CIESC Journal ›› 2020, Vol. 71 ›› Issue (8): 3797-3806.DOI: 10.11949/0438-1157.20200418
• Material science and engineering, nanotechnology • Previous Articles Next Articles
Shuang WEN1( ),Xiaojie JU1,2(
),Xiaojie JU1,2( ),Rui XIE1,2,Wei WANG1,2,Zhuang LIU1,2,Liangyin CHU1,2
),Rui XIE1,2,Wei WANG1,2,Zhuang LIU1,2,Liangyin CHU1,2
Received:2020-04-21
Revised:2020-05-13
Online:2020-08-05
Published:2020-08-05
Contact:
Xiaojie JU
温霜1( ),巨晓洁1,2(
),巨晓洁1,2( ),谢锐1,2,汪伟1,2,刘壮1,2,褚良银1,2
),谢锐1,2,汪伟1,2,刘壮1,2,褚良银1,2
通讯作者:
巨晓洁
作者简介:温霜(1995—),女,硕士研究生,基金资助:CLC Number:
Shuang WEN, Xiaojie JU, Rui XIE, Wei WANG, Zhuang LIU, Liangyin CHU. Fabrication and controlled-release properties of intestinal-targeted Ca-alginate-based capsules[J]. CIESC Journal, 2020, 71(8): 3797-3806.
温霜, 巨晓洁, 谢锐, 汪伟, 刘壮, 褚良银. 肠靶向海藻酸钙基微胶囊的制备及控释性能研究[J]. 化工学报, 2020, 71(8): 3797-3806.
Add to citation manager EndNote|Ris|BibTeX
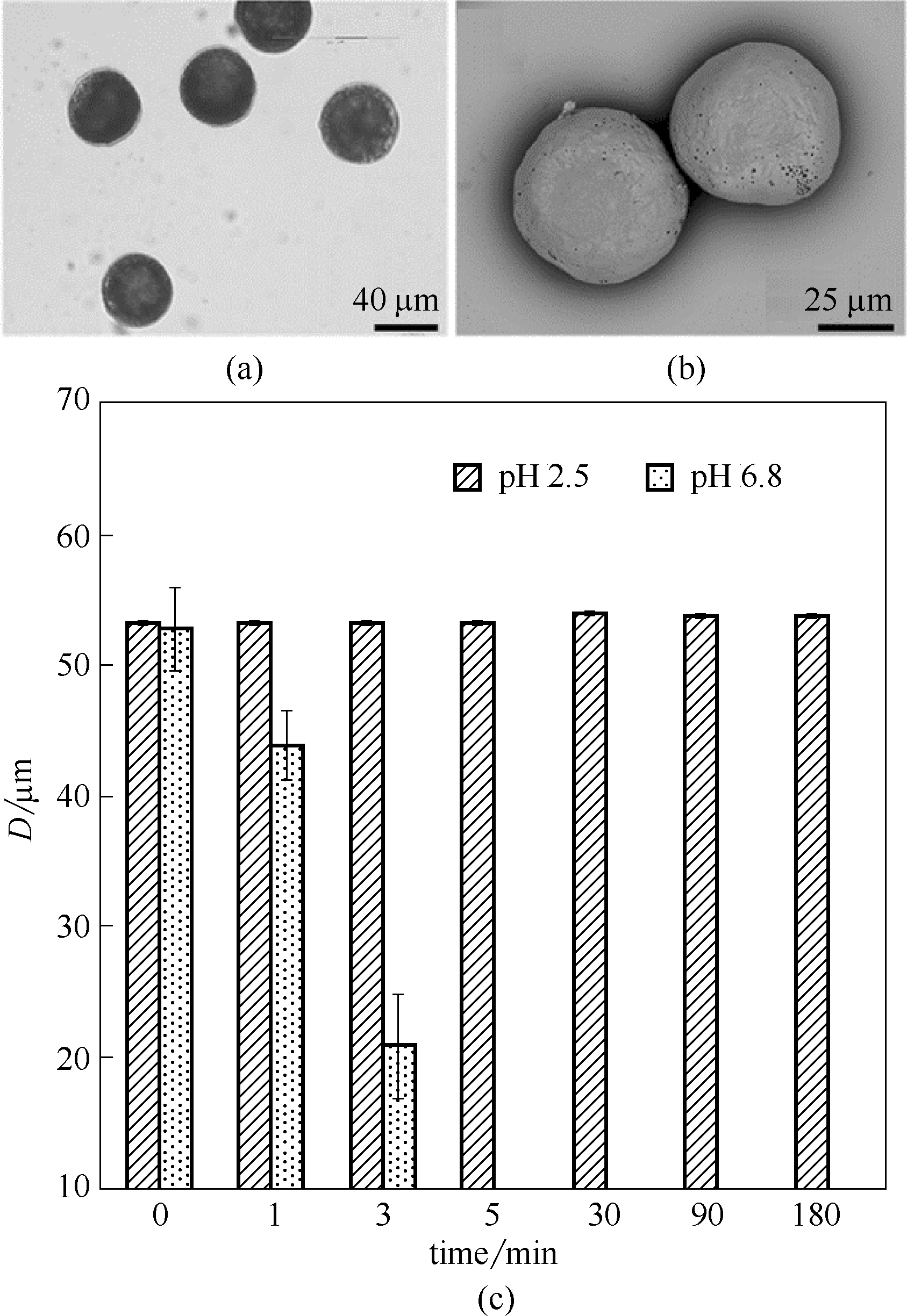
Fig.4 Microscopical photo (a) and SEM image (b) of HPMCP microspheres in pure water, and size changes of HPMCP microspheres at different pH conditions (c)

Fig.5 The stress-strain curves of AC and ACPSi capsules when they are extruded (a); the photos of ACPSi capsule starts to be squeezed (b) and finally burst (c)(the scale bar is 1 cm)
| 1 | Pinto J F. Site-specific drug delivery systems within the gastro-intestinal tract: from the mouth to the colon[J]. International Journal of Pharmaceutics, 2010, 395(1/2): 44-52. |
| 2 | Yun Y, Cho Y W, Park K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery[J]. Advanced Drug Delivery Reviews, 2013, 65(6): 822-832. |
| 3 | Kim T H, Shin S, Bulitta J B, et al. Development of a physiologically relevant population pharmacokinetic in vitro-in vivo correlation approach for designing extended-release oral dosage formulation[J]. Molecular Pharmaceutics, 2017, 14(1): 53-65. |
| 4 | Banerjee A, Qi J, Gogoi R, et al. Role of nanoparticle size, shape and surface chemistry in oral drug delivery[J]. Journal of Controlled Release, 2016, 238: 176-185. |
| 5 | Amidon S, Brown J E, Dave V S. Colon-targeted oral drug delivery systems: design trends and approaches[J]. AAPS PharmSciTech, 2015, 16(4): 731-741. |
| 6 | 吴庆喜, 姚善泾. 口服结肠靶向给药系统和制备方法的研究进展[J]. 化工学报, 2013, 64(1): 210-222. |
| Wu Q X, Yao S J. Oral colon-specific drug delivery system and its preparation[J]. CIESC Journal, 2013, 64(1): 210-222. | |
| 7 | Zhang H, Liu D, Shahbazi M A, et al. Fabrication of a multifunctional nano-in-micro drug delivery platform by microfluidic templated encapsulation of porous silicon in polymer matrix[J]. Advanced Materials, 2014, 26: 4497-4503. |
