CIESC Journal ›› 2019, Vol. 70 ›› Issue (10): 3956-3966.DOI: 10.11949/0438-1157.20190648
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Wuji LIJIANG( ),Qiaoying ZHU,Lifang CHEN(
),Qiaoying ZHU,Lifang CHEN( ),Hongye CHENG,Zhiwen QI
),Hongye CHENG,Zhiwen QI
Received:2019-06-10
Revised:2019-09-18
Online:2019-10-05
Published:2019-10-05
Contact:
Lifang CHEN
通讯作者:
陈立芳
作者简介:李姜无忌(1997—),男,硕士研究生,基金资助:CLC Number:
Wuji LIJIANG,Qiaoying ZHU,Lifang CHEN,Hongye CHENG,Zhiwen QI. Preparation of oxygen defect vacancies MoO3- x and its adsorption properties[J]. CIESC Journal, 2019, 70(10): 3956-3966.
李姜无忌,朱巧影,陈立芳,成洪业,漆志文. 氧缺陷位MoO3- x 的制备及其吸附性能研究[J]. 化工学报, 2019, 70(10): 3956-3966.
Add to citation manager EndNote|Ris|BibTeX
| Sample | Mass decrease/% | Oxygen vacancy concentration |
|---|---|---|
| MoO3 | 0 | MoO3 |
| MoO3- x -120-150 | 0.314 | MoO2.97 |
| MoO3- x -150-45 | 0.634 | MoO2.94 |
| MoO3- x -180-10 | 1.293 | MoO2.88 |
| MoO3- x -180-20 | 1.266 | MoO2.89 |
Table 1 Oxygen vacancy concentration of MoO3– x characterized by thermogravimetric analysis
| Sample | Mass decrease/% | Oxygen vacancy concentration |
|---|---|---|
| MoO3 | 0 | MoO3 |
| MoO3- x -120-150 | 0.314 | MoO2.97 |
| MoO3- x -150-45 | 0.634 | MoO2.94 |
| MoO3- x -180-10 | 1.293 | MoO2.88 |
| MoO3- x -180-20 | 1.266 | MoO2.89 |
| Absorbent sample | Q max/(mg·g-1) | Ref. |
|---|---|---|
| graphene | 153.8 | [ |
| Fe-MOF | 187 | [ |
| activated carbon | 207 | [ |
| biomassed bamboo | 606 | [ |
| Fe(Ⅲ)/Cr(Ⅲ) hydroxide | 22.8 | [ |
| zeolite | 53.1 | [ |
| clay | 300 | [ |
| MoO3 | 629 | this work |
| MoO3- x | 758 | this work |
Table 2 Summary of adsorption capacity of various materials for MB
| Absorbent sample | Q max/(mg·g-1) | Ref. |
|---|---|---|
| graphene | 153.8 | [ |
| Fe-MOF | 187 | [ |
| activated carbon | 207 | [ |
| biomassed bamboo | 606 | [ |
| Fe(Ⅲ)/Cr(Ⅲ) hydroxide | 22.8 | [ |
| zeolite | 53.1 | [ |
| clay | 300 | [ |
| MoO3 | 629 | this work |
| MoO3- x | 758 | this work |
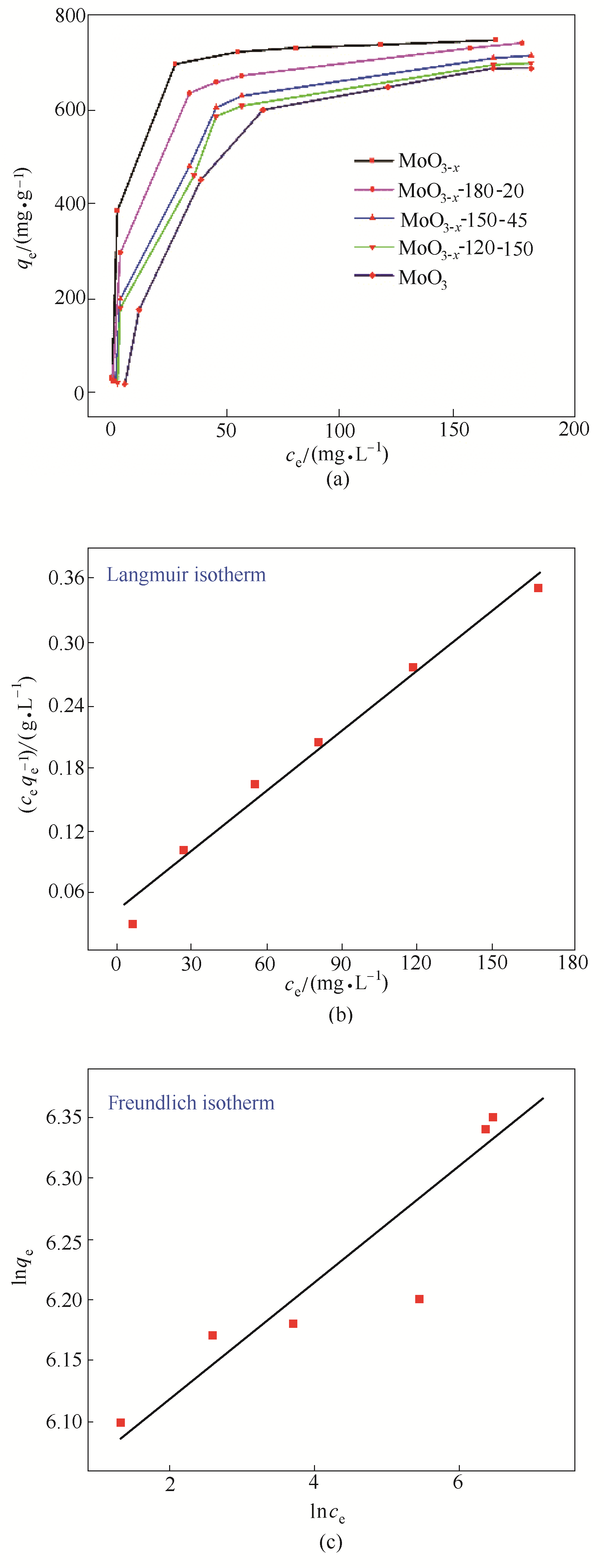
Fig.7 (a) Adsorption isotherm of MoO3- x with different oxygen vacancies concentration to MB under 25℃, (b) Langmuir isothermal equations of MoO3- x to MB, (c) Freundlich isothermal equations of MoO3- x to MB
| Adsorbent | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| q m/(mg·g-1) | R L | R 2 | 1/n | K F/((mg·g-1)(L·mg-1)1/ n ) | R 2 | ||
| MoO3- x | 748 | 0.042 | 0.978 | 0.24 | 134.3 | 0.844 | |
| MoO3- x -180-20 | 739 | 0.096 | 0.993 | 2.36 | 126.8 | 0.568 | |
| MoO3- x -150-45 | 721 | 0.356 | 0.976 | 4.56 | 263.4 | 0.497 | |
| MoO3- x -120-150 | 704 | 0.569 | 0.965 | 8.21 | 85.55 | 0.368 | |
