CIESC Journal ›› 2020, Vol. 71 ›› Issue (5): 2076-2087.DOI: 10.11949/0438-1157.20191536
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Fang JIN1( ),Peng ZHANG1(
),Peng ZHANG1( ),Guiying WU1,Di WU2
),Guiying WU1,Di WU2
Received:2019-12-18
Revised:2020-02-11
Online:2020-05-05
Published:2020-05-05
Contact:
Fang JIN
通讯作者:
金放
作者简介:金放(1980—),男,博士, 教授,基金资助:CLC Number:
Fang JIN,Peng ZHANG,Guiying WU,Di WU. Brönsted equation kinetic modeling for quantitative relationship between activity and acidity strength distribution in oligomerization and aromatization of ethylene over ZSM-5 catalyst[J]. CIESC Journal, 2020, 71(5): 2076-2087.
金放,张鹏,吴桂英,吴迪. Brönsted方程动力学模型研究ZSM-5催化乙烯齐聚及芳构化活性和酸强度分布之间的定量关系[J]. 化工学报, 2020, 71(5): 2076-2087.
Add to citation manager EndNote|Ris|BibTeX
| T0/K | (T0+ΔT)/K | E/(kJ·mol-1) | KE/s-1 |
|---|---|---|---|
| 348 | 398 | 51.9 | 1.1×10-7 |
| 373 | 423 | 62.9 | 1.3×10-8 |
| 398 | 448 | 71.7 | 1.8×10-8 |
| 423 | 473 | 88.9 | 5.1×10-9 |
| 448 | 498 | 105.6 | 9.4×10-11 |
| 473 | 523 | 128.5 | 2.2×10-11 |
| 523 | 573 | 145.5 | 6.8×10-12 |
| 573 | 623 | 176.2 | 1.10×10-13 |
Table 1 E and KEvalues estimated by fitting Eq. (9) from acid sites with uniform acid strength over ZSM-5(Si/Al molar ratio 12.5)
| T0/K | (T0+ΔT)/K | E/(kJ·mol-1) | KE/s-1 |
|---|---|---|---|
| 348 | 398 | 51.9 | 1.1×10-7 |
| 373 | 423 | 62.9 | 1.3×10-8 |
| 398 | 448 | 71.7 | 1.8×10-8 |
| 423 | 473 | 88.9 | 5.1×10-9 |
| 448 | 498 | 105.6 | 9.4×10-11 |
| 473 | 523 | 128.5 | 2.2×10-11 |
| 523 | 573 | 145.5 | 6.8×10-12 |
| 573 | 623 | 176.2 | 1.10×10-13 |
| 样品 | TOF/(mmol·h-1·g-1) | Aci/(mmol·h-1·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C3= | C4= | C6A | C7A | C8A | ||
| 19ZSM-5 | 67.76 | 0.51 | 0.37 | 28.20 | 22.67 | 1.93 | 12.66 | 11.65 |
| 25ZSM-5 | 62.06 | 0.45 | 0.54 | 23.68 | 15.36 | 1.75 | 9.80 | 12.46 |
| 60ZSM-5 | 48.91 | 0.25 | 0.24 | 18.08 | 10.68 | 1.32 | 6.17 | 6.23 |
| 70ZSM-5 | 45.41 | 0.16 | 0.25 | 13.78 | 9.87 | 0.83 | 5.33 | 5.63 |
Table 2 Activity of xZSM-5 with different Si/Al molar ratio for ethylene oligomerization and aromatization
| 样品 | TOF/(mmol·h-1·g-1) | Aci/(mmol·h-1·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C3= | C4= | C6A | C7A | C8A | ||
| 19ZSM-5 | 67.76 | 0.51 | 0.37 | 28.20 | 22.67 | 1.93 | 12.66 | 11.65 |
| 25ZSM-5 | 62.06 | 0.45 | 0.54 | 23.68 | 15.36 | 1.75 | 9.80 | 12.46 |
| 60ZSM-5 | 48.91 | 0.25 | 0.24 | 18.08 | 10.68 | 1.32 | 6.17 | 6.23 |
| 70ZSM-5 | 45.41 | 0.16 | 0.25 | 13.78 | 9.87 | 0.83 | 5.33 | 5.63 |
| 产物 | δ | γ | R2 | RSS | TSS |