| 8 | Ghaffarian R, Herrero E P, Oh H, et al. Chitosan-alginate microcapsules provide gastric protection and intestinal release of ICAM-1-targeting nanocarriers, enabling GI targeting in vivo[J]. Advanced Functional Materials, 2016, 26(20): 3382-3393. |
| 9 | 朱颖, 乔颖玉, 杨钰娜, 等. 缓释制剂不同制备技术的研究及其应用进展[J]. 中国医院药学杂志, 2018, 38(20): 94-99. |
| Zhu Y, Qiao Y Y, Yang Y N, et al. Research and application progress of different preparation techniques for sustained release preparation[J]. Chinese Journal of Hospital Pharmacy, 2018, 38(20): 94-99. | |
| 10 | Song S W, Hidajat K, Kawi S. pH-Controllable drug release using hydrogel encapsulated mesoporous silica[J]. Chemical Communications, 2007, (42): 4396-4398. |
| 11 | Lee C H, Lo L W, Mou C Y, et al. Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug[J]. Advanced Functional Materials, 2008, 18(20): 3283-3292. |
| 12 | 张笑含, 刘琳, 张紫茜, 等. 微胶囊技术及其应用[J]. 黑龙江医药, 2014, 27(5): 1051-1055. |
| Zhang X H, Liu L, Zhang Z X, et al. Microencapsulation technology and its application[J]. Heilongjiang Medicine Journal, 2014, 27(5): 1051-1055. | |
| 13 | 蔡涛, 王丹, 宋志祥, 等. 微胶囊的制备技术及其应用[J]. 化学中间体, 2009, (12): 6-13. |
| Cai T, Wang D, Song Z X, et al. Preparation of microcapsules and their application[J]. Chemical Intermediate, 2009, (12): 6-13. | |
| 14 | Mei L, Xie R, Yang C, et al. Bio-inspired mini-eggs with pH-responsive membrane for enzyme immobilization[J]. Journal of Membrane Science, 2013, 429: 313-322. |
| 15 | 何帆, 谢锐, 巨晓洁, 等. 超薄壁结构海藻酸钙胶囊膜制备及其功能化研究新进展[J]. 化工学报, 2015, 66(8): 2817-2823. |
| He F, Xie R, Ju X J, et al. Recent progress in fabrication and functionalization of Ca-alginate capsules with ultrathin membranes[J]. CIESC Journal, 2015, 66(8): 2817-2823. | |
| 16 | Wang J Y, Jin Y, Xie R, et al. Novel calcium-alginate capsules with aqueous core and thermo-responsive membrane[J]. Journal of Colloid and Interface Science, 2011, 353(1): 61-68. |
| 17 | George M, Abraham T E. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review[J]. Journal of Controlled Release, 2006, 114(1): 1-14. |
| 18 | He F, Wang W, He X H, et al. Controllable multicompartmental capsules with distinct cores and shells for synergistic release[J]. ACS Applied Materials & Interfaces, 2016, 8(13): 8743-8754. |
| 19 | Mei L, He F, Zhou R Q, et al. Novel intestinal-targeted Ca-alginate-based carrier for pH-responsive protection and release of lactic acid bacteria[J]. ACS Applied Materials & Interfaces, 2014, 6(8): 5962-5970. |
| 20 | Mei L, Xie R, Yang C, et al. pH-responsive Ca-alginate-based capsule membranes with grafted poly(methacrylic acid) brushes for controllable enzyme reaction[J]. Chemical Engineering Journal, 2013, 232: 573-581. |
| 21 | 贺龙. 以海藻酸钠/壳聚糖为壁材的三种甜橙微胶囊的制备与性能研究[D]. 上海: 上海应用技术学院, 2015. |
