CIESC Journal ›› 2021, Vol. 72 ›› Issue (1): 14-26.DOI: 10.11949/0438-1157.20200863
• Reviews and monographs • Previous Articles Next Articles
CHEN Rundao1( ),ZHENG Fang1,GUO Lidong1,YANG Qiwei1,2,ZHANG Zhiguo1,2,YANG Yiwen1,2,REN Qilong1,2,BAO Zongbi1,2(
),ZHENG Fang1,GUO Lidong1,YANG Qiwei1,2,ZHANG Zhiguo1,2,YANG Yiwen1,2,REN Qilong1,2,BAO Zongbi1,2( )
)
Received:2020-07-01
Revised:2020-07-16
Online:2021-01-05
Published:2021-01-05
Contact:
BAO Zongbi
陈润道1( ),郑芳1,郭立东1,杨启炜1,2,张治国1,2,杨亦文1,2,任其龙1,2,鲍宗必1,2(
),郑芳1,郭立东1,杨启炜1,2,张治国1,2,杨亦文1,2,任其龙1,2,鲍宗必1,2( )
)
通讯作者:
鲍宗必
作者简介:陈润道(1998—),男,博士研究生,基金资助:CLC Number:
CHEN Rundao, ZHENG Fang, GUO Lidong, YANG Qiwei, ZHANG Zhiguo, YANG Yiwen, REN Qilong, BAO Zongbi. Advancements in adsorption separation of Xe/Kr noble gases[J]. CIESC Journal, 2021, 72(1): 14-26.
陈润道, 郑芳, 郭立东, 杨启炜, 张治国, 杨亦文, 任其龙, 鲍宗必. 稀有气体Xe/Kr吸附分离研究进展[J]. 化工学报, 2021, 72(1): 14-26.
Add to citation manager EndNote|Ris|BibTeX
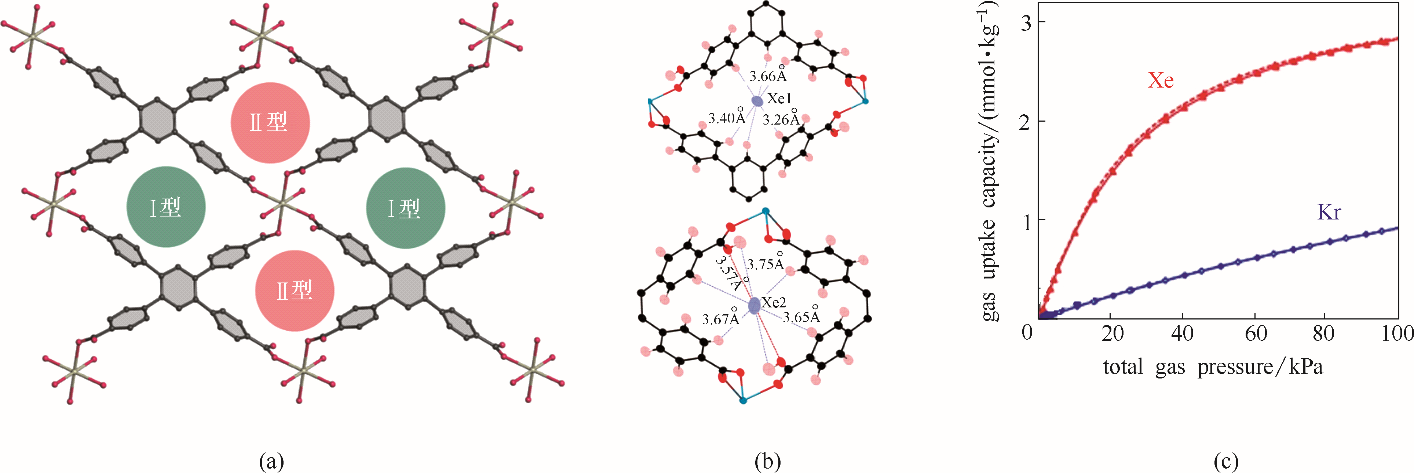
Fig.2 Pore structure of SBMOF-2(a); Binding sites of Xe with pore Ⅰ (upper) and pore II (lower) in SBMOF-2 (b); Isotherms of Xe/Kr in SBMOF-2 collected at 298 K (c)
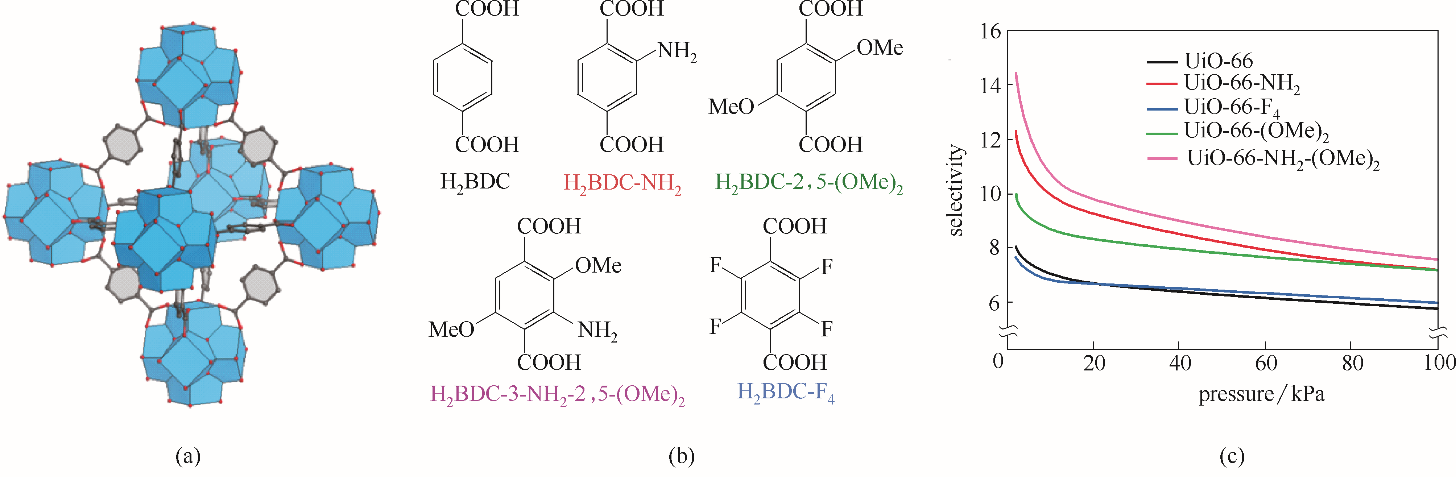
Fig.3 Structure of UiO-66(a); Functionalized ligands for UiO-66 (b); IAST-predicted Xe/Kr selectivity at 283 K for pristine and functionalized UiO-66 materials (Xe/Kr=20∶80) (c)

Fig.6 Co squarate structure with -OH groups decorated in channels (a); Xe binding site in Co squarate (b); Xe adsorption isotherms of Co squarate at low pressure at 298 K and comparison with other materials (c); Comparison of the IAST selectivity of Co squarate versus other materials for Xe/Kr (20/80) mixtures at 298 K (d)
| 吸附剂 | Xe吸附容量/ (mmol·g-1) | IAST选择性 (Xe/Kr=20∶80) | Xe等量吸附热Qst/ (kJ·mol-1) | Xe Henry系数/(mmol·g-1·bar-1) | Henry选择性 |
|---|---|---|---|---|---|
| MOF-5 | 1.98 | 3 | 13.75 | — | 8.56 |
| HKUST-1 | 3.3 | 2.6 | 17.5 | 12.2 | 8.5 |
| Ni-MOF-74 | 4.2 | 4 | 22 | 8.4 | 5.8 |
| Ag@Ni-MOF-74 | 4.6 | 11.5 | 23.6 | — | — |
| MOF-505 | 6.31 | 9~10 | — | 10.26 | 6.8 |
| Co3(HCOO)6 | 2 | 12 | 28 | 9.9 | 8.7 |
| MOF-Cu-H | 3.19 | 15.8 | 33.4 | 39.74 | 15.8 |
| UiO-66 | 1.58 | ~7 | ~22 | — | — |
| NU-403-PSDH | 2.23 | ~9 | ~26 | — | — |
| CPM-6 | 2.89 | 7.3 | 25.1 | 6.15 | 7.2 |
| Co2+-CPM-6 | 3.20 | 9.3 | 25.9 | 6.28 | 7.8 |
| SBMOF-2 | 2.83 | 10 | 26.4 | 10.5 | 8.6 |
| SBMOF-1 | 1.38 | 16① | 35 | 38.42 | 16.2 |
| CROFOUR-1-Ni | 1.8 | 22 | 37.4 | 18.73 | 24.3 |
| CROFOUR-2-Ni | 1.5 | 15.5 | 30.5 | 15.95 | 18.5 |
| Co squarate | 1.35 | 69.7 | 43.6 | 192.06 | 51.4 |
| UTSA-49 | 3 | 9.2 | 23.53 | — | — |
| ZU-62 | 3.76 | 9.72② | 35.2③ | — | — |
| FMOF-Cu | 0.8 | 2④ | 15 | — | — |
| NaA | 1.52~2.28 | 4.5⑤ | — | — | — |
| AgZ-PAN | 0.46⑥ | 4.6⑦ | — | — | — |
| Ag@ZSM-5 | ~1.2 | ~40 | 65 | — | ~3 |
| activated carbon | 4.2 | 2.9 | 28.2 | 16.2 | 9.1 |