|---|---|---|---|---|---|
| C2 | 0.0005 | 0.0031 | 0.95 | 3.36×10-9 | 6.35×10-8 |
| C3 | 0.0003 | 0.0072 | 0.77 | 1.17×10-8 | 5.15×10-8 |
| C3= | 0.0350 | 0.0025 | 0.86 | 1.62×10-5 | 1.20×10-4 |
| C4= | 0.0250 | 0.0021 | 0.85 | 1.15×10-5 | 7.49×10-5 |
| C6A | 0.0026 | 0.0018 | 0.99 | 1.24×10-8 | 1.03×10-6 |
| C7A | 0.0186 | 0.0010 | 0.94 | 2.07×10-6 | 3.40×10-5 |
| C8A | 0.0057 | 0.0100 | 0.98 | 1.29×10-7 | 3.91×10-5 |
Table 3 δ, γ values of Br?nsted relation on average strength acid sites calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization on different ZSM-5
| 产物 | δ | γ | R2 | RSS | TSS |
|---|---|---|---|---|---|
| C2 | 0.0005 | 0.0031 | 0.95 | 3.36×10-9 | 6.35×10-8 |
| C3 | 0.0003 | 0.0072 | 0.77 | 1.17×10-8 | 5.15×10-8 |
| C3= | 0.0350 | 0.0025 | 0.86 | 1.62×10-5 | 1.20×10-4 |
| C4= | 0.0250 | 0.0021 | 0.85 | 1.15×10-5 | 7.49×10-5 |
| C6A | 0.0026 | 0.0018 | 0.99 | 1.24×10-8 | 1.03×10-6 |
| C7A | 0.0186 | 0.0010 | 0.94 | 2.07×10-6 | 3.40×10-5 |
| C8A | 0.0057 | 0.0100 | 0.98 | 1.29×10-7 | 3.91×10-5 |
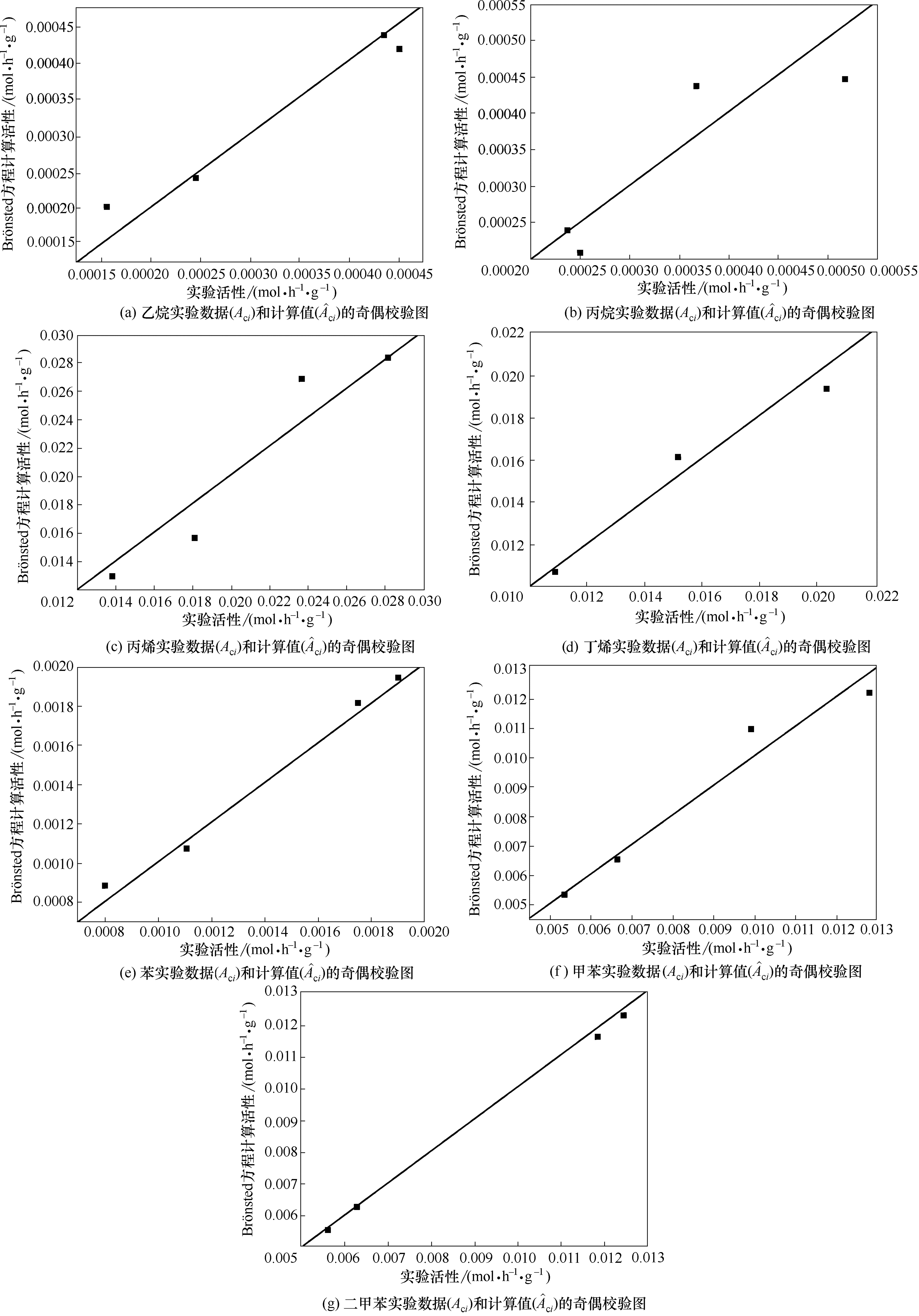
Fig.4 Parity plots between experimental Aci and model calculated date for ?ciwith Br?nsted ethane, propane, propylane, butene, benzene, toluene, xylene
| 产物 | 19ZSM-5 | 25ZSM-5 | 60ZSM-5 | 70ZSM-5 | R2 | |
|---|---|---|---|---|---|---|
| C2 | δ | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.99 |
| γ | 0.0030 | 0.0029 | 0.0032 | 0.0031 | ||
| C3 | δ | 0.0003 | 0.0003 | 0.0003 | 0.0003 | 0.99 |
| γ | 0.0060 | 0.0080 | 0.0072 | 0.0085 | ||
| C3= | δ | 0.0350 | 0.0320 | 0.0380 | 0.0360 | 0.99 |
| γ | 0.0025 | 0.0022 | 0.0030 | 0.0028 | ||
| C4= | δ | 0.0250 | 0.0210 | 0.0240 | 0.0270 | 0.99 |
| γ | 0.0025 | 0.0021 | 0.0026 | 0.0025 | ||
| C6A | δ | 0.0026 | 0.0025 | 0.0024 | 0.0026 | 0.99 |
| γ | 0.0017 | 0.0018 | 0.0019 | 0.0016 | ||
| C7A | δ | 0.0186 | 0.0160 | 0.0170 | 0.0170 | 0.99 |
| γ | 0.0010 | 0.0010 | 0.0011 | 0.0012 | ||
| C8A | δ | 0.0057 | 0.0056 | 0.0055 | 0.0056 | 0.99 |
| γ | 0.0100 | 0.0100 | 0.0100 | 0.0095 |
Table 4 δ,γ values of Br?nsted relation on average strength acid site calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization with ZSM-5 of different Si/Al molar ratio
| 产物 | 19ZSM-5 | 25ZSM-5 | 60ZSM-5 | 70ZSM-5 | R2 | |
|---|---|---|---|---|---|---|
| C2 | δ | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.99 |
| γ | 0.0030 | 0.0029 | 0.0032 | 0.0031 | ||
| C3 | δ | 0.0003 | 0.0003 | 0.0003 | 0.0003 | 0.99 |
| γ | 0.0060 | 0.0080 | 0.0072 | 0.0085 | ||
| C3= | δ | 0.0350 | 0.0320 | 0.0380 | 0.0360 | 0.99 |