| MoO3 | 629 | 0.875 | 0.998 | 13 | 0.7658 | 0.426 | |
Table 3 Adsorption isothermal equation parameters of MoO3- x and MoO3 to MB
| Adsorbent | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| q m/(mg·g-1) | R L | R 2 | 1/n | K F/((mg·g-1)(L·mg-1)1/ n ) | R 2 | ||
| MoO3- x | 748 | 0.042 | 0.978 | 0.24 | 134.3 | 0.844 | |
| MoO3- x -180-20 | 739 | 0.096 | 0.993 | 2.36 | 126.8 | 0.568 | |
| MoO3- x -150-45 | 721 | 0.356 | 0.976 | 4.56 | 263.4 | 0.497 | |
| MoO3- x -120-150 | 704 | 0.569 | 0.965 | 8.21 | 85.55 | 0.368 | |
| MoO3 | 629 | 0.875 | 0.998 | 13 | 0.7658 | 0.426 | |
| Adsorbent | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| k 1/(g·mg-1·h-1) | q e,cal/(mg·g-1) | R 2 | k 2/(g·mg-1·min-1) | q e,cal/(mg·g-1) | R 2 | |
| MoO3- x | 0.536 | 6.9×1014 | 0.2882 | 0.040 | 762 | 0.99973 |
| MoO3 | 1.822×10-4 | 7.12×1022 | 0.3554 | 0.00252 | 785 | 0.99628 |
Table 4 Kinetics equation parameters under 25℃
| Adsorbent | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| k 1/(g·mg-1·h-1) | q e,cal/(mg·g-1) | R 2 | k 2/(g·mg-1·min-1) | q e,cal/(mg·g-1) | R 2 | |
| MoO3- x | 0.536 | 6.9×1014 | 0.2882 | 0.040 | 762 | 0.99973 |
| MoO3 | 1.822×10-4 | 7.12×1022 | 0.3554 | 0.00252 | 785 | 0.99628 |
| 1 | Shannon M A , Bohn P W , Elimelech M , et al . Science and technology for water purification in the coming decades[J]. Nature, 2008, 452(7185): 301-310. |
| 2 | Ghasemi M , Mashhadi S , Asif M , et al . Microwave-assisted synthesis of tetraethylenepentamine functionalized activated carbon with high adsorption capacity for Malachite green dye[J]. Journal of Molecular Liquids, 2016, 213(1): 317-325. |
| 3 | 任南琪, 周显娇, 郭婉茜, 等 . 染料废水处理技术研究进展[J]. 化工学报, 2013, 64(1): 84-94. |
| Ren N Q , Zhou X J , Guo W Q , et al . A review on treatment methods of dye wastewater[J]. CIESC Journal, 2013, 64(1): 84-94. | |
| 4 | Hoffmann M R , Martin S T , Choi W , et al . Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95(1): 69-96. |
| 5 | Pathania D , Sharma S , Singh P . Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast[J]. Arabian Journal of Chemistry, 2017, 10(1): S1445-S1451. |
| 6 | Yagub M T , Sen T K , Ang H M . Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves[J]. Water, Air, & Soil Pollution, 2012, 223(8): 5267-5282. |
| 7 | Dabrowski A . Adsorption from theory to practice[J]. Advances in Colloid and Interface Science, 2001, 93(1): 135-224. |
| 8 | Ali I , Asim M , Khan T A . Low cost adsorbents for the removal of organic pollutants from wastewater[J]. Journal of Environmental Management, 2012, 113(1): 170-183. |