| Z11CBF-1000-2 | 4.87 | 13.0 | 32.1 | 80.0 | 19.7 |
| CC3 | 2.69 | 20.4 | 31.3 | ~19 | ~2 |
| Noria | 1.55 | 9.4 | 24.5~26.9 | ~9 | ~9.5 |
Table 1 Xe adsorption capacity and Xe/Kr selectivity for various materials at 298 K and 100 kPa
| 吸附剂 | Xe吸附容量/ (mmol·g-1) | IAST选择性 (Xe/Kr=20∶80) | Xe等量吸附热Qst/ (kJ·mol-1) | Xe Henry系数/(mmol·g-1·bar-1) | Henry选择性 |
|---|---|---|---|---|---|
| MOF-5 | 1.98 | 3 | 13.75 | — | 8.56 |
| HKUST-1 | 3.3 | 2.6 | 17.5 | 12.2 | 8.5 |
| Ni-MOF-74 | 4.2 | 4 | 22 | 8.4 | 5.8 |
| Ag@Ni-MOF-74 | 4.6 | 11.5 | 23.6 | — | — |
| MOF-505 | 6.31 | 9~10 | — | 10.26 | 6.8 |
| Co3(HCOO)6 | 2 | 12 | 28 | 9.9 | 8.7 |
| MOF-Cu-H | 3.19 | 15.8 | 33.4 | 39.74 | 15.8 |
| UiO-66 | 1.58 | ~7 | ~22 | — | — |
| NU-403-PSDH | 2.23 | ~9 | ~26 | — | — |
| CPM-6 | 2.89 | 7.3 | 25.1 | 6.15 | 7.2 |
| Co2+-CPM-6 | 3.20 | 9.3 | 25.9 | 6.28 | 7.8 |
| SBMOF-2 | 2.83 | 10 | 26.4 | 10.5 | 8.6 |
| SBMOF-1 | 1.38 | 16① | 35 | 38.42 | 16.2 |
| CROFOUR-1-Ni | 1.8 | 22 | 37.4 | 18.73 | 24.3 |
| CROFOUR-2-Ni | 1.5 | 15.5 | 30.5 | 15.95 | 18.5 |
| Co squarate | 1.35 | 69.7 | 43.6 | 192.06 | 51.4 |
| UTSA-49 | 3 | 9.2 | 23.53 | — | — |
| ZU-62 | 3.76 | 9.72② | 35.2③ | — | — |
| FMOF-Cu | 0.8 | 2④ | 15 | — | — |
| NaA | 1.52~2.28 | 4.5⑤ | — | — | — |
| AgZ-PAN | 0.46⑥ | 4.6⑦ | — | — | — |
| Ag@ZSM-5 | ~1.2 | ~40 | 65 | — | ~3 |
| activated carbon | 4.2 | 2.9 | 28.2 | 16.2 | 9.1 |
| Z11CBF-1000-2 | 4.87 | 13.0 | 32.1 | 80.0 | 19.7 |
| CC3 | 2.69 | 20.4 | 31.3 | ~19 | ~2 |
| Noria | 1.55 | 9.4 | 24.5~26.9 | ~9 | ~9.5 |
| 1 | Bussiahn R, Gortchakov S, Lange H, et al. Experimental and theoretical investigations of a low-pressure He-Xe discharge for lighting purpose[J]. Journal of Applied Physics, 2004, 95(9): 4627-4634. |
| 2 | Tsuchiya A, Obara N, Miwa M, et al. Hematoporphyrin derivative and laser photoradiation in the diagnosis and treatment of bladder cancer[J]. Journal of Urology, 1983, 130(1): 79-82. |
| 3 | Cullen S C, Gross E G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton[J]. Science, 1951, 113(2942): 580-582. |
| 4 | Albert M S, Cates G D, Driehuys B, et al. Biological magnetic resonance imaging using laser-polarized 129Xe[J]. Nature, 1994, 370(6486): 199-201. |
| 5 | 孙小兵. 核电在中国中长期能源供应体系中的作用[J]. 南方能源建设, 2016, 3(3): 6-15. |
| Sun X B. The role of nuclear power in China's medium and long term energy supply system[J]. Southern Energy Construction, 2016, 3(3): 6-15. | |
| 6 | Blue Ribbon Commission on America's Energy Future. Report to the secretary of energy[R]. Washington D C: U.S. Department of Energy, 2012. |
| 7 | Blue Ribbon Commission on America's Energy Future. Strategy for the management and disposal of used nuclear fuel and high-level radioactive waste[R]. Washington D C: U.S. Department of Energy, 2013. |