| γ | 0.0025 | 0.0022 | 0.0030 | 0.0028 | ||
| C4= | δ | 0.0250 | 0.0210 | 0.0240 | 0.0270 | 0.99 |
| γ | 0.0025 | 0.0021 | 0.0026 | 0.0025 | ||
| C6A | δ | 0.0026 | 0.0025 | 0.0024 | 0.0026 | 0.99 |
| γ | 0.0017 | 0.0018 | 0.0019 | 0.0016 | ||
| C7A | δ | 0.0186 | 0.0160 | 0.0170 | 0.0170 | 0.99 |
| γ | 0.0010 | 0.0010 | 0.0011 | 0.0012 | ||
| C8A | δ | 0.0057 | 0.0056 | 0.0055 | 0.0056 | 0.99 |
| γ | 0.0100 | 0.0100 | 0.0100 | 0.0095 |
| Parameter | Ei/(kJ·mol-1) | C2 | C3 | C3= | C4= | C6A | C7A | C8A |
|---|---|---|---|---|---|---|---|---|
| δ | 0.0004 | 0.00015 | 0.0436 | 0.0119 | 0.0019 | 0.011 | 0.015 | |
| γi | 63 | 0.0008 | -0.0326 | -0.0211 | -0.0243 | -0.0055 | -0.0007 | 0.002 |
| 90 | 0.0324 | 0.0488 | 0.0144 | -0.0161 | 0.0003 | 0.008 | -0.0279 | |
| 124 | -0.0001 | 0.0036 | -0.0043 | 0.0133 | 0.0019 | -0.0031 | -0.0179 | |
| 150 | 0.0064 | 0.0144 | 0.007 | 0.0121 | 0.0084 | 0.01 | 0.003 | |
| 175 | -0.0058 | 0.0021 | -0.0078 | -0.0131 | 0.0021 | -0.0053 | 0.005 | |
| R2 | 0.954 | 0.935 | 0.912 | 0.980 | 0.915 | 0.919 | 0.996 |
Table 5 δ, γi values of Br?nsted relation on different strength acid sites calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization on ZSM-5 of different Si/Al molar ratio
| Parameter | Ei/(kJ·mol-1) | C2 | C3 | C3= | C4= | C6A | C7A | C8A |
|---|---|---|---|---|---|---|---|---|
| δ | 0.0004 | 0.00015 | 0.0436 | 0.0119 | 0.0019 | 0.011 | 0.015 | |
| γi | 63 | 0.0008 | -0.0326 | -0.0211 | -0.0243 | -0.0055 | -0.0007 | 0.002 |
| 90 | 0.0324 | 0.0488 | 0.0144 | -0.0161 | 0.0003 | 0.008 | -0.0279 | |
| 124 | -0.0001 | 0.0036 | -0.0043 | 0.0133 | 0.0019 | -0.0031 | -0.0179 | |
| 150 | 0.0064 | 0.0144 | 0.007 | 0.0121 | 0.0084 | 0.01 | 0.003 | |
| 175 | -0.0058 | 0.0021 | -0.0078 | -0.0131 | 0.0021 | -0.0053 | 0.005 | |
| R2 | 0.954 | 0.935 | 0.912 | 0.980 | 0.915 | 0.919 | 0.996 |

Fig.5 Parity plots between experimental Aci and model calculated date for ?ciwith modified Br?nsted equation ethane, propane, propylane, butene, benzene, toluene, xylene
| 1 | 张惠明. 甲醇制低碳烯烃工艺技术新进展[J]. 化学反应工程与工艺, 2008, 24(2): 178-182. |
| Zhang H M. Advances in process research of methanol to light olefins[J]. Chemical Reaction Engineering and Technology, 2008, 24(2): 178-182. | |
| 2 | Chen J, Bozzano A, Glover B, et al. Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process[J]. Catalysis Today, 2005, 106(1/2/3/4): 103-107. |
| 3 | Koempel H, Liebner W. Lurgi’s methanol to propylene (MTP®) report on a successful commercialisation[J]. Studies in Surface Science and Catalysis, 2007, 167: 261-267. |