| 9 | Zhu Q , Wang Z , Chen L , et al . Ionic-liquid-controlled two-dimensional monolayer Bi2MoO6 and its adsorption of azo molecules[J]. ACS Applied Nano Materials, 2018, 1(9): 5083-5091. |
| 10 | 赵亚红, 薛振华, 王喜明, 等 . 羧甲基纤维素/蒙脱土纳米复合材料对刚果红染料的吸附及解吸性能[J]. 化工学报, 2012, 63(8): 2655-2660. |
| Zhao Y H , Xue Z H , Wang X M , et al . Adsorption and desorption properties for Congo red dye of carboxymethylcellulose/montmorillonite nanocomposites[J].CIESC Journal, 2012, 63(8): 2655-2660. | |
| 11 | Allen S J , Koumanova B . Decolourisation of water/wastewater using adsorption[J]. Journal of the University of Chemical Technology and Metallurgy, 2005, 40(3): 175-192. |
| 12 | Gougoulias N , Papachatzis A , Kalorizou H . Role of acid blue 25 dye as active site for the adsorption of Cd2+, and Zn2+, using activated carbons[J]. Dyes & Pigments, 2013, 96(2): 459-466. |
| 13 | Sarkar M , Majumdar P . Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead[J]. Chemical Engineering Journal, 2011, 175(1): 376-387. |
| 14 | Lee M Y , Hong K J , Kajiuchi T , et al . Synthesis of chitosan-based polymeric surfactants and their adsorption properties for heavy metals and fatty acids[J]. International Journal of Biological Macromolecules, 2005, 36(3): 152-158. |
| 15 | Liu S , Ding Y , Li P . Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N, N-dimethyl dehydroabietylamine oxide[J]. Chemical Engineering Journal, 2014, 248(1): 135-144. |
| 16 | Nie L H , Tan Q , Zhu W . Fast adsorption removal of Congo red on hierarchically porous γ-Al2O3 hollow microspheres prepared by microwave-assisted hydrothermal method[J]. Acta Physico-Chimica Sinica, 2015, 31(9): 1815-1822. |
| 17 | He Q , Ni Y , Ye S . Preparation of flowerlike BiOBr/Bi2MoO6 composite superstructures and the adsorption behavior to dyes[J]. Journal of Physics and Chemistry of Solids, 2017, 104(1): 286-292. |
| 18 | 汪泽华, 蔡卫权, 郭蕾, 等 . P123辅助SB粉溶胶制备大孔径介孔γ-Al2O3及其对甲基蓝的强化吸附性能[J]. 化工学报, 2012, 63(8): 2623-2628. |
| Wang Z H , Cai W Q , Guo L , et al . P123-assisted synthesis of enlarged mesoporous γ-Al2O3 from SB pseudoboehmite sol and its enhanced adsorption performance towards methyl blue [J]. CIESC Journal, 2012, 63(8): 2623-2628. | |
| 19 | 徐刚, 郜洪文 . 甜菜碱OSB-12@高岭土杂化材料对染料吸附机理研究[J]. 化学学报, 2012, 70(24): 2496-2500. |
| Xu G , Gao H W . Betaine OSB-12@kaolin hybrid material synthesized for adsorption of dyes[J]. Acta Chimica Sinica, 2012, 70(24): 2496-2500. | |
| 20 | Lou X W , Zeng H C . Hydrothermal synthesis of α-MoO3 nanorods via acidification of ammonium heptamolybdate tetrahydrate [J]. Chemistry of Materials, 2002, 14(11): 4781-4789. |