| He L. The preparation and properties of three types of microencapsulations of sweet orange oil using alginate-chitosan as wall material[D]. Shanghai: Shanghai Institute of Technology, 2015. | |
| 22 | 王伟浩, 杨鑫, 李飞, 等. 载酶海藻酸钙复合微球稳定水包油型Pickering乳液及其强化界面酶催化反应[J]. 化工学报, 2019, 70(12): 4777-4786. |
| Wang W H, Yang X, Li F, et al. E@Alg@s-TiO2 microsphere stabilized O/W Pickering emulsion and the enhancement of interfacial enzymatic catalysis[J]. CIESC Journal, 2019, 70(12): 4777-4786. | |
| 23 | Ping Y, Guo J, Ejima H, et al. pH-Responsive capsules engineered from metal-phenolic networks for anticancer drug delivery[J]. Small, 2015, 11(17): 2032-2036. |
| 24 | Wei J, Ju X节J, Zou X Y, et al. Multi-stimuli-responsive microcapsules for adjustable controlled-release[J]. Advanced Functional Materials, 2014, 24(22): 3312-3323. |
| 25 | Zarket B C, Raghavan S R. Onion-like multilayered polymer capsules synthesized by a bioinspired inside-out technique[J]. Nature Communications, 2017, 8(1): 1-10. |
| 26 | Zhang Y, Wang Q C, Yu H, et al. Evaluation of alginate-whey protein microcapsules for intestinal delivery of lipophilic compounds in pigs[J]. Journal of the Science of Food and Agriculture, 2016, 96(8): 2674-2681. |
| 27 | Işıklan N, İnal M, Kurşun F, et al. pH responsive itaconic acid grafted alginate microspheres for the controlled release of nifedipine[J]. Carbohydrate Polymers, 2011, 84(3): 933-943. |
| 28 | He F, Mei L, Ju X J, et al. pH-responsive controlled release characteristics of solutes with different molecular weights diffusing across membranes of Ca-alginate/protamine/silica hybrid capsules[J]. Journal of Membrane Science, 2015, 474: 233-243. |
| 29 | Dowling M B, Bagal A S, Raghavan S R. Self-destructing “mothership” capsules for timed release of encapsulated contents[J]. Langmuir, 2013, 29(25): 7993-7998. |
| 30 | Lee D W, Hwang S J, Park J B, et al. Preparation and release characteristics of polymer-coated and blended alginate microspheres[J]. Journal of Microencapsulation, 2003, 20(2): 179-192. |
| 31 | 郭海丽, 阎志飞, 丁红, 等. 吲达帕胺肠溶微球的制备及影响因素考察[J]. 中国现代应用药学, 2012, 29(11): 993-997. |
| Guo H L, Yan Z F, Ding H, et al. Preparation of the enteric microspheres of indapamide and investigation of the influencing factors[J]. Chinese Journal of Modern Applied Pharmacy, 2012, 29(11): 993-997. |
| [1] | Jingwei CHAO, Jiaxing XU, Tingxian LI. Investigation on the heating performance of the tube-free-evaporation based sorption thermal battery [J]. CIESC Journal, 2023, 74(S1): 302-310. |
| [2] | Yitong LI, Hang GUO, Hao CHEN, Fang YE. Study on operating conditions of proton exchange membrane fuel cells with non-uniform catalyst distributions [J]. CIESC Journal, 2023, 74(9): 3831-3840. |
| [3] | Xuanzhi HE, Yongqing HE, Guiye WEN, Feng JIAO. Ferrofluid droplet neck self-similar breakup behavior [J]. CIESC Journal, 2023, 74(7): 2889-2897. |