| 4 | Ding W, Li S, Meitzner G, et al. Methane conversion to aromatics on Mo/H-ZSM5: structure of molybdenum species in working catalysts[J]. The Journal of Physical Chemistry B, 2000, 105(2): 506-513. |
| 5 | Okolie C, Lyu Y, Kovarik L, et al. Coupling of methane to ethane, ethylene and aromatics over nickel on ceria-zirconia at low temperatures[J]. ChemCatChem. , 2018, 10(12): 2700-2708. |
| 6 | 孙晓轩. 生物质气化合成甲醇二甲醚技术现状及展望[J]. 中外能源, 2007, 12(4): 29-36. |
| Sun X X. Status & Prospect of biomass gasfication methnol/dimethyl ether synthesis system[J]. Sino-global Energy, 2007, 12(4): 29-36. | |
| 7 | Bai P T, Rajmohan K S, Prasad P S S, et al. Oxidative dehydrogenation of ethane to ethylene over metal oxide catalysts using carbon dioxide[M]//Winter F, Agarwal R A, Hrdlicka J, et al. CO2 Separation, Purification and Conversion to Chemicals and Fuels. Singapore: Springer Singapore, 2019: 93-117. |
| 8 | Zhang M, Yu Y. Dehydration of ethanol to ethylene[J]. Industrial & Engineering Chemistry Research, 2013, 52(28): 9505-9514. |
| 9 | 钱伯章. 甲醇制汽油路线及其应用[J]. 化工设计通讯, 2009, 35(4): 31-36. |
| Qian B Z. Methanol to gasoline process and application[J]. Chemical Engineering Design Communications, 2009, 35(4): 31-36. | |
| 10 | Chang C, Chu C, Socha R. Methanol conversion to olefins over ZSM-5(I): Effect of temperature and zeolite SiO2/Al2O3 [J]. Journal of Catalysis, 1984, 86(2): 289-296. |
| 11 | Bröonsted J. Acid and basic catalysis[J]. Chem. Rev. , 1928, 5(3): 231-338. |
| 12 | Jin F, Li Y. A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule[J]. Catalysis Today, 2009, 145(1/2): 101-107. |
| 13 | Yi X, Liu K, Chen W, et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites[J]. Journal of the American Chemical Society, 2018, 140(34): 10764-10774. |
| 14 | Zheng A, Li S, Liu S, et al. Acidic properties and structure-activity correlations of solid acid catalysts revealed by solid-state NMR spectroscopy[J]. Accounts of Chemical Research, 2016, 49(4): 655-663. |
| 15 | Hashimoto K, Masuda T, Mori T. A method for calculating activation energy distribution of desorption from temperature-programmed desorption spectrum of ammonia[J]. Studies in Surface Science and Catalysis, 1986, 28: 503-510. |
| 16 | Auroux A. Calorimetry and Thermal Methods in Catalysis[M]. Hull R, Jagadish C, et al. Berlin: Springer Series in Materials Science, 2013: 131-171. |
| 17 | Smith A, Aranoff S. Thermodesorption of gases from solids[J]. The Journal of Physical Chemistry, 1958, 62(6): 684-686. |