| 21 | Sheehan P E , Lieber C M . Nanotribology and nanofabrication of MoO3 structures by atomic force microscopy[J]. Science, 1996, 272(5265): 1158-1161. |
| 22 | Song J , Ni X , Gao L , et al . Synthesis of metastable h-MoO3 by simple chemical precipitation[J]. Materials Chemistry and Physics, 2007, 102(2/3): 245-248. |
| 23 | Siciliano T , Tepore A , Filippo E , et al . Characteristics of molybdenum trioxide nanobelts prepared by thermal evaporation technique[J]. Materials Chemistry and Physics, 2009, 114(2/3): 687-691. |
| 24 | Schöllhorn R , Kuhlmann R , Besenhard J O . Topotactic redox reactions and ion exchange of layered MoO3 bronzes[J]. Materials Research Bulletin, 1976, 11(1): 83-90. |
| 25 | Prasad A K , Kubinski D J , Gouma P I . Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection [J]. Sensors and Actuators B: Chemical, 2003, 93(1/2/3): 25-30. |
| 26 | Kim H S , Cook J B , Lin H , et al . Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3- x [J]. Nature Materials, 2017, 16(4): 454. |
| 27 | Dieterle M , Weinberg G , Mestl G . Raman spectroscopy of molybdenum oxides (Ⅰ): Structural characterization of oxygen defects in MoO3- x by DR UV/VIS, Raman spectroscopy and X-ray diffraction [J]. Physical Chemistry Chemical Physics, 2002, 4(5): 812-821. |
| 28 | Tao P , Xu Y , Zhou Y , et al . Nitrogen oxide (NO) gas-sensing properties of Bi2MoO6 nanosheets synthesized by a hydrothermal method[J]. Materials Research, 2017, 20(3): 786-790. |
| 29 | Vasilopoulou M , Douvas A M , Georgiadou D G , et al . The influence of hydrogenation and oxygen vacancies on molybdenum oxides work function and gap states for application in organic optoelectronics[J]. Journal of the American Chemical Society, 2012, 134(39): 16178-16187. |
| 30 | Greiner M T , Chai L , Helander M G , et al . Transition metal oxide work functions: the influence of cation oxidation state and oxygen vacancies[J]. Advanced Functional Materials, 2012, 22(21): 4557–4568. |
| 31 | Kong X Y , Ng B J , Tan K H , et al . Simultaneous generation of oxygen vacancies on ultrathin BiOBr nanosheets during visible-light-driven CO2, photoreduction evoked superior activity and long-term stability[J]. Catalysis Today, 2018, 314: 20-27. |
| 32 | Zhang L , Wang W , Jiang D , et al . Photoreduction of CO2 on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies[J]. Nano Research, 2015, 8(3): 821-831. |
| 33 | Cervantes F J , Garciaespinosa A , Morenoreynosa M A , et al . Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes[J]. Environmental Science & Technology, 2010, 44(5): 1747-1753. |