| [4] | Yuanyuan ZHANG, Jiangyuan QU, Xinxin SU, Jing YANG, Kai ZHANG. Gas-liquid mass transfer and reaction characteristics of SNCR denitration in CFB coal-fired unit [J]. CIESC Journal, 2023, 74(6): 2404-2415. |
| [5] | Chi YIN, Zhengguo ZHANG, Ziye LING, Xiaoming FANG. Combining paraffin@silica nanocapsules with carbon fiber to develop a phase change thermal interface material for efficient heat dissipation [J]. CIESC Journal, 2023, 74(4): 1795-1804. |
| [6] | Hao WANG, Siyang TANG, Shan ZHONG, Bin LIANG. An investigation of the enhancing effect of solid particle surface on the CO2 desorption behavior in chemical sorption process with MEA solution [J]. CIESC Journal, 2023, 74(4): 1539-1548. |
| [7] | Lu DENG, Xiaojie JU, Wenjie ZHANG, Rui XIE, Wei WANG, Zhuang LIU, Dawei PAN, Liangyin CHU. Controllable preparation of radioactive chitosan embolic microspheres by microfluidic method [J]. CIESC Journal, 2023, 74(4): 1781-1794. |
| [8] | Lufan JIA, Yiying WANG, Yuman DONG, Qinyuan LI, Xin XIE, Hao YUAN, Tao MENG. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction [J]. CIESC Journal, 2023, 74(3): 1239-1246. |
| [9] | Zhiguang QIAN, Yue FAN, Shixue WANG, Like YUE, Jinshan WANG, Yu ZHU. Effect of purging conditions on the impedance relaxation phenomenon and low temperature start-up of PEMFC [J]. CIESC Journal, 2023, 74(3): 1286-1293. |
| [10] | Yang HE, Senhu GAO, Qingyun WU, Mingli ZHANG, Tao LONG, Pei NIU, Jinghui GAO, Yingqi MENG. Numerical study on heat and mass transfer characteristics of straight slotted fins under wet conditions [J]. CIESC Journal, 2023, 74(3): 1073-1081. |
| [11] | Wanyuan HE, Yiyu CHEN, Chunying ZHU, Taotao FU, Xiqun GAO, Youguang MA. Study on gas-liquid mass transfer characteristics in microchannel with array bulges [J]. CIESC Journal, 2023, 74(2): 690-697. |
| [12] | Hao ZHANG, Ziyue WANG, Yujie CHENG, Xiaohui HE, Hongbing JI. Progress in the mass production of single-atom catalysts [J]. CIESC Journal, 2023, 74(1): 276-289. |
| [13] | Xintong HUANG, Yuhao GENG, Hengyuan LIU, Zhuo CHEN, Jianhong XU. Research progress on new functional nanoparticles prepared by microfluidic technology [J]. CIESC Journal, 2023, 74(1): 355-364. |
| [14] | Xuqing WANG, Shenglin YAN, Litao ZHU, Xibao ZHANG, Zhenghong LUO. Research progress on the mass transfer process of CO2 absorption by amines in a packed column [J]. CIESC Journal, 2023, 74(1): 237-256. |
| [15] | Yuelin WANG, Wei CHAO, Xiaocheng LAN, Zhipeng MO, Shuhuan TONG, Tiefeng WANG. Review of ethanol production via biological syngas fermentation [J]. CIESC Journal, 2022, 73(8): 3448-3460. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||