| 18 | Cvetanovic R, Amenomiya Y. Application of a temperature-programmed desorption technique to catalyst studies[J]. Adv. Catal. , 1967, 17: 103-149. |
| 19 | Cvetanovic R, Amenomiya Y. A temperature programmed desorption technique for investigation of practical catalysts[J]. Catalysis Reviews, 1972, 6(1): 21-48. |
| 20 | Konvalinka J A. Analysis of second-order desorption kinetics in temperature-programmed desorption[J]. Journal of Catalysis, 1977, 48(1/2/3): 365-373. |
| 21 | Hunger B, Hoffmann J. Kinetic analysis of NH3 temperature programmed desorption (TPD) on a HZSM-5 zeolite[J]. Thermochimica Acta, 1986, 106: 133-140. |
| 22 | Forni L, Magni E. Temperature-programmed desorption study of ammonia desorption-diffusion in molecular sieves: I. Theory[J]. Journal of Catalysis, 1988, 112(2): 437-443. |
| 23 | Leary K, Michaels J, Stacy A. Temperature‐programmed desorption: multisite and subsurface diffusion models[J]. AIChE Journal, 1988, 34(2): 263-271. |
| 24 | Kuipers H, Leuven H, Visser W. The characterization of heterogeneous catalysts by XPS based on geometrical probability(1): Monometallic catalysts[J]. Surface and Interface Analysis, 1986, 8(6): 235-242. |
| 25 | Bhatia S, Beltramini J, Do D D. Temperature programmed analysis and its applications in catalytic systems[J]. Catalysis Today, 1990, 7(3): 309-438. |
| 26 | Falconer J L, Madix R J. Desorption rate isotherms in flash desorption analysis[J]. Journal of Catalysis, 1977, 48(1/2/3): 262-268. |
| 27 | Yang S R. Shangrun Y. Relationship between peak height, peak temperature and activation energy of desorption in tpd spectrum of first order desorption kinetics[J]. Acta Physico-Chimica Sinica, 1987, 3(3): 313-320. |
| 28 | Xu W X, Tao Y G, Yang S R. Determination of thermal desorption parameters Ed and a by linear analysis method[J]. Chin. J. Catal. , 1993, 14(1): 77-80. |
| 29 | Duan X W Q. A new method of quantitative analysis of TPD spectrograms[J]. Chin. J. Catal. , 1986, 7(2): 169-176. |
| 30 | Sawa M, Niwa M, Murakami Y. One-point method for determining acid strength of zeolite by temperature-programmed desorption of ammonia[J]. Zeolites, 1991, 11(1): 93-94. |
| 31 | Katada N, Igi H, Kim J. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium[J]. Journal of Physical Chemistry B, 1997, 101(31): 5969-5977. |
| 32 | Costa C, Lopes J M, Lemos F, et al. Activity-acidity relationship in zeolite Y(part Ⅲ): Application of Brönsted type equations[J]. Journal of Molecular Catalysis A: Chemical, 1999, 144(1): 233-238. |