| 34 | Liu T , Li Y , Du Q , et al . Adsorption of methylene blue from aqueous solution by graphene[J]. Colloids and Surfaces B- Biointerfaces, 2012, 90: 197-203. |
| 35 | Haque E , Jun J W , Jhung S H . Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235)[J]. Journal of Hazardous Materials, 2011, 185(1): 507-511. |
| 36 | Li Y , Du Q , Liu T , et al . Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes[J]. Chemical Engineering Research & Design: Transactions of the Institution of Chemical Engineers, 2013, 91(2): 361-368. |
| 37 | Guo J Z , Li B , Liu L , et al . Removal of methylene blue from aqueous solutions by chemically modified bamboo[J]. Chemosphere, 2014, 111: 225-231. |
| 38 | Namasivayam A , Sumithra S . Removal of direct red 12B and methylene blue from water by adsorption onto Fe(Ⅲ)/Cr(Ⅲ) hydroxide, an industrial solid waste[J]. Journal of Environmental Management, 2005, 74(3): 207-215. |
| 39 | Dogan M , Alkan M , Onager Y . Adsorption of methylene blue from aqueous solution onto perlite[J]. Water Air & Soil Pollution, 2000, 120(3/4): 229-248. |
| 40 | Bagane M , Guiza S . Removal of a dye from textile effluents by adsorption[J]. Annales de Chimie-Science des Materiaux, 2000, 25(8): 615-626. |
| 41 | Tian P , Han X , Ning G , et al . Synthesis of porous hierarchical MgO and its superb adsorption properties[J]. ACS Applied Materials & Interfaces, 2013, 5(23): 12411-12418. |
| 42 | 陈自正, 沈卫华, 陈立芳, 等 . 纳米H2TiO3锂吸附剂的水热合成及其吸附性能[J]. 中国有色金属学报, 2017, 27(3): 547-554. |
| Chen Z Z , Shen W H , Chen L F , et al . Hydrothermal synthesis and adsorption properties of nano scale H2TiO3 adsorbent [J].The Chinese Journal of Nonferrous Metals, 2017, 27(3): 547-554. | |
| 43 | Atia A A , Donia A M , Al-Amrani W A . Adsorption/desorption behavior of acid orange 10 on magnetic silica modified with amine groups[J]. Chemical Engineering Journal, 2009, 150(1): 55-62. |
| 44 | Senturk H B , Ozdes D , Gundogdu A , et al . Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: equilibrium, kinetic and thermodynamic study[J]. Journal of Hazardous Materials, 2009, 172(1): 353-362. |
| 45 | Zhang J , Xiao H , Yang Y . Preparation of hemicellulose-containing latex and its application as absorbent toward dyes[J]. Journal of Materials Science, 2015, 50(4): 1673-1678. |
| [1] | Cheng CHENG, Zhongdi DUAN, Haoran SUN, Haitao HU, Hongxiang XUE. Lattice Boltzmann simulation of surface microstructure effect on crystallization fouling [J]. CIESC Journal, 2023, 74(S1): 74-86. |
| [2] | Yan GAO, Peng WU, Chao SHANG, Zejun HU, Xiaodong CHEN. Preparation of magnetic agarose microspheres based on a two-fluid nozzle and their protein adsorption properties [J]. CIESC Journal, 2023, 74(8): 3457-3471. |