| 8 | Auer M, Kumberg T, Sartorius H, et al. Ten years of development of equipment for measurement of atmospheric radioactive xenon for the verification of the CTBT[J]. Pure and Applied Geophysics, 2010, 167(4): 471-486. |
| 9 | Kerry F G. Industrial Gas Handbook: Gas Separation and Purification[M]. Boca Raton, FL: CRC Press, 2007. |
| 10 | Jameson C J, Jameson A K, Lim H M. Competitive adsorption of xenon and krypton in zeolite NaA: 129Xe nuclear magnetic resonance studies and grand canonical Monte Carlo simulations[J]. The Journal of Chemical Physics, 1997, 107(11): 4364-4372. |
| 11 | Munakata K, Yamatsuki S, Tanaka K, et al. Screening test of adsorbents for recovery of krypton[J]. Journal of Nuclear Science and Technology, 2000, 37(1): 84-89. |
| 12 | Munakata K, Kanjo S, Yamatsuki S, et al. Adsorption of noble gases on silver-mordenite[J]. Journal of Nuclear Science and Technology, 2003, 40(9): 695-697. |
| 13 | Garn T G, Law J D, Greenhalgh M R, et al. Composite media for fluid stream processing, a method of forming the composite media, and a related method of processing a fluid stream: US8686083[P]. 2014-04-01. |
| 14 | Garn T G, Law J D, Greenhalgh M R. FY-12 INL krypton capture activities supporting the off-gas sigma team[R]. Idaho Falls, Idaho, USA: Idaho National Laboratory, 2012. |
| 15 | Daniel C, Elbaraoui A, Aguado S, et al. Xenon capture on silver-loaded zeolites: characterization of very strong adsorption sites[J]. Journal of Physical Chemistry C, 2013, 117(29): 15122-15129. |
| 16 | Deliere L, Coasne B, Topin S, et al. Breakthrough in xenon capture and purification using adsorbent-supported silver nanoparticles[J]. Chemistry-A European Journal, 2016, 22(28): 9660-9666. |
| 17 | Bazan R E, Bastos-Neto M, Moeller A, et al. Adsorption equilibria of O2, Ar, Kr and Xe on activated carbon and zeolites: single component and mixture data[J]. Adsorption-Journal of the International Adsorption Society, 2011, 17(2): 371-383. |
| 18 | Thallapally P K, Grate J W, Motkuri R K. Facile xenon capture and release at room temperature using a metal-organic framework: a comparison with activated charcoal[J]. Chemical Communications, 2012, 48(3): 347-349. |
| 19 | Gong Y J, Tang Y M, Mao Z H, et al. Metal-organic framework derived nanoporous carbons with highly selective adsorption and separation of xenon[J]. Journal of Materials Chemistry A, 2018, 6(28): 13696-13704. |
| 20 | 冯淑娟, 周崇阳, 周国庆, 等. 氙在活性炭和碳分子筛上的动态吸附性能[J]. 核化学与放射化学, 2010, 32(5): 274-279. |
| Feng S J, Zhou C Y, Zhou G Q, et al. Dynamic adsorption property of xenon on activated carbon and carbon molecular sieves[J]. Journal of Nuclear and Radiochemistry, 2010, 32(5): 274-279. | |
| 21 | 刘孟, 张莉, 王茜. 碳分子筛和活性炭吸附氙气性能的研究[J]. 湘潭大学自然科学学报, 2015, 37(2): 27-32. |