| 33 | Costa C, Lopes J, Lemos F, et al. Acidity-activity relationship in zeolite Y: a preliminary study for n-heptane transformation[J]. Catalysis Letters, 1997, 44(3): 255-257. |
| 34 | Borges P, Ramos P R, Lemos A, et al. Activity-acidity relationship for alkane cracking over zeolites: n-hexane cracking over HZSM-5[J]. Journal of Molecular Catalysis A: Chemical, 2005, 229(1/2): 127-135. |
| 35 | Costa C, Dzikh I P, Lopes J M, et al. Activity-acidity relationship in zeolite ZSM-5. Application of Brönsted-type equations[J]. Journal of Molecular Catalysis A: Chemical, 2000, 154(1): 193-201. |
| 36 | Jin F, Fan Y, Wu G, et al. Modified Brönsted type equation with ammonia as probe molecule: quantitative acidity-activity relationship for pyridine synthesis with ZSM-5 catalyst[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 123(2): 517-527. |
| 37 | Wang C M, Brogaard R Y, Weckhuysen B M, et al. Reactivity descriptor in solid acid catalysis: predicting turnover frequencies for propene methylation in zeotypes[J]. The Journal of Physical Chemistry Letters, 2014, 5(9): 1516-1521. |
| 38 | Masuda T, Fujikata Y, Ikeda H, et al. A method for calculating the activation energy distribution for desorption of ammonia using a TPD spectrum obtained under desorption control conditions[J]. Appl. Catal. A, 1997, 162(1): 29-40. |
| 39 | Karge H, Dondur V. Investigation of the distribution of acidity in zeolites by temperature-programmed desorption of probe molecules(Ⅰ): Dealuminated mordenites[J]. The Journal of Physical Chemistry, 1990, 94(2): 765-772. |
| 40 | Wang C, Wang L, Wu G, et al. Quantitative relationship between activity and acid site distribution in the oligomerization of ethylene over MCM-41 catalyst[J]. Catalysis Letters, 2020, 150: 429-437. |
| 41 | Jin F, Fan Y, Yuan M, et al. Single-event kinetic modeling of ethene oligomerization on ZSM-5[J]. Catalysis Today, 2018, 316: 129-141. |
| [1] | Zhenghao JIN, Lijie FENG, Shuhong LI. Energy and exergy analysis of a solution cross-type absorption-resorption heat pump using NH3/H2O as working fluid [J]. CIESC Journal, 2023, 74(S1): 53-63. |
| [2] | Yepin CHENG, Daqing HU, Yisha XU, Huayan LIU, Hanfeng LU, Guokai CUI. Application of ionic liquid-based deep eutectic solvents for CO2 conversion [J]. CIESC Journal, 2023, 74(9): 3640-3653. |
| [3] | Pan LI, Junyang MA, Zhihao CHEN, Li WANG, Yun GUO. Effect of the morphology of Ru/α-MnO2 on NH3-SCO performance [J]. CIESC Journal, 2023, 74(7): 2908-2918. |
