig.12 (a) CV curves at 0.1 mV·s-1; (b) Ex-situ Mn 2p XPS spectra of the KMOd electrode at different charge/discharge states; (c) Adsorption energies of Zn2+ and H+ in KMOd, KMO, and α-MnO2[63]; (d) XPS spectra of N 1s of MnO2@N electrode; (e) CV curves of MnO2 and MnO2@N electrodes at a scan rate of 0.1 mV·s-1; (f) GITT curves of ion diffusion coefficient during discharge for MnO2 and MnO2@N[64]; (g) XAS curves of Od-MnO2 and C-MnO2 with reference to standard MnO, Mn3O4, Mn2O3, and MnO2; (h) Schematic diagram of Zn2+ intercalation/extraction of initial MnO2 and MnO2 with oxygen vacancies[65]
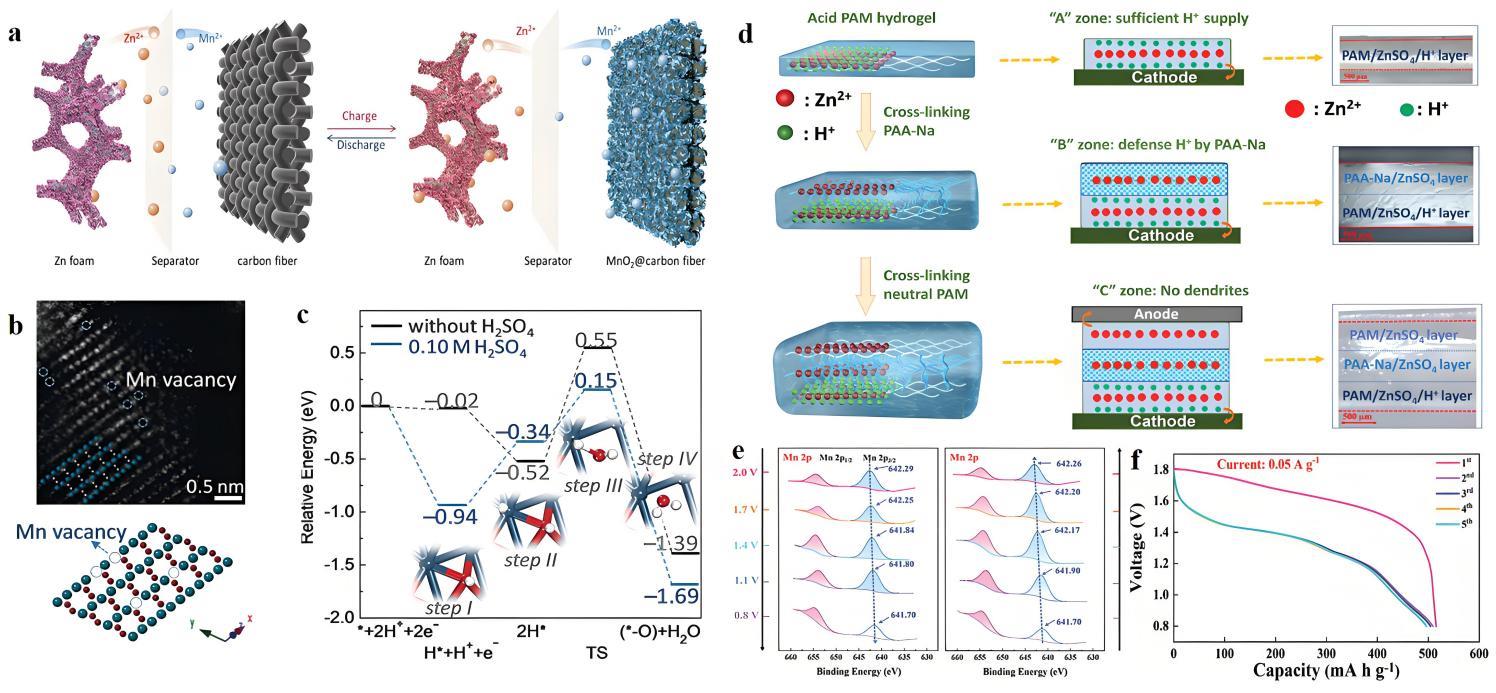
图13 (a) 新型电解Zn/MnO2电池示意图;(b) 具有暴露的(101)晶面和Mn空位(虚线圆圈)的HAADF-STEM图像和晶体结构[66];(c) 反应路径的相对能量分布(步骤I~IV);(d) ABC-H电解质的合成方法;(e) MnO2正极在不同充/放电深度下Mn 2p的XPS图谱;(f) 采用ABC-H电解质的Zn/MnO2电池在0.05 A·g-1下初始五个循环中的放电容量[67]
Fig.13 (a) Schematic of the new electrolytic Zn/MnO2 battery; (b) HAADF-STEM image and crystal structure with exposed (101) facets and Mn vacancies (dotted circles)[66]; (c) Relative energy profiles of the reaction pathway (steps I~IV); (d) Synthesis route of the ABC-H electrolyte (A: a strongly acidic “Acid zone” (A zone), B: a mildly alkaline “Buffer zone” (B zone), C: a neutral “Conservation zone” (C zone), H: hydrogel); (e) XPS spectra of Mn 2p at different charge/discharge depth of MnO2 cathode; (f) Discharge capacity of Zn/MnO2 cell with ABC-H electrolyte in the initial five cycles at 0.05 A·g-1[67]
| [1] | Zhao Y J, Zheng M L. Battery management system for zinc-based flow batteries: a review[J]. Renewable and Sustainable Energy Reviews, 2025, 215: 115604. |
| [2] | Raugei M, Hutchinson A, Morrey D. Can electric vehicles significantly reduce our dependence on non-renewable energy? Scenarios of compact vehicles in the UK as a case in point[J]. Journal of Cleaner Production, 2018, 201: 1043-1051. |
| [3] | Sun Y W, Wang C, Gao L, et al. Anion-regulated deposition-dissolution chemistry in acidic aqueous indium─MnO2 batteries[J]. Angewandte Chemie International Edition, 2025, 64(38): e202512463. |
| [4] | Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices[J]. Science, 2011, 334(6058): 928-935. |
| [5] | Palacín M R. Recent advances in rechargeable battery materials: a chemist's perspective[J]. Chemical Society Reviews, 2009, 38(9): 2565-2575. |
| [6] | Mei W X, Cheng Z X, Wang L B, et al. Thermal hazard comparison and assessment of Li-ion battery and Na-ion battery[J]. Journal of Energy Chemistry, 2025, 102: 18-26. |
| [7] | Tarascon J M, Armand M. Issues and challenges facing rechargeable lithium batteries[J]. Nature, 2001, 414(6861): 359-367. |
| [8] | Peljo P, Villevieille C, Girault H H. The redox aspects of lithium-ion batteries[J]. Energy & Environmental Science, 2025, 18(4): 1658-1672. |
| [9] | Jia X X, Liu C F, Neale Z G, et al. Active materials for aqueous zinc ion batteries: synthesis, crystal structure, morphology, and electrochemistry[J]. Chemical Reviews, 2020, 120(15): 7795-7866. |
| [10] | Peng Z, Yan H, Zhang Q Q, et al. Stabilizing zinc anode through ion selection sieving for aqueous Zn-ion batteries[J]. Nano Letters, 2024, 24(30): 9137-9146. |