| Liu M, Zhang L, Wang Q. Adsorption properties of xenon by carbon molecular sieve and activated charcoal[J]. Natural Science Journal of Xiangtan University, 2015, 37(2): 27-32. | |
| 22 | Zhou H C, Long J R, Yaghi O M. Introduction to metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 673-674. |
| 23 | Banerjee D, Cairns A J, Liu J, et al. Potential of metal-organic frameworks for separation of xenon and krypton[J]. Accounts of Chemical Research, 2015, 48(2): 211-219. |
| 24 | Furukawa H, Ko N, Go Y B, et al. Ultrahigh porosity in metal-organic frameworks[J]. Science, 2010, 329(5990): 424-428. |
| 25 | Grzesiak A L, Uribe F J, Ockwig N W, et al. Polymer-induced heteronucleation for the discovery of new extended solids[J]. Angewandte Chemie International Edition, 2006, 45(16): 2553-2556. |
| 26 | Cohen S M. Modifying MOFs: new chemistry, new materials[J]. Chemical Science, 2010, 1(1): 32-36. |
| 27 | Wang B, Xie L H, Wang X Q, et al. Applications of metal-organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal[J]. Green Energy & Environment, 2018, 3(3): 191-228. |
| 28 | Lv X L, Wang K C, Wang B, et al. A base-resistant metalloporphyrin metal-organic framework for C—H bond halogenation[J]. Journal of the American Chemical Society, 2017, 139(1): 211-217. |
| 29 | Yang F, Xu G, Dou Y B, et al. A flexible metal-organic framework with a high density of sulfonic acid sites for proton conduction[J]. Nature Energy, 2017, 2(11): 877-883. |
| 30 | Horcajada P, Chalati T, Serre C, et al. Porous metal-organic framework nanoscale carriers as a potential platform for drug delivery and imaging[J]. Nature Materials, 2010, 9(2): 172-178. |
| 31 | Li J R, Yu J M, Lu W G, et al. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption[J]. Nature Communications, 2013, 4(1): 1538. |
| 32 | Chen K J, Madden D G, Pham T, et al. Tuning pore size in square-lattice coordination networks for size-selective sieving of CO2[J]. Angewandte Chemie International Edition, 2016, 55(35): 10268-10272. |
| 33 | Chen B L, Ockwig N W, Millward A R, et al. High H2 adsorption in a microporous metal-organic framework with open metal sites[J]. Angewandte Chemie International Edition, 2005, 44(30): 4745-4749. |
| 34 | Dinca M, Long J R. Hydrogen storage in microporous metal-organic frameworks with exposed metal sites[J]. Angewandte Chemie International Edition, 2008, 47(36): 6766-6779. |
| 35 | Wood C D, Tan B, Trewin A, et al. Microporous organic polymers for methane storage[J]. Advanced Materials, 2008, 20(10): 1916-1921. |
| 36 | Niu Z, Cui X L, Pham T, et al. A metal-organic framework based methane nano-trap for the capture of coal-mine methane[J]. Angewandte Chemie International Edition, 2019, 58(30): 10138-10141. |
| 37 | Nijem N, Wu H, Canepa P, et al. Tuning the gate opening pressure of metal-organic frameworks (MOFs) for the selective separation of hydrocarbons[J]. Journal of the American Chemical Society, 2012, 134(37): 15201-15204. |