| [4] | Yaxin CHEN, Hang YUAN, Guanzhang LIU, Lei MAO, Chun YANG, Ruifang ZHANG, Guangya ZHANG. Advances in enzyme self-immobilization mediated by protein nanocages [J]. CIESC Journal, 2023, 74(7): 2773-2782. |
| [5] | Xiaoling TANG, Jiarui WANG, Xuanye ZHU, Renchao ZHENG. Biosynthesis of chiral epichlorohydrin by halohydrin dehalogenase based on Pickering emulsion system [J]. CIESC Journal, 2023, 74(7): 2926-2934. |
| [6] | Lei MAO, Guanzhang LIU, Hang YUAN, Guangya ZHANG. Efficient preparation of carbon anhydrase nanoparticles capable of capturing CO2 and their characteristics [J]. CIESC Journal, 2023, 74(6): 2589-2598. |
| [7] | Tan ZHANG, Guang LIU, Jinping LI, Yuhan SUN. Performance regulation strategies of Ru-based nitrogen reduction electrocatalysts [J]. CIESC Journal, 2023, 74(6): 2264-2280. |
| [8] | Kuikui HAN, Xianglong TAN, Jinzhi LI, Ting YANG, Chun ZHANG, Yongfen ZHANG, Hongquan LIU, Zhongwei YU, Xuehong GU. Four-channel hollow fiber MFI zeolite membrane for the separation of xylene isomers [J]. CIESC Journal, 2023, 74(6): 2468-2476. |
| [9] | Chengze WANG, Kaili GU, Jinhua ZHANG, Jianxuan SHI, Yiwei LIU, Jinxiang LI. Sulfidation couples with aging to enhance the reactivity of zerovalent iron toward Cr(Ⅵ) in water [J]. CIESC Journal, 2023, 74(5): 2197-2206. |
| [10] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [11] | Zijian WANG, Ming KE, Jiahan LI, Shuting LI, Jinru SUN, Yanbing TONG, Zhiping ZHAO, Jiaying LIU, Lu REN. Progress in preparation and application of short b-axis ZSM-5 molecular sieve [J]. CIESC Journal, 2023, 74(4): 1457-1473. |
| [12] | Tianhao BAI, Xiaowen WANG, Mengzi YANG, Xinwei DUAN, Jie MI, Mengmeng WU. Study on release and inhibition behavior of COS during high-temperature gas desulfurization process using Zn-based oxide derived from hydrotalcite [J]. CIESC Journal, 2023, 74(4): 1772-1780. |
| [13] | Yin XU, Jie CAI, Lu CHEN, Yu PENG, Fuzhen LIU, Hui ZHANG. Advances in heterogeneous visible light photocatalysis coupled with persulfate activation for water pollution control [J]. CIESC Journal, 2023, 74(3): 995-1009. |
| [14] | Runzhu LIU, Tiantian CHU, Xiaoa ZHANG, Chengzhong WANG, Junying ZHANG. Synthesis and properties of phenylene-containing α,ω-hydroxy-terminated fluorosilicone polymers [J]. CIESC Journal, 2023, 74(3): 1360-1369. |
| [15] | Jieyuan ZHENG, Xianwei ZHANG, Jintao WAN, Hong FAN. Synthesis and curing kinetic analysis of eugenol-based siloxane epoxy resin [J]. CIESC Journal, 2023, 74(2): 924-932. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||