| [11] | Zhao Q, Huang W W, Luo Z Q, et al. High-capacity aqueous zinc batteries using sustainable quinone electrodes[J]. Science advances, 2018, 4(3): eaao1761. |
| [12] | Zhu J, Ge X M, Peng Z, et al. Interfacial regulation for zinc metal anode of aqueous zinc-ion battery[J]. Green Energy & Environment, 2025, 10(4): 689-708. |
| [13] | Kundu D, Adams B D, Duffort V, et al. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode[J]. Nature Energy, 2016, 1: 16119. |
| [14] | Fang G Z, Zhou J, Pan A Q, et al. Recent advances in aqueous zinc-ion batteries[J]. ACS Energy Letters, 2018, 3(10): 2480-2501. |
| [15] | Xu C J, Li B H, Du H D, et al. Energetic zinc ion chemistry: the rechargeable zinc ion battery[J]. Angewandte Chemie International Edition, 2011, 51(4): 933-935. |
| [16] | Cheng Z J, Dong Q Y, Pu G Q, et al. A durable and high-voltage Mn–graphite dual-ion battery using Mn-based hybrid electrolytes[J]. Small, 2024, 20(28): 2400389. |
| [17] | Zhang N, Cheng F Y, Liu Y C, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery[J]. Journal of the American Chemical Society, 2016, 138(39): 12894-12901. |
| [18] | Yan M Y, He P, Chen Y, et al. Water-lubricated intercalation in V2O5 ·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries[J]. Advanced Materials, 2018, 30(1): 1703725. |
| [19] | Zhang X Q, Zhang X, Miao Y, et al. A rechargeable aqueous phenazine-Prussian blue proton battery with long cycle life[J]. Journal of Materials Chemistry A, 2023, 11(13): 7152-7158. |
| [20] | Jia Z J, Wang B G, Wang Y. Copper hexacyanoferrate with a well-defined open framework as a positive electrode for aqueous zinc ion batteries[J]. Materials Chemistry and Physics, 2015, 149/150: 601-606. |
| [21] | Li S Q, Zhang G L, Jing G L, et al. Aqueous zinc–polyaniline secondary battery[J]. Synthetic Metals, 2008, 158(6): 242-245. |
| [22] | Chen S, Zhu J W, Wu X D, et al. Graphene oxide–MnO2 nanocomposites for supercapacitors[J]. ACS Nano, 2010, 4(5): 2822-2830. |
| [23] | Lai G J, Ruan P C, Hu X T, et al. Dynamic compensation of MnOOH to mitigate the irregular dissolution of MnO2 in rechargeable aqueous Zn/MnO2 batteries[J]. Journal of Materials Chemistry A, 2023, 11(28): 15211-15218. |
| [24] | Panda M R, El Meragawi S, Mirshekarloo M S, et al. Acidity-aided surface modification strategy to enhance in situ MnO2 deposition for high performance Zn-MnO2 battery prototypes[J]. Small, 2025, 21(28): 2311933. |
| [25] | Guo X T, Li J M, Jin X, et al. A hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries[J]. Nanomaterials, 2018, 8(5): 301. |
| [26] | Li W J, Han C, Wang Y, et al. Structural modulation of manganese oxides for zinc-ion batteries[J]. Chinese Journal of Structural Chemistry, 2020, 39(1): 31-35. |
| [27] | Lee B, Yoon C S, Lee H R, et al. Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide[J]. Scientific Reports, 2014, 4: 6066. |
| [28] | Shi H Y, Sun X Q. Interlayer engineering of layered cathode materials for advanced Zn storage[J]. Chem, 2020, 6(4): 817-819. |