| [3] | Linzheng WANG, Yubing LU, Ruizhi ZHANG, Yonghao LUO. Analysis on thermal oxidation characteristics of VOCs based on molecular dynamics simulation [J]. CIESC Journal, 2023, 74(8): 3242-3255. |
| [4] | Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent [J]. CIESC Journal, 2023, 74(8): 3375-3385. |
| [5] | Ruihang ZHANG, Pan CAO, Feng YANG, Kun LI, Peng XIAO, Chun DENG, Bei LIU, Changyu SUN, Guangjin CHEN. Analysis of key parameters affecting product purity of natural gas ethane recovery process via ZIF-8 nanofluid [J]. CIESC Journal, 2023, 74(8): 3386-3393. |
| [6] | Mengmeng ZHANG, Dong YAN, Yongfeng SHEN, Wencui LI. Effect of electrolyte types on the storage behaviors of anions and cations for dual-ion batteries [J]. CIESC Journal, 2023, 74(7): 3116-3126. |
| [7] | Ji CHEN, Ze HONG, Zhao LEI, Qiang LING, Zhigang ZHAO, Chenhui PENG, Ping CUI. Study on coke dissolution loss reaction and its mechanism based on molecular dynamics simulations [J]. CIESC Journal, 2023, 74(7): 2935-2946. |
| [8] | Jie WANG, Xiaolin QIU, Ye ZHAO, Xinyang LIU, Zhongqiang HAN, Yong XU, Wenhan JIANG. Preparation and properties of polyelectrolyte electrostatic deposition modified PHBV antioxidant films [J]. CIESC Journal, 2023, 74(7): 3068-3078. |
| [9] | Jipeng ZHOU, Wenjun HE, Tao LI. Reaction engineering calculation of deactivation kinetics for ethylene catalytic oxidation over irregular-shaped catalysts [J]. CIESC Journal, 2023, 74(6): 2416-2426. |
| [10] | Guangyu WANG, Kai ZHANG, Kaihua ZHANG, Dongke ZHANG. Heat and mass transfer and energy consumption for microwave drying of coal slime [J]. CIESC Journal, 2023, 74(6): 2382-2390. |
| [11] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [12] | Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation [J]. CIESC Journal, 2023, 74(5): 2057-2066. |
| [13] | Quanbi ZHANG, Yijin YANG, Xujing GUO. Catalytic degradation of dissolved organic matter in rifampicin pharmaceutical wastewater by Fenton oxidation process [J]. CIESC Journal, 2023, 74(5): 2217-2227. |
| [14] | Shaoyun CHEN, Dong XU, Long CHEN, Yu ZHANG, Yuanfang ZHANG, Qingliang YOU, Chenglong HU, Jian CHEN. Preparation and adsorption properties of monolayer polyaniline microsphere arrays [J]. CIESC Journal, 2023, 74(5): 2228-2238. |
| [15] | Simin YI, Yali MA, Weiqiang LIU, Jinshuai ZHANG, Yan YUE, Qiang ZHENG, Songyan JIA, Xue LI. Study on ammonia evaporation and hydration kinetics of microcrystalline magnesite [J]. CIESC Journal, 2023, 74(4): 1578-1586. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||