| 38 | Li B, Cui X L, Daniel O'Nolan, et al. An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity[J]. Advanced Materials, 2017, 29(47): 1704210. |
| 39 | Bao Z B, Wang J W, Zhang Z G, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 40 | Mueller U, Schubert M, Teich F, et al. Metal-organic frameworks-prospective industrial application[J]. Journal of Materials Chemistry, 2006, 16(7): 626-636. |
| 41 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 42 | Liu J, Strachan D M, Thallapally P K. Enhanced noble gas adsorption in Ag@MOF-74Ni[J]. Chemical Communications, 2013, 50(4): 466-468. |
| 43 | Chui S Y, Lo M F, Charmant J P H, et al. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n[J]. Science, 1999, 283(5405): 1148-1150. |
| 44 | Liu J, Thallapally P K, Strachan D. Metal-organic frameworks for removal of Xe and Kr from nuclear fuel reprocessing plants[J]. Langmuir, 2012, 28(31): 11584-11589. |
| 45 | Hulvey Z, Lawler K V, Qiao Z, et al. Noble gas adsorption in copper trimesate, HKUST-1: an experimental and computational study[J]. The Journal of Physical Chemistry C, 2013, 117(39): 20116-20126. |
| 46 | Böhlmann W, Pöppl A, Sabo M, et al. Characterization of the metal-organic framework compound Cu3(benzene1,3,5-tricarboxylate)2 by means of 129Xe nuclear magnetic and electron paramagnetic resonance spectroscopy[J]. The Journal of Physical Chemistry B, 2006, 110(41): 20177-20181. |
| 47 | Liu Z, Wu Y, Liu B, et al. Tuning the adsorption and separation properties of noble gases and N2 in CuBTC by ligand functionalization[J]. RSC Advances, 2016, 6(94): 91093-91101. |
| 48 | Chen X, Plonka A M, Banerjee D, et al. Direct observation of Xe and Kr adsorption in a Xe-selective microporous metal-organic framework[J]. Journal of the American Chemical Society, 2015, 137(22): 7007-7010. |
| 49 | Dan-Hardi M, Serre C, Frot T, et al. A new photoactive crystalline highly porous titanium (Ⅳ) dicarboxylate[J]. Journal of the American Chemical Society, 2009, 131(31): 10857-10859. |
| 50 | Fu Y, Sun D, Chen Y, et al. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction[J]. Angewandte Chemie International Edition, 2012, 51(14): 3364-3367. |
| 51 | Wang Z, Cohen S M. Postsynthetic covalent modification of a neutral metal-organic framework[J]. Journal of the American Chemical Society, 2007, 129(41): 12368-12369. |
| 52 | Tanabe K K, Wang Z, Cohen S M. Systematic functionalization of a metal-organic framework via a postsynthetic modification approach[J]. Journal of the American Chemical Society, 2008, 130(26): 8508-8517. |
| 53 | Yaghi O M, Kalmutzki M J, Diercks C S. Introduction to Reticular Chemistry[M]. Weinheim, Germany: Wiley-VCH, 2019: 145-173. |