| [29] | Pan H L, Shao Y Y, Yan P F, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions[J]. Nature Energy, 2016, 1: 16039. |
| [30] | Sun W, Wang F, Hou S, et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion[J]. Journal of the American Chemical Society, 2017, 139(29): 9775-9778. |
| [31] | Alfaruqi M H, Gim J, Kim S, et al. A layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications[J]. Electrochemistry Communications, 2015, 60: 121-125. |
| [32] | Alfaruqi M H, Gim J, Kim S, et al. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode[J]. Journal of Power Sources, 2015, 288: 320-327. |
| [33] | Alfaruqi M H, Mathew V, Gim J, et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system[J]. Chemistry of Materials, 2015, 27(10): 3609-3620. |
| [34] | Wei C G, Xu C J, Li B H, et al. Preparation and characterization of manganese dioxides with nano-sized tunnel structures for zinc ion storage[J]. Journal of Physics and Chemistry of Solids, 2012, 73(12): 1487-1491. |
| [35] | Stoller M D, Park S, Zhu Y, et al. Graphene-based ultracapacitors[J]. Nano Letters, 2008, 8(10): 3498-3502. |
| [36] | Wu B K, Zhang G B, Yan M Y, et al. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery[J]. Small, 2018, 14(13): 1703850. |
| [37] | Zhang Y, Deng S J, Li Y H, et al. Anchoring MnO2 on nitrogen-doped porous carbon nanosheets as flexible arrays cathodes for advanced rechargeable Zn–MnO2 batteries[J]. Energy Storage Materials, 2020, 29: 52-59. |
| [38] | Zhao Q, Huang X J, Zhou M M, et al. Proton insertion promoted a polyfurfural/MnO2 nanocomposite cathode for a rechargeable aqueous Zn-MnO2 battery[J]. ACS Applied Materials & Interfaces, 2020, 12(32): 36072-36081. |
| [39] | Zhang S L, He J J, Zheng J, et al. Porous graphdiyne applied for sodium ion storage[J]. Journal of Materials Chemistry A, 2017, 5(5): 2045-2051. |
| [40] | Li J F, Chen Y H, Guo J, et al. Graphdiyne oxide-based high-performance rechargeable aqueous Zn–MnO2 battery[J]. Advanced Functional Materials, 2020, 30(42): 2004115. |
| [41] | Li Y, Wang S Y, Salvador J R, et al. Reaction mechanisms for long-life rechargeable Zn/MnO2 batteries[J]. Chemistry of Materials, 2019, 31(6): 2036-2047. |
| [42] | Zhao Q H, Chen X, Wang Z Q, et al. Unravelling H+/Zn2+ synergistic intercalation in a novel phase of manganese oxide for high-performance aqueous rechargeable battery[J]. Small, 2019, 15(47): 1904545. |
| [43] | Hashem A M, Abdel-Latif A M, Abuzeid H M, et al. Improvement of the electrochemical performance of nanosized α-MnO2 used as cathode material for Li-batteries by Sn-doping[J]. Journal of Alloys and Compounds, 2011, 509(40): 9669-9674. |
| [44] | Gao X, Wu H W, Li W J, et al. H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery[J]. Small, 2020, 16(5): 1905842. |
| [45] | Pang Q, Kundu D, Cuisinier M, et al. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries[J]. Nature Communications, 2014, 5: 4759. |
| [46] | Song J X, Xu T, Gordin M L, et al. Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium-sulfur batteries[J]. Advanced Functional Materials, 2014, 24(9): 1243-1250. |
| [47] | Huang J, Tang X, Liu K, et al. Interfacial chemical binding and improved kinetics assisting stable aqueous Zn–MnO2 batteries[J]. Materials Today Energy, 2020, 17: 100475. |
| [48] | Gao S Y, Li B M, Lu K, et al. Modulating MnO2 interface with flexible and self-adhering alkylphosphonic layers for high-performance Zn-MnO2 batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(20): 23724-23731. |
| [49] | Peng L L, Peng X, Liu B R, et al. Ultrathin two-dimensional MnO2/graphene hybrid nanostructures for high-performance, flexible planar supercapacitors[J]. Nano Letters, 2013, 13(5): 2151-2157. |
| [50] | Chen J Y, Liang J X, Zhou Y, et al. A vertical graphene enhanced Zn–MnO2 flexible battery towards wearable electronic devices[J]. Journal of Materials Chemistry A, 2021, 9(1): 575-584. |
| [51] | Guo W, Yu C, Li S F, et al. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: Challenges and perspectives[J]. Nano Energy, 2019, 57: 459-472. |
| [52] | Li X D, Wu G X, Liu X, et al. Orderly integration of porous TiO2(B) nanosheets into bunchy hierarchical structure for high-rate and ultralong-lifespan lithium-ion batteries[J]. Nano Energy, 2017, 31: 1-8. |
| [53] | Chen X J, Li W, Zeng Z P, et al. Engineering stable Zn-MnO2 batteries by synergistic stabilization between the carbon nanofiber core and birnessite-MnO2 nanosheets shell[J]. Chemical Engineering Journal, 2021, 405: 126969. |
| [54] | Zhang X Y, Wu S W, Deng S J, et al. 3D CNTs networks enable MnO2 cathodes with high capacity and superior rate capability for flexible rechargeable Zn–MnO2 batteries[J]. Small Methods, 2019, 3(12): 1900525. |
| [55] | Jiang Y Q, Ba D L, Li Y Y, et al. Noninterference revealing of "layered to layered" zinc storage mechanism of δ-MnO2 toward neutral Zn–Mn batteries with superior performance[J]. Advanced Science, 2020, 7(6): 1902795. |
| [56] | Huang J H, Wang Z, Hou M Y, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery[J]. Nature Communications, 2018, 9: 2906. |
| [57] | Ren Q Y, Qin N, Liu B, et al. An oxygen-deficient vanadium oxide@N-doped carbon heterostructure for sodium-ion batteries: insights into the charge storage mechanism and enhanced reaction kinetics[J]. Journal of Materials Chemistry A, 2020, 8(6): 3450-3458. |
| [58] | Wang H E, Li X C, Qin N, et al. Sulfur-deficient MoS2 grown inside hollow mesoporous carbon as a functional polysulfide mediator[J]. Journal of Materials Chemistry A, 2019, 7(19): 12068-12074. |
| [59] | Cai Y, Chua R, Huang S Z, et al. Amorphous manganese dioxide with the enhanced pseudocapacitive performance for aqueous rechargeable zinc-ion battery[J]. Chemical Engineering Journal, 2020, 396: 125221. |
| [60] | Cheng F Y, Zhang T R, Zhang Y, et al. Enhancing electrocatalytic oxygen reduction on MnO2 with vacancies[J]. Angewandte Chemie International Edition, 2013, 52(9): 2474-2477. |
| [61] | Zhai T, Lu X H, Wang F X, et al. MnO2 nanomaterials for flexible supercapacitors: performance enhancement via intrinsic and extrinsic modification[J]. Nanoscale Horizons, 2016, 1(2): 109-124. |