| 54 | Chen R, Zhang J, Chelora J, et al. Ruthenium(Ⅱ) complex incorporated UiO-67 metal-organic framework nanoparticles for enhanced two-photon fluorescence imaging and photodynamic cancer therapy[J]. ACS Applied Materials & Interfaces, 2017, 9(7): 5699-5708. |
| 55 | Kim M, Cahill J F, Fei H, et al. Postsynthetic ligand and cation exchange in robust metal-organic frameworks[J]. Journal of the American Chemical Society, 2012, 134(43): 18082-18088. |
| 56 | Kim M, Cahill J F, Su Y, et al. Postsynthetic ligand exchange as a route to functionalization of ‘inert' metal-organic frameworks[J]. Chemical Science, 2011, 3(1): 126-130. |
| 57 | Lee S J, Kim S, Kim E J, et al. Adsorptive separation of xenon/krypton mixtures using ligand controls in a zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 335: 345-351. |
| 58 | Sikora B J, Wilmer C E, Greenfield M L, et al. Thermodynamic analysis of Xe/Kr selectivity in over 137000 hypothetical metal-organic frameworks[J]. Chemical Science, 2012, 3(7): 2217-2223. |
| 59 | Wang H, Yao K X, Zhang Z J, et al. The first example of commensurate adsorption of atomic gas in a MOF and effective separation of xenon from other noble gases[J]. Chemical Science, 2014, 5(2): 620-624. |
| 60 | Xiong S S, Gong Y J, Hu S L, et al. A microporous metal-organic framework with commensurate adsorption and highly selective separation of xenon[J]. Journal of Materials Chemistry A, 2018, 6(11): 4752-4758. |
| 61 | Li T, Kozlowski M T, Doud E A, et al. Stepwise ligand exchange for the preparation of a family of mesoporous MOFs[J]. Journal of the American Chemical Society, 2013, 135(32): 11688-11691. |
| 62 | Idrees K B, Chen Z, Zhang X, et al. Tailoring pore aperture and structural defects in zirconium-based metal-organic frameworks for krypton/xenon separation[J]. Chemistry of Materials, 2020, 32(9): 3776-3782. |
| 63 | 刘博煜, 龚有进, 刘强, 等. 新型多孔材料在惰性气体Xe/Kr分离中的应用[J]. 材料导报, 2017, 31(19): 54-62. |
| Liu B Y, Gong Y J, Liu Q, et al. Application of novel porous materials in noble gas Xe/Kr separation[J]. Materials Reports, 2017, 31(19): 54-62. | |
| 64 | Liu B Y, Gong Y J, Wu X N, et al. Enhanced xenon adsorption and separation with an anionic indium-organic framework by ion exchange with Co2+[J]. RSC Advances, 2017, 7(87): 55012-55019. |
| 65 | Banerjee D, Simon C M, Plonka A M, et al. Metal-organic framework with optimally selective xenon adsorption and separation[J]. Nature Communications, 2016, 7: ncomms11831. |
| 66 | Mohamed M H, Elsaidi S K, Pham T, et al. Hybrid ultra-microporous materials for selective xenon adsorption and separation[J]. Angewandte Chemie International Edition, 2016, 55(29): 8285-8289. |
| 67 | Li L Y, Guo L D, Zhang Z G, et al. A robust squarate-based metal-organic framework demonstrates record-high affinity and selectivity for xenon over krypton[J]. Journal of the American Chemical Society, 2019, 141(23): 9358-9364. |
| 68 | Witman M, Ling S, Jawahery S, et al. The influence of intrinsic framework flexibility on adsorption in nanoporous materials[J]. Journal of the American Chemical Society, 2017, 139(15): 5547-5557. |
| 69 | Xiong S S, Liu Q, Wang Q, et al. A flexible zinc tetrazolate framework exhibiting breathing behaviour on xenon adsorption and selective adsorption of xenon over other noble gases[J]. Journal of Materials Chemistry A, 2015, 3(20): 10747-10752. |
| 70 | Wang Q J, Ke T,Yang L F, et al. Separation of Xe from Kr with record selectivity and productivity in anion-pillared ultramicroporous materials by inverse size-sieving effect[J]. Angewandte Chemie International Edition, 2020, 59(9): 3423-3428. |
| 71 | Fernandez C A, Liu J, Thallapally P K, et al. Switching Kr/Xe selectivity with temperature in a metal-organic framework[J]. Journal of the American Chemical Society, 2012, 134(22): 9046-9049. |
| 72 | Sun X D, Yao S, Li G H, et al. A flexible doubly interpenetrated metal-organic framework with breathing behavior and tunable gate opening effect by introducing Co2+ into Zn4O clusters[J]. Inorganic Chemistry, 2017, 56(11): 6645-6651. |
| 73 | Zhu A X, Yang Q Y, Mukherjee S, et al. Tuning the gate-opening pressure in a switching pcu coordination network, X-pcu-5-Zn, by pillar-ligand substitution[J]. Angewandte Chemie International Edition, 2019, 58(50): 18212-18217. |
| 74 | Elsaidi S K, Mohamed M H, Banerjee D, et al. Flexibility in metal-organic frameworks: a fundamental understanding[J]. Coordination Chemistry Reviews, 2018, 358: 125-152. |
| 75 | Kong G Q, Wu C D. Four novel coordination polymers based on a flexible zwitterionic ligand and their framework dependent luminescent properties[J]. Crystal Growth & Design, 2010, 10(10): 4590-4595. |
| 76 | Holst J R, Trewin A, Cooper A I. Porous organic molecules[J]. Nature Chemistry, 2010, 2(11): 915-920. |
| 77 | Chen L J, Reiss P S, Chong S Y, et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages[J]. Nature Materials, 2014, 13(10): 954-960. |
| 78 | Patil R S, Banerjee D, Simon C M, et al. Noria: a highly Xe-selective nanoporous organic solid[J]. Chemistry-A European Journal, 2016, 22(36): 12618-12623. |
| 79 | Tozawa T, Jones J T A, Swamy S I, et al. Porous organic cages[J]. Nature Materials, 2009, 8(12): 973-978. |
| 80 | Bezzu C G, Helliwell M, Warren J E, et al. Heme-like coordination chemistry within nanoporous molecular crystals[J]. Science, 2010, 327(5973): 1627-1630. |
| [1] | Caihong LIN, Li WANG, Yu WU, Peng LIU, Jiangfeng YANG, Jinping LI. Effect of alkali cations in zeolites on adsorption and separation of CO2/N2O [J]. CIESC Journal, 2023, 74(5): 2013-2021. |