| [62] | Huang Y, Li Y Y, Hu Z Q, et al. A carbon modified MnO2 nanosheet array as a stable high-capacitance supercapacitor electrode[J]. Journal of Materials Chemistry A, 2013, 1(34): 9809-9813. |
| [63] | Han K, An F Q, Yan F S, et al. High-performance aqueous Zn–MnO2 batteries enabled by the coupling engineering of K+ pre-intercalation and oxygen defects[J]. Journal of Materials Chemistry A, 2021, 9(28): 15637-15647. |
| [64] | Zhang Y A, Liu Y P, Liu Z H, et al. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries[J]. Journal of Energy Chemistry, 2022, 64: 23-32. |
| [65] | Xiong T, Yu Z G, Wu H J, et al. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous zinc ion battery[J]. Advanced Energy Materials, 2019, 9(14): 1803815. |
| [66] | Chao D L, Zhou W H, Ye C, et al. An electrolytic Zn–MnO2 battery for high-voltage and scalable energy storage[J]. Angewandte Chemie International Edition, 2019, 58(23): 7823-7828. |
| [67] | Shen Z X, Tang Z Q, Li C W, et al. Precise proton redistribution for two-electron redox in aqueous zinc/manganese dioxide batteries[J]. Advanced Energy Materials, 2021, 11(41): 2102055. |
| [68] | Dai Y J, Zhang J Z, Yan X R, et al. Investigating the electrochemical performance of MnO2 polymorphs as cathode materials for aqueous proton batteries[J]. Chemical Engineering Journal, 2023, 471: 144158. |
| [69] | Liu Y Z, Qin Z M, Yang X P, et al. Voltage induced lattice contraction enabling superior cycling stability of MnO2 cathode in aqueous zinc batteries[J]. Energy Storage Materials, 2023, 56: 524-531. |
| [70] | Ding Y X, Cai C, Ma L T, et al. Tailoring MnO2 cathode interface via organic–inorganic hybridization engineering for ultra-stable aqueous zinc-ion batteries[J]. Advanced Energy Materials, 2025, 15(3): 2402819. |
| [71] | Zhao L L, Yin J W, Liu B C, et al. Construction of high-performance aqueous zinc-ion batteries by guest pre-intercalation MnO2-based cathodes[J]. Advances in Colloid and Interface Science, 2025, 341: 103499. |
| [1] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [2] | 刘卓龙, 甘云华, 屈可扬, 陈宁光, 潘铭晖. 均匀电场对生物柴油小尺度射流扩散燃烧特性影响研究[J]. 化工学报, 2025, 76(9): 4800-4808. |
| [3] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [4] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [5] | 王钰, 冯英楠, 王涛, 赵之平. 原位生长构筑纳米复合纳滤膜:膜制备与应用[J]. 化工学报, 2025, 76(9): 4723-4736. |
| [6] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [7] | 李相海, 赖德林, 孔纲, 周健. 双仿生表面水下疏油协同机制的分子动力学模拟研究[J]. 化工学报, 2025, 76(9): 4551-4562. |
| [8] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [9] | 叶鑫煌, 薛嘉豪, 赵玉来. 可聚型Gemini表面活性剂的制备、表征及其稳定高内相乳液的研究[J]. 化工学报, 2025, 76(8): 4331-4340. |
| [10] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [11] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [12] | 吴阿强, 诸葛祥群, 刘通, 王明星, 罗鲲. 纳米普鲁士蓝悬浮电解液对锂氧电池性能的影响[J]. 化工学报, 2025, 76(8): 4310-4317. |
| [13] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [14] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [15] | 李姿睿, 齐凯, 王军, 夏国栋. 基于Janus纳米通道的脱盐过程分子动力学模拟研究[J]. 化工学报, 2025, 76(7): 3531-3538. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号