| [2] | Xue’an LIU, Liyi TANG, Jian QIN, Dajiang TANG, Zhangfa TONG, Huiying QU. Preparation of carbon nanotube bridged porous carbon by Ni/Co-ZIF-8 pyrolysis and its application to supercapacitors [J]. CIESC Journal, 2022, 73(7): 3287-3297. |
| [3] | Heng MAO, Yue WANG, Sen WANG, Weimin LIU, Jing LYU, Fuxue CHEN, Zhiping ZHAO. APTES-modified ZIF-L/PEBA mixed matrix membranes for enhancing phenol perm-selective pervaporation [J]. CIESC Journal, 2022, 73(3): 1389-1402. |
| [4] | Chenxu GENG, Yuxiu SUN, Hongliang HUANG, Xiangyu GUO, Zhihua QIAO, Chongli ZHONG. Mechanochemically synthesized small sized MOF fillers assisted for highly efficient CO2 separation [J]. CIESC Journal, 2021, 72(9): 4750-4758. |
| [5] | WANG Jiexiang, LI Hongguo, YE Songshou, ZHENG Jinbao, CHEN Binghui. Halogen-rich zinc-adeninate framework construction and its catalytic performance on CO2 cycloaddition without cocatalyst [J]. CIESC Journal, 2021, 72(7): 3686-3695. |
| [6] | HAN Xiao,CHEN Yuting,SU Baogen,BAO Zongbi,ZHANG Zhiguo,YANG Yiwen,REN Qilong,YANG Qiwei. Advances in adsorbents for hexane isomers separation [J]. CIESC Journal, 2021, 72(7): 3445-3465. |
| [7] | Puxu LIU, Chaohui HE, Libo LI, Jinping LI. Stable mixed metal-organic framework for efficient C2H6/C2H4 separation [J]. CIESC Journal, 2020, 71(9): 4211-4218. |
| [8] | Tong YANG, Xiaobo HE, Fengxiang YIN. Preparation of M-MOF-74 (M = Ni, Co, Zn) and its performance in electrocatalytic synthesis of ammonia [J]. CIESC Journal, 2020, 71(6): 2857-2870. |
| [9] | Ye YUAN, Ming WANG, Yunqi ZHOU, Zhi WANG, Jixiao WANG. Progress in pore size regulation of metal-organic frameworks [J]. CIESC Journal, 2020, 71(2): 429-450. |
| [10] | Yanqin XU, Liyue XIAO, Yuan CAO, Changguo CHEN, Dan WANG. Research on synthesis and application of metal-organic frame composites in supercapacitors [J]. CIESC Journal, 2020, 71(10): 4473-4490. |
| [11] | Miao CHANG, Lei LIU, Qingyuan YANG, Dahuan LIU, Chongli ZHONG. Study on efficient separation of SF6/N2 mixture using a hydrothermally stable metal-organic framework [J]. CIESC Journal, 2020, 71(1): 320-328. |
| [12] | Bo SONG,Min YANG,Ran AN,Xiaopo WANG. Accurate calculations of second dielectric virial coefficient of noble gases [J]. CIESC Journal, 2019, 70(S2): 50-53. |
| [13] | Lei WANG, Guiying FANG, Qingyuan YANG. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening [J]. CIESC Journal, 2019, 70(3): 1135-1143. |
| [14] | Ke AN, Dong YANG, Zhanfeng ZHAO, Hanjie REN, Yao CHEN, Zhiyuan ZHOU, Zhongyi JIANG. Research progress on microenvironment regulation of metal-organic framework photocatalyst [J]. CIESC Journal, 2019, 70(10): 3776-3790. |
| [15] | XU Jiaxing, CHAO Jingwei, LI Tingxian, WANG Ruzhu. Preparation and characterization of expanded graphite/metal organic frameworks composite sorbent [J]. CIESC Journal, 2018, 69(S2): 492-499. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||